TEN YEARS ago in this journal’s Landmark Article series of reproduced historical publications, the remarkable impact was described1 of the 1956 report in JAMA by Merrill et al2 of an identical twin renal transplantation. The operation was performed in December 1954, by Joseph E. Murray (Nobel Laureate, 1990) and his associates at the Peter Bent Brigham Hospital, Boston, Mass. By avoiding problems with rejection, such cases symbolized what might some-day be accomplished if the immunologic reaction could be controlled. Then, on January 24, 1959, the barrier posed by genetic nonidentity was breached for the kidneys with a successful fraternal twin transplantation3 by the same team.
Over the ensuing 35 years, the beach-head has been expanded by the successful allotransplantation of the liver,4 heart,5 lung,6 pancreas,7 intestine,8 multiple abdominal viscera,9 and bone marrow.10,11 Such milestones are never eroded from the landscape, but their appearance changes as the days wear on. The field of organ transplantation has been full of such transitions as insight has deepened into what actually has been accomplished. These shifting perceptions are the subject of this discussion, with emphasis on their potential future application.
BACKGROUND
Although details are obscure, there was little mystery after 1944 about the general meaning of transplant rejection, following its elucidation by Medawar (Nobel Laureate, 1960) as an immunologic event.12 In contrast, why allografts or xenografts can escape from rejection with or without the aid of immunosuppression has been one of the most arcane subjects in biology ever since the description of acquired tolerance by Billingham, Brent, and Medawar13,14 more than four decades ago.
A simple explanation for the tolerance in their special model was at first beguiling. Immunocompetent adult spleen cells were injected in utero or perinatally into mice that had not yet evolved the immunologic equipment to reject them. The engrafted cells flourished, perpetuated themselves, and in effect endowed the recipient with the donor immune system. Thereafter, the chimeric mice failed to recognize donor strain skin or other tissues as alien.
THE ONE-WAY PARADIGM
In essence, the perception of tolerance as a switch in immunologic apparatus defined the one-way paradigm of transplantation immunology. The concept of a unidirectional immune reaction in these experiments was strengthened by the studies of Main and Prehn15 who demonstrated the same outcome in irradiated adult mice, whose cytoablated hematolymphopoietic cells were reconstituted with bone marrow. Hundreds of subsequent tolerance induction experiments in animals and eventually clinical bone marrow transplantation seemingly depended on a similar natural, or iatrogenically imposed, defenseless recipient state.
The anticipated clinical application of such tolerance induction was temporarily derailed in 1957 when it was realized that an immunologically active graft could turn the tables and reject the recipient. Billingham and Brent16 showed in their mouse model and Simonsen17 in chickens that this risk of graft-vs-host disease (GVHD) (also called runt disease) was roughly proportional to the extent of the major histocompatibility complex barrier. Such disparities became measurable in humans after identification of HLA by Dausset18 (Nobel Laureate, 1980) and by Terasaki and others.19 The complication of GVHD in rodent20 and large animal irradiation chimera models21–24 forestalled for many years the clinical use of HLA-mismatched bone marrow cells or other mature immunocytes, either for immunologic reconstitution for purely hematologic purposes or as a means of facilitating whole organ graft acceptance.
Nevertheless, a strategy for clinical bone marrow transplantation eventually was assembled directly from the rodent experiments, but with similar histocompatibility-imposed restrictions.21 After recipient cytoablation with total body irradiation or cytotoxic drugs, stable chimerism could be induced in humans by the infusion of donor bone marrow if there was a good HLA match, but otherwise there was an intolerable incidence of GVHD. After successful engraftment, maintenance immunosuppression frequently was not needed, mimicking the kind of acquired immunologic tolerance originally described by Billingham, Brent, and Medawar,13,14 and then by Main and Prehn.15
The eventual success of clinical bone marrow transplantation in 196810,11 was supremely gratifying because it had been so logical, as Thomas (Nobel Laureate, 1990) has summarized.21 However, the achievement effectively detached from a scientific base those who by this time already had recorded thousands of human whole organ transplantations (mostly kidneys) under continuous immunosuppression—without host preconditioning, dependence on HLA matching, or problems with GVHD. In the case described by Merrill et al in 1960,3 the renal recipient, whose donor was his fraternal (dizygotic) twin brother, was preconditioned with sublethal total body irradiation. However, donor bone marrow was not given, already a significant departure from the Billingham-Brent-Medawar framework. The recipient’s own bone marrow recovered, and the transplanted kidney and patient survived for 20 years. Six additional examples of protracted kidney graft survival (≥1 year) after recipient irradiation without marrow were recorded in Paris over the next 36 months.25,26 Five of the six donors were more distant than a fraternal twin and two were genetically unrelated.26
These were isolated successes in a sea of failures. The frustration continued after the introduction for human renal transplantation of mercaptopurine and its analogue azathioprine by Murray et al27 following extensive experimental studies, first with rodent skin transplantation28,29 and then with canine kidney transplant models.27,30–32 The drugs originally had been developed as anti-leukemic agents by Elion, Bieber, and Hitchings33 (Elion and Hitchings, Nobel Laureates, 1988) and were first demonstrated to be immunosuppressive by Schwartz and Dameshek.34
Although the sixth patient treated by Murray et al with one or the other of these myelotoxic drugs had function of a nonrelated renal allograft for 17 months, the clinical results were poor at first,27,35 similar to those with total body irradiation. The tidal wave of whole organ cases began in earnest in 1962 when azathioprine was combined with prednisone.36 A characteristic cycle was identified in which rejection could be reversed surprisingly easily with prednisone. More important, the need later on for maintenance immunosuppression frequently declined and in occasional cases treatment could be stopped. The same sequence has been seen since with all other organs transplanted and with all of the immunosuppressive regimens. Agents introduced later were more potent and reliable in chaperoning the desired chain of events: antilymphocyte globulin,37 cyclosporine,38 and tacrolimus (FK 506).39 Notwithstanding their diversity, all of the drugs seemed in a fundamentally similar way to have allowed something to change in the host, the graft, or both. But what?
Answers were not provided by the one-way paradigm of transplantation immunology that had gained ascendency by 1960 and remained unchallenged for more than 30 years. In this conceptualization of a unidirectional immune reaction, rejection of whole organs was construed as a mirror image of GVHD; the destroyers were the recipient immunocytes, and the allografts were the defenseless victims. The introduction of an in vitro model, the one-way mixed lymphocyte reaction of Bach and Hirschorn40 and Bain et al,41 made possible the detailed examination of this proposition and generated thousands of increasingly sophisticated cellular and ultimately molecular studies of immunologic reactions.
However, the plethora of new information resembled at times an exponentially expanding phone book without a coherent theme. Most seriously, this context lured successive generations of investigators into the trap of believing that tolerance induction for whole organ recipients (the “holy grail”) lay in variations on the HLA-limiting strategy used for bone marrow transplantation, namely, host preconditioning in preparation for a variety of donor leukocyte preparations.
THE TWO-WAY PARADIGM
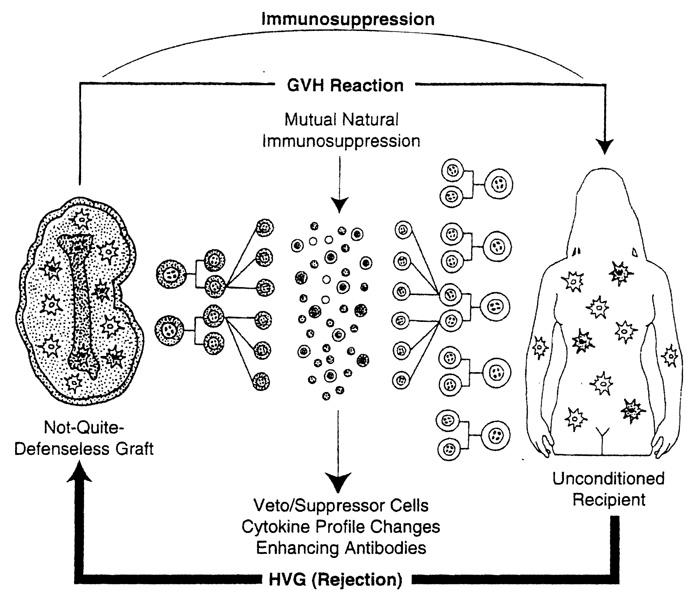
Thirty years and a revolution in immunology passed before a plausible explanation emerged for the success of the empirically developed whole organ transplantation practices, contrary to the initially pessimistic predictions of most experts of the earlier time. In 1992, it was discovered in a study of pioneer kidney and liver recipients who were still extant from the early days that donor leukocytes of bone marrow origin, which are part of the structure of all complex grafts (“passenger leukocytes”),42,43 had migrated from the organs and survived ubiquitously in these patients for up to 30 years.44,45 Thus, organ allograft acceptance was associated with the cryptic survival including stem cells of a small fragment of extramedullary donor marrow (depicted as a bone silhouette in Figure 1), which was assimilated into the overwhelmingly larger immunologic network of the host. The cell movement was in both directions, with small numbers of residual donor cells (microchimerism) in both the graft and host.
Figure 1.
Two-way paradigm (organ). Bidirectional mechanism of whole organ graft acceptance involving a graft-vs-host (GVH) reaction by the bone marrow–derived donor leukocytes in the graft that are pitted against the whole recipient immunologic apparatus (host-vs-graft [HVG], rejection). For standard whole organ clinical transplantation, the recipient is not preconditioned.
From this information, a revision of transplantation immunology was possible in which the immunologic confrontation following whole organ transplantation could be seen as bidirectional (GVH as well as host-vs-graft) and mutually canceling, providing the two participants in the David and Goliath mismatch could survive the initial onslaught. In a clinical context, but not in several animal models, this survival requires an umbrella of immunosuppression that protects both cell populations equally (Figure 1). Current research is targeted to understanding the amplification device by which a small number of cells can so profoundly affect the immunologic vision of the vast army against which it is arrayed. Although the chimeric leukocytes are multilineage,44–47 the antigen-presenting dendritic cells of Steinman and Cohn48 and Steinman49 are thought to be critical because they can modify the expression of cell interaction, major histocompatibility complex, and adhesion molecules—all of which determine how antigen signals are heeded by T cells.49
With the two-way paradigm, virtually every previously enigmatic problem seen clinically after experimental or clinical whole organ transplantation becomes either transparent, or at least susceptible to experimental inquiry44,45: why HLA matching is so poorly predictive of outcome, why organ grafts are inherently tolerogenic, and why GVHD does not develop after the transplantation of immunologically active grafts such as the liver and intestine. In addition, inexplicable results from a variety of earlier studies might be rationally reinterpreted.
For example, historical efforts to give extra donor antigen in the form of bone marrow50,51 or donor blood transfusions52–54 had been hampered in design or execution by the assumption that the infused cells would be destroyed without recipient preconditioning, justifiable anxiety about GVHD if host preconditioning was provided, and a lack of information about the appropriate timing of the infusions. The new information that chimerism is a naturally occurring event after whole organ transplantation44,45 exposed a perioperative window of opportunity during which unaltered HLA-incompatible bone marrow or donor-specific blood transfusion was predicted to be safe without recipient preparation or deviation from the generic practices of immunosuppression for whole organ transplantation that had evolved over the years from the original azathioprine-prednisone formula.36
The validity of this strategy was verified recently in nonpreconditioned recipients of cadaveric kidneys, livers, hearts, and lungs who were given 3 × 108/kg adjuvant bone marrow cells at the same time as organ transplantation under standard tacrolimus-prednisone treatment.55 Chimerism estimated to be more than 1000 times that occurring in whole organ recipients not given bone marrow could be reliably and safely produced and sustained. The persistent blood chimerism (usually > 1%), trend toward donor-specific nonreactivity, and high rate of patient and graft survival has marked the bone marrow-augmented recipients as an advantaged cohort. They are the first to undergo HLA-mismatched cadaveric organ transplantation with the reasonable prospect of eventually becoming drug free. The process of tolerance induction and drug weaning is expected to take 5 to 10 years in most patients who are given mismatched organs, and in some the drug-free state may never be attainable.
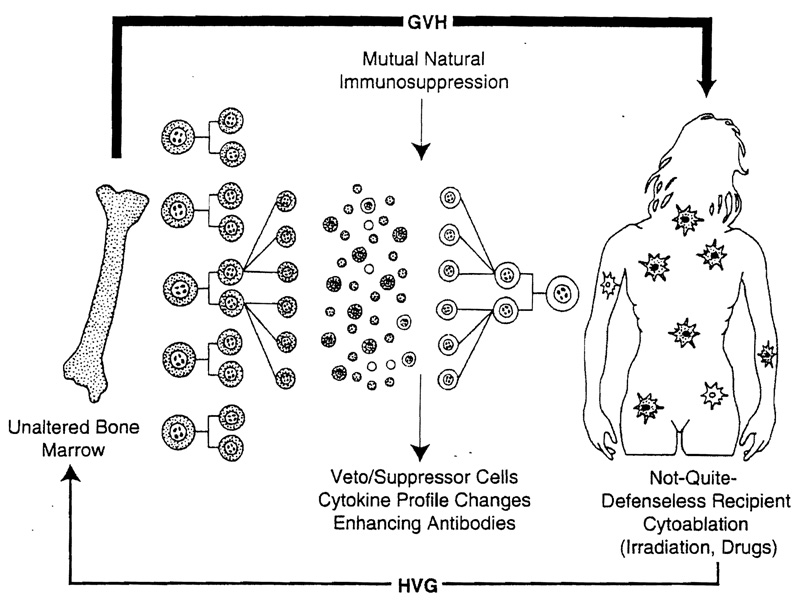
Thus, the seemingly vast gap between the bone marrow and whole organ transplantation fields was realized to reflect entrenched differences of treatment strategy—leaving intact the mutually censoring immunologic limbs with organ transplantation and deliberately trying to remove one of the limbs for bone marrow grafting procedures. It is doubtful that it is ever possible (much less desirable) with the cytoablation techniques of bone marrow transplantation to completely eliminate the entire recipient immune system (Figure 2). Although this was long assumed to have occurred in successful cases, a trace population of recipient leukocytes has been almost invariably detected with sensitive techniques in patients previously thought to have complete bone marrow replacement.56,57 These bone marrow recipients were in fact mirror images of successfully treated whole organ recipients, the difference being that their own rather than donor leukocytes constituted the trace population. In either kind of patient, the appearance of major histocompatibility–restricted veto and suppressor cells, enhancing antibodies, and changes in cytokine profile could be construed as by-products of and accessory to the seminal event of mixed chimerism (Figures 1 and 2).
Figure 2.
Two-way paradigm (bone marrow). Bidirectional paradigm in bone marrow transplantation where cytoablation is used to precondition the recipient. However, complete elimination of the recipient leukocyte population is almost never possible (see text). Note that the result in the recipient is a mirror-image version of whole organ transplantation (compare with Figure 1).
Beyond an adjuvant role for whole organ transplantation, an important question is whether HLA-mismatched bone marrow without an accompanying organ can be engrafted in patients whose disease can be corrected with a minimal or even microchimeric state, using the same immunosuppression as for marrow-augmented kidney, liver, and heart recipients, The potential list of indications in which complete marrow replacement is unnecessary is a long one, exemplified by the Iysozomal enzyme deficiencies.58 Another look into the future has been provided by the demonstration that xenograft transplantation is followed by the same cell migration process as that seen with allografts.59
CONCLUSION
The fusion of whole organ and bone marrow transplantation into a unitarian world may clarify the meaning of acquired transplantation tolerance. Experience from three decades of whole organ transplantation has shown that the end result of slowly evolving narrow immunologic nonreactivity can be produced in humans at any age, including patients with undetectable or surgically excised thymus glands, or after splenectomy. Ironically, the explanation of graft acceptance via the bidirectional immune transaction herein described has direct analogies to a hypothesis proposed from splenocyte and bone marrow transplant experiments by Simonsen60,61 and Michie, Woodruff, and Zeiss62 a third of a century ago, but abandoned because it could not be proved. This insight has not diminished the significance of the historical milestones of transplantation. However, the new information has displayed these landmarks in a truer light and it should make feasible better clinical strategies to achieve drug-free graft acceptance.
Acknowledgments
This work was aided by research grants from the Department of Veterans Affairs, Washington, DC, and Project Grant DK 29961 from the National Institutes of Health, Bethesda, Md.
References
- 1.Starzl TE. The landmark identical twin case. JAMA. 1984;251:2572–2573. [PMC free article] [PubMed] [Google Scholar]
- 2.Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. JAMA. 1956;160:277–282. doi: 10.1001/jama.1956.02960390027008. [DOI] [PubMed] [Google Scholar]
- 3.Merrill JP, Murray JE, Harrison JH, Friedman EA, Dealy JB, Jr, Dammin GJ. Successful homotransplantation of the kidney between non-identical twins. N Engl J Med. 1960;262:1251–1260. [Google Scholar]
- 4.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard CN. What we have learned about heart transplants. J Thorac Cardiovasc Surg. 1968;56:457–468. [PubMed] [Google Scholar]
- 6.Derom F, Barbier F, Ringoir S, et al. Ten-month survival after lung homotransplantation in man. J Thornc Cardiovasc Surg. 1971;61:835–846. [PubMed] [Google Scholar]
- 7.Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827–837. [PubMed] [Google Scholar]
- 8.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–943. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 10.Bach FH. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet. 1968;2:1364–1366. doi: 10.1016/s0140-6736(68)92672-x. [DOI] [PubMed] [Google Scholar]
- 11.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 12.Medawar PB. The behavior and fate of skin autografts and skin homografts in rabbits. J Anat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- 13.Billingham RE, Brent L, Medawar PB. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 14.Billingham R, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity, III: actively acquired tolerance. Philos Trans R Soc Lond (Biol) 1956;239:357–412. [Google Scholar]
- 15.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023–1029. [PubMed] [Google Scholar]
- 16.Billingham R, Brent L. A simple method for inducing tolerance of skin homografts in mice. Transpl Bull. 1957;4:67–71. [PubMed] [Google Scholar]
- 17.Simonsen M. The impact on the developing embryo and newborn animal of adult homologous cells. Acta Path Microbial Scand. 1957;40:480–500. [PubMed] [Google Scholar]
- 18.Dausset J. The HLA adventure. In: Terasaki PI, editor. History of HLA: Ten Recollections. Los Angeles, Calif: UCLA Tissue Typing Laboratory; 1990. pp. 1–20. [Google Scholar]
- 19.Terasaki PI. History of HLA: Ten Recollections. Los Angeles, Calif: UCLA Tissue Typing Laboratory; 1990. [Google Scholar]
- 20.Trentin JJ. Induced tolerance and ‘homologous disease’ in X-irradiated mice protected with homologous bone marrow. Proc Soc Exp Biol Med. 1957;96:139–144. doi: 10.3181/00379727-96-23414. [DOI] [PubMed] [Google Scholar]
- 21.Thomas ED. Allogeneic marrow grafting: a story of man and dog. In: Terasaki PI, editor. History of Transplantation: Thirty-Five Recollections. Los Angeles, Calif: UCLA Press; 1991. pp. 379–394. [Google Scholar]
- 22.Mannick JA, Lochte HL, Ashley CA, Thomas ED, Ferrebee JW. A functioning kidney homotransplant in the dog. Surgery. 1959;46:821–828. [PubMed] [Google Scholar]
- 23.Hume DM, Jackson BT, Zukoski CF, Lee HM, Kauffman HM, Egdahl RH. The homotransplantation of kidneys and of fetal liver and spleen after total body irradiation. Ann Surg. 1960;152:354–373. [PMC free article] [PubMed] [Google Scholar]
- 24.Rapaport FT, Bachvaroff RJ, Mollen N, Hirasawa H, Asano T, Ferrebee JW. Induction of unresponsiveness to major transplantable organs in adult mammals. Ann Surg. 1979;190:461–473. doi: 10.1097/00000658-197910000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamburger J, Vaysse J, Crosnier J, Auvert J, LaLanne AM, Hopper J., Jr Renal homotransplantation in man after radiation of the recipient. Am J Med. 1962;32:854–871. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 26.Kuss R, Legrain M, Mathe G, Nedey R, Camey M. Homologous human kidney transplantation: experience with six patients. Postgrad Med J. 1962;38:528–531. doi: 10.1136/pgmj.38.443.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Jr, Alexandre GW, Harrison JH. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337–355. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker W, Condie R, Weiner D, Varco RL, Good RA. Prolongation of skin homograft survival in rabbits by 6-mercaptopurine. Proc Soc Exp Biol Med. 1959;102:459–461. [Google Scholar]
- 29.Schwartz R, Dameshek W. The effects of 6-mercaptopurine on homograft reactions. J Clin Invest. 1960;39:952–958. doi: 10.1172/JCI104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calne RY. The rejection of renal homografts: inhibition in dogs by 6-mercaptopurine. Lancet. 1960;1:417–418. doi: 10.1016/s0140-6736(60)90343-3. [DOI] [PubMed] [Google Scholar]
- 31.Zukoski CF, Lee HM, Hume DM. The prolongation of functional survival of Canine renal homografts by 6-mercaptopurine. Surg Forum. 1960;11:470–472. [PubMed] [Google Scholar]
- 32.Calne RY. Inhibition of the rejection of renal homografts in dogs with purine analogues. Transpl Bull. 1961;28:445–461. [PubMed] [Google Scholar]
- 33.Elion GB, Bieber S, Hitchings GH. The fate of 6-mercaptopurine in mice. Ann N Y Acad Sci. 1955;60:297–303. doi: 10.1111/j.1749-6632.1954.tb40020.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 35.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 36.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 38.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 39.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach F, Hirschorn K. Lymphocyte interaction: a potential histocompatibility test in vitro. Science. 1964;143:813–814. doi: 10.1126/science.143.3608.813. [DOI] [PubMed] [Google Scholar]
- 41.Bain B, Vas MR, Lowenstein L. The development of large immature mononuclear cells in mixed leukocyte cultures. Blood. 1964;23:108–116. [PubMed] [Google Scholar]
- 42.Snell GD. The homograft reaction. Ann Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 43.Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967;158:127–129. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- 44.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 46.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 47.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice, I: morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 50.Monaco AP, Clark AW, Brown RW. Active enhancement of a human cadaver renal allograft with ALS and donor bone marrow: case report of an initial attempt. Surgery. 1976;79:384–392. [PubMed] [Google Scholar]
- 51.Barber WH, Mankin JA, Laskow DA, et al. Long-term results of a controlled prospective study with transfusion of donor specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Salvatierra O, Jr, Vincenti F, Amend WJ, et al. Deliberate donor-specific blood transfusions prior to living related renal transplantation: a new approach. Ann Surg. 1980;192:543–552. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson CB, Sicard GA, Etheredge EE. Pretreatment of renal allograft recipients with azathioprine and donor-specific blood products. Surgery. 1982;92:315–341. [PubMed] [Google Scholar]
- 54.Sollinger HW, Burlingham WJ, Sparks EM, Glass NR, Belzer FO. Donor-specific transfusions in unrelated and related HLA-mismatched donor-recipient combinations. Transplantation. 1984;38:612–615. doi: 10.1097/00007890-198412000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Przepiorka D, Thomas ED, Durham DM, Fisher L. Use of a probe to repeat sequence of the Y chromosome for detection of host cells in peripheral blood of bone marrow transplant recipients. Am J Clin Pathol. 1991;95:201–206. doi: 10.1093/ajcp/95.2.201. [DOI] [PubMed] [Google Scholar]
- 57.Wessman M, Popp S, Ruutu T, Volin L, Cremer T, Knuutila S. Detection of residual host cells after bone marrow transplantation using non-isotopic in situ hybridization and karyotype analysis. Bone Marrow Transplant. 1993;11:279–284. [PubMed] [Google Scholar]
- 58.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher’s disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starzl TE, Fung J, Tzakis A, et al. Baboon to human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simonsen M. On the acquisition of tolerance by adult cells. Ann N Y Acad Sci. 1960;87:382–390. doi: 10.1111/j.1749-6632.1960.tb23207.x. [DOI] [PubMed] [Google Scholar]
- 61.Simonsen M. Graft versus host reactions: their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467. [PubMed] [Google Scholar]
- 62.Michie D, Woodruff MFA, Zeiss IM. An investigation of immunological tolerance based on chimera analysis. Immunology. 1961;4:413–424. [PMC free article] [PubMed] [Google Scholar]