Abstract
Objective
Exposure to a number of drugs, chemicals or environmental factors can cause parkinsonism. Epidemiologic evidence supports a causal link between the consumption of flour made from the washed seeds of the plant, Cycas micronesica, by the Chamorro population of Guam and the development of Amyotrophic Lateral Sclerosis/Parkinsonism Dementia Complex (ALS/PDC).
Methods
We now report that consumption of washed cycad flour pellets by Sprague-Dawley male rats induces progressive parkinsonism.
Results
Cycad-fed rats displayed motor abnormalities after two to three months of feeding such as spontaneous unilateral rotation, shuffling gait and stereotypy. Histological and biochemical examination of brains from cycad-fed rats revealed an initial decrease in the levels of dopamine and its metabolites in the striatum (STR), followed by neurodegeneration of dopaminergic (DAergic) cell bodies in the substantia nigra pars compacta (SNc). α-synuclein (α-syn; proteinase K-resistant) and ubiquitin aggregates were found in the DAergic neurons of the SNc and neurites in the STR. In addition, we identified α-syn aggregates in neurons of the locus coeruleus and cingulate cortex. No loss of motor neurons in the spinal cord was found after chronic consumption of cycad flour. In an organotypic slice culture of the rat substantia nigra and the striatum, an organic extract of cycad causes a selective loss of DA neurons and α-synuclein aggregates in the substantia nigra.
Interpretation
Cycad-fed rats exhibit progressive behavioral, biochemical, and histological hallmarks of parkinsonism, coupled with a lack of fatality.
Keywords: α-synuclein, progressive neurodegeneration, cycad neurotoxins
Introduction
Parkinson’s disease (PD) afflicts an increasing number of older individuals worldwide.1–2 The etiology of PD remains largely unknown except for a minority of genetic related disorders and several well-documented environmental toxins or drugs known to induce parkinsonism.1,3 There is a provocative idea suggesting that exposure to environmental toxins, possibly primed by genetic susceptibility, can cause parkinsonism.4,5,11 One such example is the toxin(s) found in the flour of washed seeds from the plant Cycas micronesica, (cycad) which has been implicated in the development of Amyotrophic Lateral Sclerosis/Parkinsonism Dementia Complex (ALS/PDC) in the Chamorro population of Guam.6–12 A high incidence of ALS in Guam was first described in the 1940’s, and by the early 1960’s another co-morbid neurodegenerative disease was identified as PDC.6–10 These diseases have been linked to the ingestion of cycad seed flour tortillas, prepared after careful washing of the seeds.8 The Chamorros knew that unwashed seeds containing neurotoxins β-N-methylamino-l-alanine (BMAA) and cycasin were poisonous.9 However, they were unaware that even washed cycad seeds contained toxins that could produce neurodegeneration years later.10, 11
We began our investigation of the behavioral and neurobiological effects of ingested cycad flour in outbred Sprague-Dawley rats in an attempt to develop a rat model of ALS with which to compare to the SOD1G93A genetic model of ALS.15 Other investigators have developed a mouse model of ALS/PDC by feeding washed cycad flour to outbred CD1 mice.12–14 These mice developed an ALS-like syndrome with a loss of motor neurons and an eventual loss of dopaminergic innervation of the striatum (STR). However, cycad-fed rats displayed a behavioral phenotype resembling that of parkinsonism, with no symptoms of ALS. It has proven especially difficult to develop progressive rodent models for parkinsonism,16 however cycad-fed rats show the gradual development of multiple PD symptoms including decreased motor activity and a progressive loss of dopamine (DA) in the basal ganglia circuit, as well as aggregation of phosphorylated α-synuclein (phospho-α-syn) in multiple brain nuclei.
Materials and Methods
Animals
Twelve-week old Sprague-Dawley (SD) male rats were obtained from Taconic (Hudson, New York). Cohorts 1 and 2 each included 8 cycad-fed and 8 flour-fed rats and Cohort 3 included 8 cycad-fed and 6 flour-fed rats (Fig 1). All rats were individually housed under a reversed 12 h:12 h light:dark cycle (lights off at 07:00 h, lights on at 19:00 h). Regular rat chow and water were provided ad libitum. All animals were treated in accordance with federal standards and institutional guidelines.
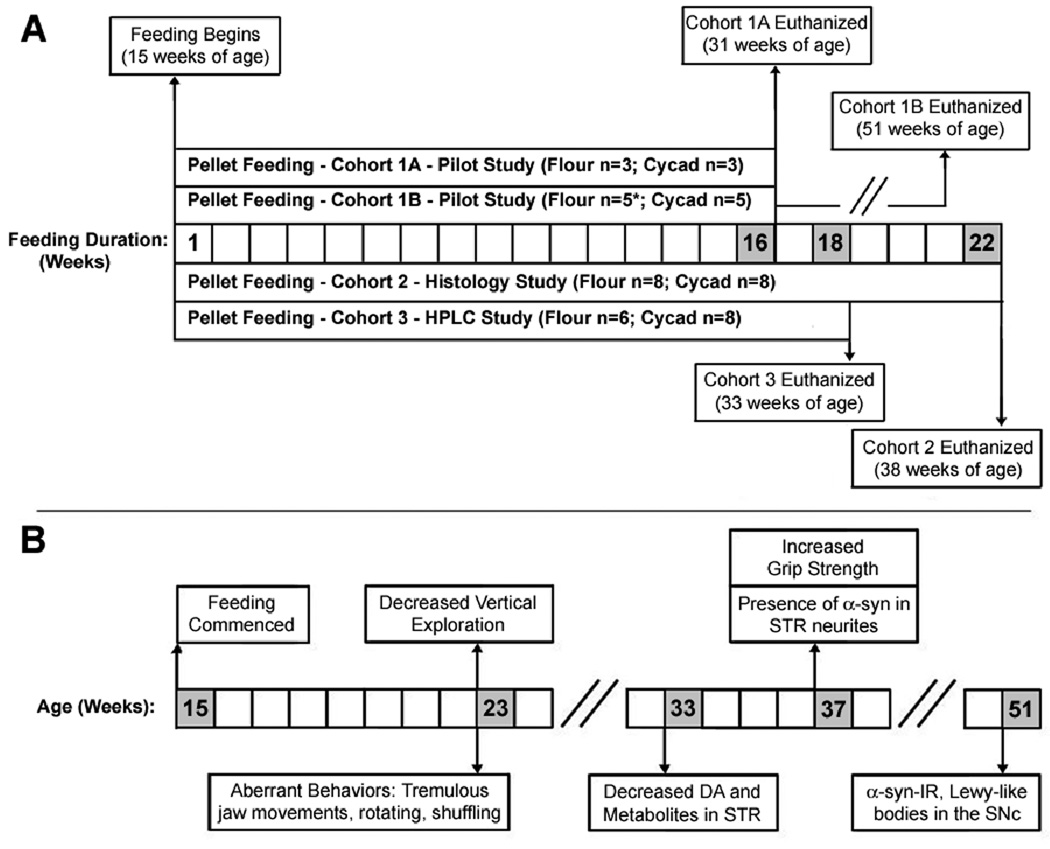
Figure 1.
Experimental paradigm and development of symptoms for cycad-induced model of parkinsonism. (A) Three cohorts of rats underwent the pellet-feeding paradigm as outlined above. Feeding began for all rats (flour-fed, n = 21; cycad-fed, n = 24) at 15 weeks of age. The pilot study consisted of Cohort 1A and 1B, in which rats were pellet fed over 16 weeks. At this time, Cohort 1A was euthanized, with the brain and spinal cord collected for subsequent analysis. To observe potential long-term effects of cycad exposure, Cohort 1B was monitored for an additional 20 weeks and then euthanized. Cohort 2 was pellet fed for 22 weeks before brains were collected for subsequent immunohistochemical analysis. Cohort 3 was pellet fed for 18 weeks, after which brains were collected for high-performance liquid chromatography (HPLC) analysis of dopamine (DA) and DA metabolite levels. See Supplementary Materials and Methods for additional details of experiments. Please note that due to the natural death of a flour-fed rat, the final count for the flour group in Cohort 1B is n = 4. (B) This figure summarizes the multiple parkinsonian-like symptoms that were observed in cycad-fed rats. Pellet feeding for all rats commenced at 15 weeks of age. At roughly 23 weeks of age, cycad-fed rats showed a variety of aberrant motor behaviors, including tremulous jaw movements and decreased vertical exploration. By 33 weeks of age, cycad-fed rats were found to have decreased levels of DA and DA metabolites in the striatum (STR) but not the substantia nigra pars compacta (SNc). At 37 weeks of age, cycad-fed rats displayed an increase in grip strength, and histological analysis revealed the presence of phosphorylated α-synuclein–immunoreactive cells (α-syn-IR) in the neurites of the STR but not in the DA neurons of the SNc. Finally, at 51 weeks of age, cycad-fed rats displayed aggregates of phosphorylated α-syn in the SNc.
Preparation of Cycad and Wheat Flour Pellets
Several batches of cycad seeds (Cycas micronesica, K.D. Hill) were obtained from Guam and washed using Khabazian’s protocol to remove all traces of BMAA and cycasin (Supplementary, S1).17 The washed seeds were ground to a flour-like consistency and formed into pellets (see supplemental methods).
Feeding Schedule
Monday through Friday, between 09:00–11:00 h, each rat was fed a 1.25 g flour pellet in addition to regular chow ad libitium. Due to the sweetened flavoring, rats readily consumed the flour pellets (food deprivation was not necessary). As outlined (Fig 1A), feeding began for all rats at 15 weeks of age. Cohorts 1A and 1B were pellet fed over a 16 week period. After 16 weeks of pellet feeding, Cohort 1A (n = 6) was euthanized and the brain and spinal cord were collected for subsequent histological analysis. To observe potential long-term effects of cycad exposure, Cohort 1B (n = 9, one flour-fed animal died of unexplained causes) was monitored, though not fed cycad, for an additional 20 weeks and then euthanized at 51 weeks of age. Cohorts 2 (n = 16) and 3 (n = 14) were pellet fed for 22 weeks and 18 weeks, then were euthanized at 38 weeks and 33 weeks of age, respectively, after which brains were either collected for histology (Cohort 2) or for High Performance Liquid Chromatography (HPLC) analysis of DA and DA metabolite levels (Cohort 3). Fig 1B provides a summary of the development of cycad-induced symptoms for all cohorts.
Behavioral Testing of Motor and Non-motor Function
Tests of motor function were performed every other week. A cylinder test was used to determine general motor activity represented by vertical exploration.18–19 The number of rears over a 3-minute interval in an enclosed cylinder was recorded for all rats throughout the feeding paradigm (including baseline recordings). Changes in gait were evaluated by paw-print analysis and grip strength was measured using a grip strength meter (Columbus Instruments).16,19 Aberrant behaviors such as tremulous jaw movements and spontaneous rotations were video recorded. Drug-induced behavioral tests were conducted by subcutaneously injecting 1 mg/kg apomorphine (Sigma) to induce either contralateral rotations in unilaterally rotating animals or to induce movement in animals demonstrating arrested movement, and 0.5 mg/kg apomorphine was used to suppress tremulous jaw movements.20
Immunohistochemistry and immunofluorescence
For fixation and tissue processing methods see Supplementary Materials and Methods.
Statistical Analysis
Data were compared using unpaired two-tailed Student's t-test or a repeated measure ANOVA as required. Values of p<0.05 were deemed significant. All statistical analysis was performed with SigmaStat and GraphPad Prism 4.0 software.
Results
Cycad-induced progressive motor dysfunction
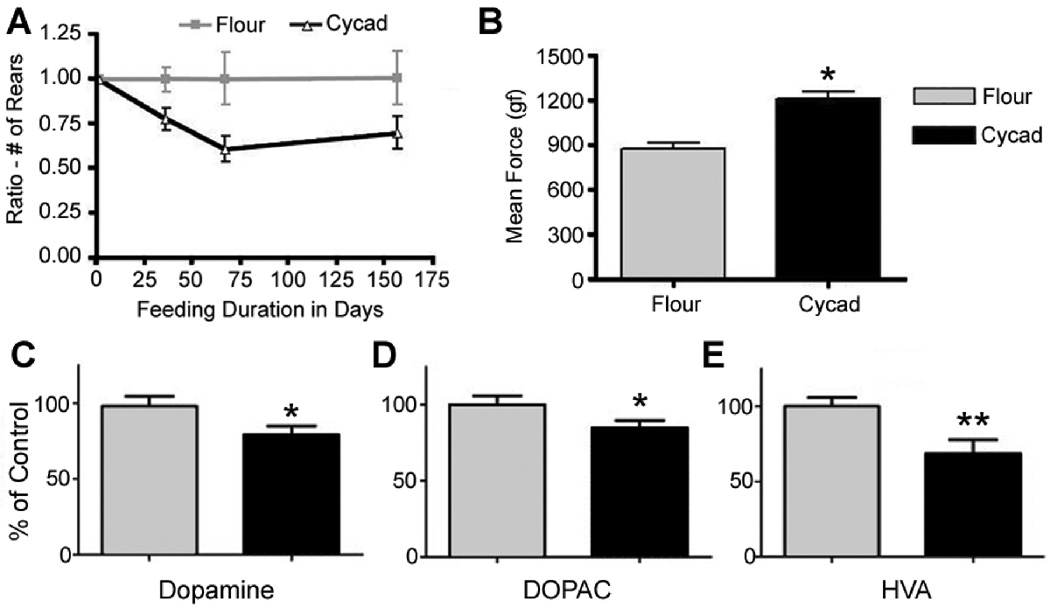
After approximately eight weeks of cycad consumption, rats began to display aberrant motor behaviors (Fig 1B). The cylinder test, used to monitor changes in motor activity, revealed a progressive decline in the number of rears completed by cycad-fed rats (F(1,21)=7.8, p=0.01; Fig 2A). This decrease in vertical exploration was accompanied by the development of slowness in the execution of movement in free range recordings. As cycad feeding continued, rats developed a variety of additional motor abnormalities (see Supplementary Videos). By week 22 of cycad feeding rats displayed a significant increase in grip strength when compared to flour-fed control rats (t(21)=4.093, p=0.0005; Fig 2B). In addition, paw print analysis revealed a blurred, short-stepped gait indicative of shuffling, although this phenotype could not be consistently recorded (Supplementary, S2). Another novel behavior seen in a number of cycad-fed rats was the presence of tremulous jaw movements at a mean frequency of 6 Hz. This frequency was quantitated using video recordings. This vacuous activity is at a characteristic frequency seen in other parkinsonian animal models.22 These jaw movements were suppressed with apomorphine (0.5 mg/kg). We also observed a spontaneous, unilateral rotating behavior in some cycad-fed rats that could be reversed upon administration of apomorphine (1.0 mg/kg; see Supplementary Videos). None of these behaviors were seen in flour-fed animals.
Figure 2.
Aberrant motor behavior and decrease of dopamine and its metabolites in the striatum of cycad-fed rats. (A) Cycad-fed rats (n = 16) showed a significant decrease in rearing behavior when compared to flour-fed rats (n = 7; p = 0.01, 2-way repeated measures analysis of variance). Data were age-normalized in the form of a ratio to monitor specific activity changes in each rat and enable comparisons between animals. In addition, age-dependent decreases in activity were eliminated by using a correction factor (inverse of averaged control ratio for each time point) applied to all data. (B) The grip strength of flour-fed (n = 7) and cycad-fed (n = 16) rats was determined using the averaged result of 6 measurements. Data were compared using a 2-tailed Student t test. Results revealed a significant increase in the grip strength of cycad-fed rats (1,212 ± 51.03 gram [gf]) when compared to flour-fed controls (880.1 ± 33.81 gf; ***p = 0.0005). (C) Cohort 3 (flour, n = 6; cycad, n = 8) was pellet fed for 18 weeks then euthanized for high-performance liquid chromatography analysis. Cycad-fed rats showed a significant decrease in the concentration of dopamine in the dorsal striatum (*p = 0.02, Student t test). (D, E) Cycad-fed rats also demonstrated significant decreases in the concentration of dopamine metabolites (3,4-dihydroxy-phenylacetic acid [DOPAC] and homovanillic acid [HVA]) in the dorsal striatum compared to flour-fed rats (*p = 0.03 and **p = 0.005, respectively). The mean DOPAC/dopamine ratio for flour-fed animals was 0.173 ± 0.004
Initial loss of striatal DA innervation in cycad-fed rats
We investigated the effects of cycad on DA and DA metabolite concentrations in the STR and SNc of Cohort 3 with HPLC analysis.22 After 18 weeks of pellet feeding, cycad-fed rats displayed significant alterations in DA metabolism in the dorsal striatum compared to flour-fed rats. There was a significant bilateral decrease in the concentration of DA (t(12)= 2.15, p = 0.0.21; Fig 2C) and its metabolites, DOPAC (t(12)= 1.95, p = 0.031; Fig 2D) and HVA (t(12)= 2.78 p = 0.005; Fig 2E) in the STR of cycad-fed rats. No significant differences in the concentration of DA and its metabolites between cycad-fed and flour-fed rats were found in the substantia nigra of these animals (data not shown).
Later degeneration of dopaminergic neurons in the substantia nigra of cycad-fed rats
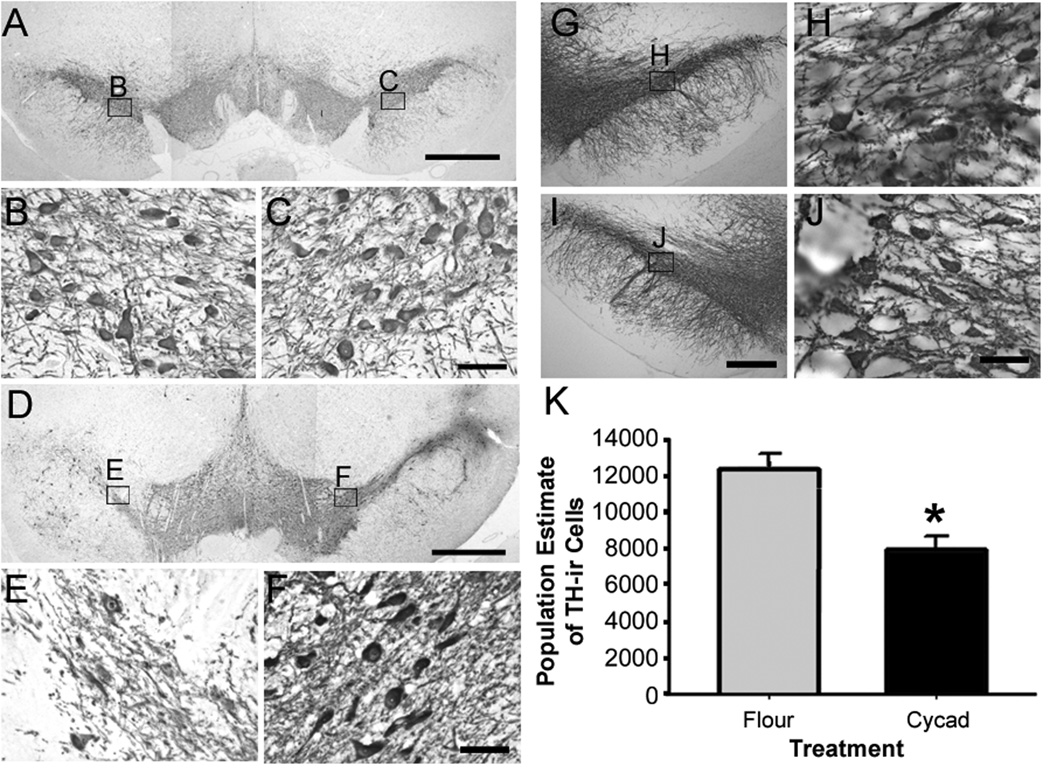
To determine if long-term cycad exposure produced a degeneration of DAergic neurons of the SNc, Cohort 1B was pellet fed for 16 weeks then fed regular rat chow for an additional 20 weeks. Immunohistochemical analysis suggested a loss of DAergic cell bodies in the SNc and their projections in cycad-fed rats (Fig 3D to J) when compared to flour-fed rats (Fig 3A to C). To quantify these changes we calculated stereological cell population estimates. There was a significant loss of TH-immunoreactive (TH-ir) neurons in cycad-fed rats (7,935 ± 779) compared to flour-fed rats (11,551 ± 708; p=0.014). This loss of TH-ir cells was concurrent with a significant decrease in SNc volume (Fig 3K).
Figure 3.
Cycad-fed rats show significant changes in the number of dopamine neurons of the substantia nigra pars compacta (SNc). Representative images of tyrosine hydroxylase-immunoreactive (TH-ir) neurons in SNc of flour-fed rats (A–C), and both hemispheres of cycad-fed rats (D–J) in Cohorts 1A and 1B. In Cohort 1A, in cycad-fed rotating animal GH, TH-ir labeling shows severe asymmetry (D–F) and a substantial loss of TH-ir neurons in this animal (E). In Cohort 1B, another cycad-fed rat shows loss of TH-ir neurons bilaterally (G–J). (K) Stereological population estimates of TH-ir neurons in the SNc determined that cycad-fed rats had significantly fewer TH-ir cells in the SNc (n = 4; 7,935 ± 708) when compared to flour-fed rats (n = 4; 11,551 ± 779; t(4)=3.434; *p = 0.014). This loss of TH-ir cells was concurrent with a significant decrease in SNc volume (cycad, 2.830 ± 0.189; flour, 3.684 ± 0.274 × 103µm3); t(4) = 4.022, *p = 0.02). The data were analyzed by a 1-way analysis of variance followed by the Holm-Sidak post hoc test. For stereological methods, see Supplementary Materials and Methods. Scale bars: A, D = 1mm; G, I = 250µm; B, C, E, F, H, J = 50µm.
As mentioned previously, some cycad-fed rats displayed a spontaneous unilateral rotating behavior. Immunohistochemical analysis of DAergic neurons in the SNc of Cohort 1A revealed a unilateral lesion in a cycad-fed rat (Fig 3D to F) when compared to flour-fed rats (Fig 3A to C). In addition, astrogliosis occurred on the same side of the lesion in cycad-fed rats and was not seen in flour-fed rats (data not shown). However, this severe loss of DAergic neurons in the SNc after only 16 weeks of cycad exposure was an unusual occurrence and in most cases a bilateral loss of DAergic fibers was found at this time point. In addition, cycad-induced neurodegeneration in the SNc was specific for DAergic neurons and neurites in the SNc and STR. Immunohistochemical staining for NeuN-ir and GABA-ir of the striatum revealed no loss of overall neurons or loss of GABAergic neurons and their processes (data not shown). In addition, cycad-fed rats showed no pathological signs of neurofibrillary tangles (NFTs) in the entorhinal cortex, hippocampus, and neocortex (data not shown). As a positive control, human neocortical Alzheimer’s tissue was immunostained for NFTs.
Aggregates of phosphorylated α-synuclein and ubiquitin in cycad-fed rats
Synucleinopathy is a well-known characteristic of several neurodegenerative diseases including Parkinson’s disease, Dementia with Lewy bodies, and multiple system atrophy. We used immunohistochemical analysis with antibodies against α-syn and phospho-α-synuclein to determine if cycad-fed rats also displayed this abnormal synuclein aggregation. Analysis of Cohort 2 revealed neurites with α-synuclein-ir aggregates in the STR of cycad-fed rats (Supplementary, S3) but not in flour-fed rats. At this time point, after 22 wk of cycad feeding, no α-synuclein aggregates were observed in the SNc of cycad-fed rats. However, five months after the cessation of cycad feeding, analysis of Cohort 1B revealed that long-term cycad exposure produced aggregates (composed of the toxic, phosphorylated form of α-synuclein23) in TH-ir neurons in the SNc (Fig 4A to C, 4G). These phospho-α-syn aggregates were proteinase K-resistant, (Fig 4M, N). SNc neurons containing aggregates of phospho α-syn also contained ubiquitin-positive aggregates (Fig 4D to I) as confirmed with confocal microscopy. No aggregates of α-synuclein/ ubiquitin were found in SNc neurons of flour-fed rats (Fig 4J to L). In addition, in the locus coeruleus and the cingulate cortex of these cycad-fed rats, we found phospho-α-synuclein inclusions (Supplementary, S4).
Figure 4.
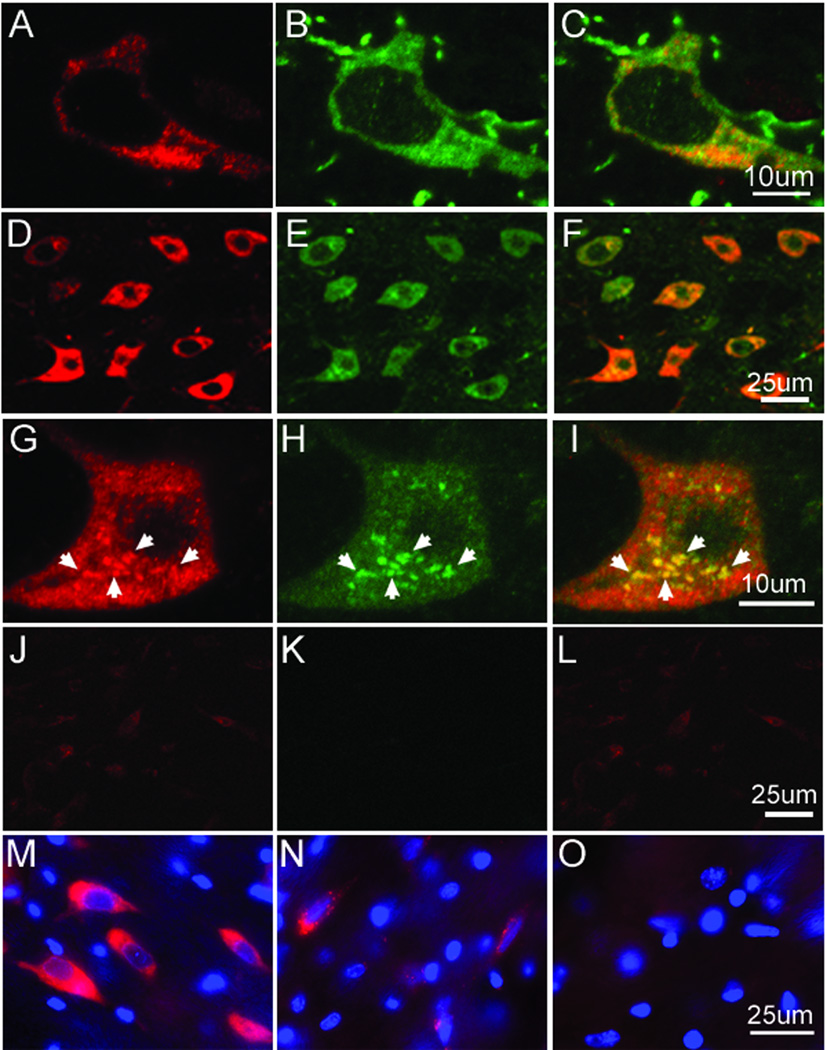
α-synuclein (α-syn) and ubiquitin aggregates in dopamine (DA) neurons of the substantia nigra pars compacta (SNc) of cycad-fed rats. Confocal microscopy with antibodies against phosphorylated α-synuclein (phospho-α-syn; A) and against tyrosine hydroxylase (B) revealed α-syn aggregates in DA neurons in the SNc of 51-week-old rats from Cohort 1B (C, merged image). Dual labeling of SNc sections of another member of cycad-fed Cohort 1B, with antibodies against phospho-α-syn and ubiquitin, revealed numerous neurons with aggregates of both α-syn and ubiquitin in the SNc (D, phospho-α-syn; E, ubiquitin; F, merged image). A confocal image of these SNc neurons revealed numerous aggregates in individual neurons (G, phospho-α-syn; H, ubiquitin; I, merged image) that were not seen in flour-fed rats (J, phospho-α-syn; K, ubiquitin; L, merged image). Phosphorylated α-syn aggregates are shown before and after proteinase K treatment. Prior to proteinase K treatment (M), immunostaining for phospho-α-syn, showed both phospho-α-syn aggregates and soluble phospho-α-syn in SNc neurons; following proteinase K treatment, phospho-α-syn aggregates were found in SNc neurons from cycad-fed animals (N), but were not found in flour-fed animals (O). Scale bars are 25µm in D–F, J––O; 10µm in A–C, G–I.
Cycad-fed rats did not display ALS phenotype
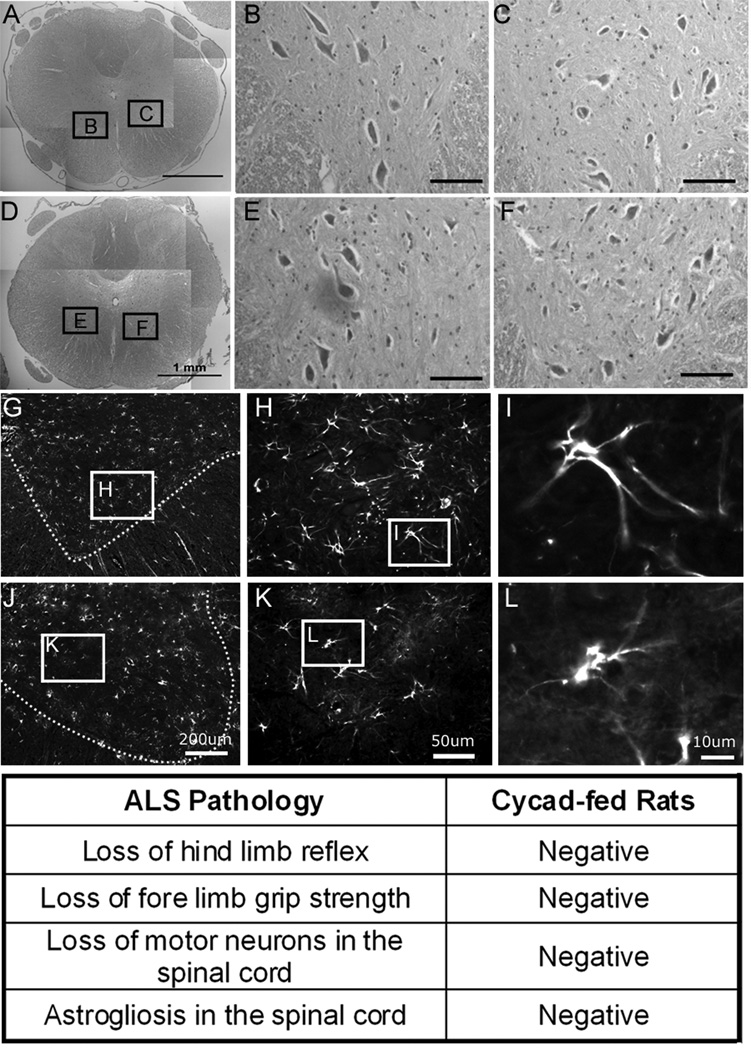
Based on the previous report of the development of an ALS-like syndrome in cycad-fed mice, we conducted behavioral tests to evaluate possible motor neuron loss in Cohort 1 (Flour n = 7; Cycad n = 8).13 No muscle weakness or change in hind limb reflexes or grip strength could be demonstrated in cycad-fed rats. In addition, we verified a lack of ALS pathology in cycad-fed rats. No loss of motor neurons or increased gliosis was observed in the lumbar spinal cords of either of the cycad-fed rats or in the flour fed rats in these cohorts (Fig 5).
Figure 5.
Cycad-fed rats do not display amyotrophic lateral sclerosis (ALS) phenotype or pathology. Cohort 1A and 1B (flour, n = 7; cycad, n = 8) were used to verify a lack of ALS pathology in cycad-fed rats. Representative images of motor neurons from the ventral horn of the lumbar spinal cord stained with hematoxylin and eosin from cycad-fed rat IJ (D–F) and flour-fed rat QR (A–C). Results indicate no differences in size of intact motor neurons on either side. There was also no astrogliosis in the spinal cord of cycad-fed rats (G–I) as compared to flour-fed rats. Scale bars: A, D = 1mm; G, J = 200µm; B, C, E, F = 100µm; H, K = 50µm; I, L = 10µm.
Cycad toxin(s) are selective for dopaminergic neurons in vitro
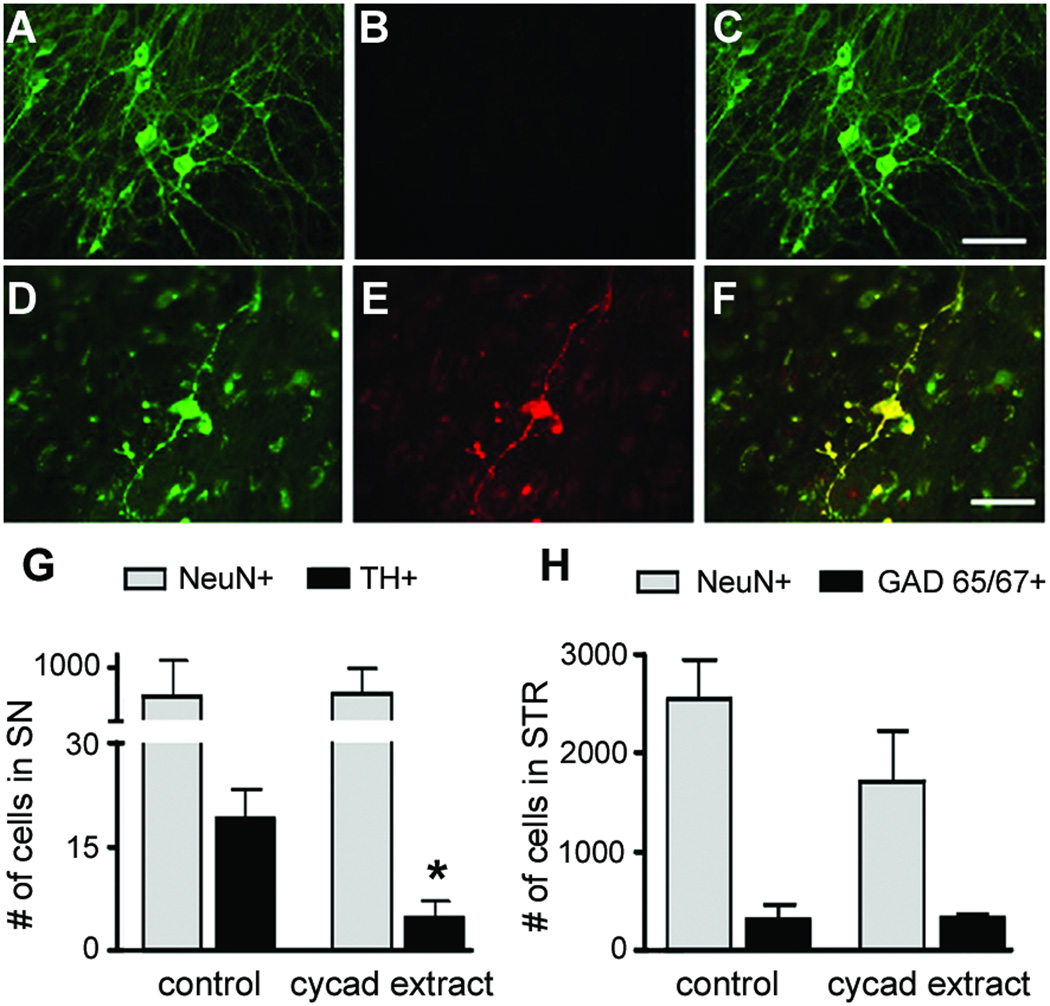
We used nigrostriatal organotypic co-cultures (NSOC) to begin the investigation into the mechanism(s) of cycad toxicity. This unique explant culture system maintains a stable number of TH-ir somata with an extensive network of dendrites, as well as axonal projections extending from the nigra to cells in the striatum up to 48 days in vitro (DIV). In NSOC, TH-ir projections in the striatum have small varicosities reminiscent of synaptic boutons.24,25 Cultures were treated with either vehicle (DMSO, 1:10,000 dilution) or DMSO with cycad flour (1.9 mg/ml) derived from an organic extract of washed cycad (Supplementary, S1) twice a week for two weeks. Exposure to cycad extract led to a significant loss of TH-ir somata in the SN slice (Fig 6A, D, G) and a change in cell morphology (Fig 6A, D). The TH-ir neurons in the NSOC appeared to have both shrunken somata. The total number of neurons (NeuN-ir) cells in the cycad-treated SN slice was unchanged Fig 6G) and there was no loss of GABAergic cells in the SN (data not shown). In addition, in the STR, the total number of neurons (NeuN and the numbers of GABAergic (GAD65/67-ir) neurons were also comparable between vehicle and cycad-treated cultures (Fig 6H). As seen in cycad-fed rats, TH-ir neurons in the SN of cycad-treated cultures contained phospho α-syn aggregates (Fig 6D to F). This localization of α-synuclein in TH-ir neurons and their projections was not seen in control cultures (Fig 6B, C) confirming our in vivo observations that cycad neurotoxins cause the formation of α-synuclein aggregates in dopamine neurons.
Figure 6.
Loss of dopamine neurons and induction of phosphorylated α-synuclein (phospho-α-syn) aggregates by incubation of an organic extract of washed cycad in nigrostriatal organotypic cocultures (NSOC). After 2 weeks of exposure to cycad extract, NSOC were analyzed for tyrosine hydroxylase-immunoreactive (TH-ir) and phospho-α-syn aggregates. In the substantia nigra (SN) in the presence of an organic cycad extract, there was a loss of TH-ir neurons and processes (D) as compared to vehicle-treated cultures (A). TH neurons (dimethyl sulfoxide [DMSO] = 20 ± 2.5; cycad = 3 ± 1.2; p < 0.01; n = 9–19 cultures/cycad or vehicle treatment; n = 12 experiments). There were phospho-α-syn aggregates in the remaining TH-ir neurons (E). Merged images show TH-ir only in vehicle-treated cultures (C) and TH-ir neurons with phospho-α-syn aggregates in cycad extract-treated cultures (F). The total number of neurons in the SN (G) and in the striatum (STR) (H; neuronal nuclei [NeuN] immunoreactive) of the cycad-treated NSOC was unchanged (G, H; SN slice: DMSO = 750 ± 304.1; cycad = 776 ± 210.9; p = 0.95; STR slice: DMSO = 2,533 ± 394.4; cycad = 1,699 ± 514.3; p = 0.24; n = 4–5 cultures/treatment group; n = 12 experiments). The number of γ-aminobutyric acid (GABA)ergic neurons (GAD65/67-ir) in the STR of cycad-fed cultures was unchanged in the presence of the extract (H; DMSO = 325 ± 125.8; cycad = 339 ± 24.7; p = 0.91; n = 4–5 cultures/treatment group; n = 12 experiments). Student t test analysis revealed a significant loss of TH-ir cells in cycad extract-treated cultures (G). Controls/DMSO 20 ± 2.6 TH-ir neurons, n = 15 cultures; cycad/DMSO, 5 ± 2.4 TH-ir neurons, n = 4 cultures, t = 2.95; *p = 0.03. Values represent mean + standard error of the mean. Scale bar = 50µm (A–F).
Discussion
The tragic development of a significant cluster of cases of ALS/PDC among the Chamorro population of Guam generated much interest as to a common etiology for these neurodegenerative disorders.6 The ingestion of washed flour prepared from seeds of the plant Cycas micronesica during a period of famine has been implicated. This is not without precedent. In Guadeloupe atypical parkinsonism has been linked to the consumption of the fruit and leaves of the tropical plant Annona muricata (soursop).26 Patients present with symptoms of progressive supranuclear palsy or Guadeloupian PDC with cerebral atrophy. Pathologically, there is neurodegeneration of both cholinergic cells and GABAergic cells of the STR and cholinergic and DAergic cells of the substantia nigra,27 differing from our observations with cycad exposure both in vivo and in vitro.
We began our investigation of cycad-induced neurodegeneration in an attempt to develop another rat model of ALS.15 In cycad-fed mice, Wilson and Shaw demonstrated an ALS-type syndrome with later denervation of DAergic neurites in the STR.13–14 However, we found parkinsonian behavioral symptoms in cycad-fed rats including slowness of movement, tremulous jaw movements, a shuffling gait, unilateral rotations and increased grip strength (Fig 2).28–29 We conducted neurochemical and histological analysis of the brains of cycad-fed rats and found that cycad ingestion causes a progressive loss of DAergic innervation of the STR (Fig 2), and progressive degeneration of nigral DAergic neurons later (Fig 3) with the remaining DAergic neurons showing ubiquitin aggregates and proteinase K-resistant aggregates of phospho α-syn (Fig 4)3,21,23,30–31. We also found phospho α-syn aggregates in the locus coeruleus and cingulate cortex of cycad-fed rats (Supplementary, S4) as has been reported in PD.32–33 Cycad ingestion causes a slow progressive loss of DAergic neurons rather than the rapid loss seen in other toxin-induced models of PD; and cycad ingestion also causes α-synuclein aggregates colocalized with ubiquitin, a common marker in PD pathology.33–34 In the Dopamine receptor 2 (D(2)R) knockout mouse, cytoplasmic inclusions containing α-synuclein and ubiquitin were present in the SNc neurons of older D(2)R(−/−) mice, and were also occasionally seen in aged wild-type mice.35 The phosphorylated α-synuclein antibody used in our studies binds to α-synuclein, phosphorylated at residue Ser-129, a post-translational modification shown to be a toxic form of the protein and indicative of increasing levels of oxidative stress in these cells.30–31,35 In cycad-fed rats, there was a time-dependent accumulation of aggregates of α-synuclein first in DAergic neurites in the STR and later in DAergic neurons in the SNc. This loss of the TH-ir neurons in the SNc could be a loss of the TH phenotype in the absence of an actual cell loss of DAergic neurons. However, our stereological counts of TH-ir neurons at 51 weeks of age (Cohort 1 B) showed a significant loss of TH-ir neurons in the presence of increased α-synuclein aggregates (Fig 4). Chu and Kordower36 found in PD patients and in non-human primates that increases in α-synuclein are age-associated and we found a similar phenomenon in older cycad-fed rats. Rats in our cohorts did not develop a loss of motor neurons in the lumbar cord or increased gliosis in the grey matter of the spinal cord (Fig 5).
Organotypic nigrostriatal co-cultures offer a useful tool for studying dopaminergic degeneration in a natural circuit. These slice cultures survive over two months, making chronic intoxication studies feasible.24,25 Chronic treatment with an organic extract of washed cycad over two weeks resulted in an 85% decrease of TH-ir somata in the SN, either by loss of TH-ir neurons or a loss of the TH phenotype. The lack of NeuN-ir cell loss in the SN from slice cultures (Fig 6) is possibly due to loss of the TH phenotype in the absence of actual TH-ir cell loss. However, the abnormal morphology of the TH-ir neurons in the presence of cycad suggests that death of DAergic neurons, which are only a small number of the total number of NeuN-ir neurons, may occur. In the STR of slice cultures, there is a small (not significant) loss of NeuN-ir cells which is clearly distinct from the 85% loss of TH-ir cells in the SN. One possible explanation is that the concentrations of cycad toxins in the slice culture differ from the dose of cycad ingested in vivo, leading to slightly different selectivity. In addition, phospho α-syn accumulation in TH-ir neurons and neurites in the NSOC was observed (Fig 6). Most notably, this preparation is the first demonstration of phospho α-syn aggregates coupled with a loss of DAergic neurons in an organotypic slice without a genetic alteration.
A number of natural and synthetic molecules exert deleterious effects on DAergic neurons including: 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and paraquat. None of these toxins causes a progressive syndrome that closely resembles idiopathic parkinsonism in humans. For example, brains of individuals who died after MPTP exposure showed evidence of DA neuronal loss in the SNc in the absence of Lewy bodies in the remaining neurons.37 The temporal loss of DAergic innervation in the STR before the loss of DAergic neurons in the SNc in cycad-fed rats mimics a similar phenomenon in non-human primates in a chronic low dose MPTP model of PD.38 Braak and colleagues have proposed that sporadic PD can be staged in the brain based upon nonrandom neuronal dysfunction and Lewy body pathology.39–40 To demonstrate similarities between the cycad model and sporadic PD we investigated whether aggregates of α-synuclein occur in other regions of the brain in addition to the SNc. We found these aggregates in the locus coeruleus and cingulate cortex, similar to sporadic PD.40 Previous studies have found both α-synuclein aggregates in the presence of rotenone and non-aggregated α-synuclein in midbrain axons of MPTP-treated squirrel monkeys.41–43 Our in vivo findings in the cycad-fed rat makes this model an interesting addition to the study of the relationship between α-synuclein aggregates and DAergic neurodegeneration in a non-genetic model, recapitulating some of the progressive behavioral and histopathological abnormalities seen in the human disorder. Unlike PDC, the cycad-fed rat showed no pathological signs of NFTs in the cortex. Thus the rat exposed to cycad flour has both similarities (parkinsonism) and differences (synuclein aggregates in the SNc and no cortical NFTs) with pathology found in patients with PDC.
The mechanism of neurotoxicity behind cycad consumption is unknown. One environmental interaction that can affect neurons is exposure to high levels of phytosterols and sterol glucosides.44–45 Plant sterols enter the mammalian body only via the diet. Relatively large amounts of plant sterols are present in plant oils, nuts, and avocados, and interestingly, cycad flour. Phytosterols have a comparable chemical structure to cholesterol and can accumulate in the brain.46 In addition, phytosterols such as sitosterol and campesterol can activate Liver X Receptors (LXRs).44 Activation of LXRs have an important role in cholesterol metabolism and it has been determined that cholesterol metabolites, like 24-hydroxycholesterol, and certain phytosterols, are ligands for the LXR-β receptor.44–45 Administration of β-sitosterol to LXR-β −/− mice accelerated the previously observed motor neuron degeneration in this knockout, and causes a loss of DAergic neurons in the SNc. β-sitosterol also reduced brain cholesterol levels in not only the LXR-β −/− mouse but in wild type mice as well.44 These studies suggest that LXR-β signaling is abnormal in the presence of β-sitosterol, leading to neuronal damage in the SNc. Thus phytosterols and sterol glucosides could be etiological agents in PD46 and our current studies are focusing on this intriguing avenue in an attempt to elucidate the mechanism of cycad toxicity.
Unlike genetic models or toxicant based end-stage models of parkinsonism that may have a great deal of phenotypic similarity among animals, there is considerable variability in the response of individual animals to cycad ingestion. This variability may be due to first-pass metabolism of cycad flour or due to the unevenness of uptake of phytosterols and other components of cycad flour into the brain. However, we believe that the novelty of this cycad-fed rat model is that it recapitulates the variability and progression of symptoms and neuropathology seen in human parkinsonism. It also allows examination of the early stages of the disorder. Based upon our observations; we believe that the cycad model in rats can uniquely serve as a progressive model of parkinsonism.
Supplementary Material
Acknowledgements
We thank Dr. Thomas Marler, University of Guam, for collection of cycad seeds; Neurodyn, Inc. for supplying the cycad seeds; Dr. Rudy Castellani, for supplying Alzheimer’s neocortical sections; Dr. Sergi Ferre for comments, and Dr. Kathryn Grumbach for help in the preparation of the manuscript. This work was funded by: Veteran’s Affairs (PY, PF); National Institutes of Health (CS, HJ) and NIEHS Training Grant (KM).
References
- 1.Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16(Spec No. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. Basic research on dopamine in Parkinson's disease and the discovery of the nigrostriatal dopamine pathway: the view of an eyewitness. Neurodegener Dis. 2008;5:114–117. doi: 10.1159/000113678. [DOI] [PubMed] [Google Scholar]
- 3.Perl DP. Handbook of clinical neurology, Vol 83, Parkinson's disease and related disorders. Part I. Philadelphia: Elsevier; 2007. The neuropathology of parkinsonism. [DOI] [PubMed] [Google Scholar]
- 4.Morris HR, Steele JC, Crook R, et al. Genome-wide analysis of the parkinsonism-dementia complex of Guam. Arch Neurol. 2004;61:1889–1897. doi: 10.1001/archneur.61.12.1889. [DOI] [PubMed] [Google Scholar]
- 5.Uversky VN. Neurotoxicant-induced models of Parkinson's disease: Understanding the role of rotenone, maneb, and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 6.Kurland LT. Amyotrophic lateral sclerosis and Parkinson's disease complex on Guam linked to an environmental neurotoxin. Trends Neurosci. 1988;11(2):51–54. doi: 10.1016/0166-2236(88)90163-4. [DOI] [PubMed] [Google Scholar]
- 7.Hirano A, Kurland LT, Krooth RS, et al. Parkinsonism-dementia complex, an endemic disease on the island of Guam: 1. Clinical Features. Brain. 1961;84:642–661. doi: 10.1093/brain/84.4.642. [DOI] [PubMed] [Google Scholar]
- 8.Plato CC, Garruto RM, Galasko D, et al. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol. 2003;157:149–157. doi: 10.1093/aje/kwf175. [DOI] [PubMed] [Google Scholar]
- 9.Borenstein AR, Mortimer JA, Schofield E, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–1771. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- 10.Hirano A, Malamud N, Kurland LT. Parkinsonism-dementia complex, an endemic disease on the island of Guam: II. Pathological Features. Brain. 1961;84:662–679. doi: 10.1093/brain/84.4.662. [DOI] [PubMed] [Google Scholar]
- 11.Ince PG, Codd GA. Return of the cycad hypothesis - does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol Appl Neurobiol. 2005;31:345–353. doi: 10.1111/j.1365-2990.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Marler TE, Lee V, Shaw CA. Cycad toxins and Neurological Diseases in Guam: Defining Theoretical and experimental Standards for Correlating Human Disease with Environmental Toxins HortScience 2005. 2005;40:1598–1605. [Google Scholar]
- 13.Wilson JM, Khabazian I, Wong MC, et al. Behavioral and neurological correlates of ALS-Parkinsonism dementia complex in adult mice fed washed cycad flour. Neuromuscular Med. 2002;1:207–221. doi: 10.1385/NMM:1:3:207. [DOI] [PubMed] [Google Scholar]
- 14.Shaw CA, Wilson JMB. Analysis of Neurological Disease in 4 Dimensions. Neurosci Biobehev Rev. 2003;27:493–505. doi: 10.1016/j.neubiorev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Howland DS, Liu J, She Y, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Nat Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olanow CW, Kordower JH. Modeling Parkinson's disease. Ann Neurol. 2009;66:432–436. doi: 10.1002/ana.21832. [DOI] [PubMed] [Google Scholar]
- 17.Khabazian J, Bains JS, Williams DE, et al. Isolation of various forms of sterol beta-d-glucoside from the seed of the Cycas circinalis: neurotoxicity and implications for ALS-Parkinsonism dementia complex. J Neurochem. 2002;82:516–528. doi: 10.1046/j.1471-4159.2002.00976.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwarting RK, Sedelis M, Hofele K, et al. Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson's disease. Neurotox Res. 1999;1:41–156. doi: 10.1007/BF03033338. [DOI] [PubMed] [Google Scholar]
- 19.Schallert T, Tillerson JL. Central nervous system diseases: innovative animal models from lab to clinic. Totowa, NJ: Humana Press; 2000. Intervention strategies for degeneration of dopamine neurons in Parkinsonism: optimizing behavioral assessment of outcome. [Google Scholar]
- 20.Salamone JD, Mayorga AJ, Trevitt JT, et al. Tremulous jaw movements in rats: A model of Parkinsonian tremor. Prog Neurobiol. 1998;56:591–611. doi: 10.1016/s0301-0082(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 21.Alerte TNM, Akinfolarin AA, Fredrich EE, et al. α-synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: lessons from viral transduction of knockout mice. Neurosci Lett. 2008;435:24–29. doi: 10.1016/j.neulet.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JM, Levey AI, Rajput A, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson's disease. Neurology. 1996;47:718–726. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- 23.Gorbatyuk OS, Shoudong L, Sullivan LF, et al. The phosphorylation state of Ser-129 in human α-synuclein determines neurodegeneration in a rat model of Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:763–769. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siebert AS, Desai V, Chandrasekaran K, et al. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal co-cultures. J Neurosci Res. 2009;87:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 25.Plenz D, Kitai ST. Organotypic cortex-striatum-mesencephalon cultures: the nigrostriatal pathway. Neurosci Lett. 1996;209:177–180. doi: 10.1016/0304-3940(96)12644-6. [DOI] [PubMed] [Google Scholar]
- 26.Lannuzel A, Höglinger GU, Verhaeghe S, et al. Atypical parkinsonism in Guadeloupe: a common risk factor for two closely related phenotypes? Brain. 2007;30:816–827. doi: 10.1093/brain/awl347. [DOI] [PubMed] [Google Scholar]
- 27.Caparros-Lefebre D, Lees AJ. Atypical unclassificable Parkinsonism in Guadeloupe: an environmental toxic hypothesis. Mov Disord. 2005 suppl 12:S114–S118. doi: 10.1002/mds.20553. [DOI] [PubMed] [Google Scholar]
- 28.Fellows SJ, Noth J. Grip force abnormalities in de novo Parkinson's disease. Mov Disord. 2004;19:560–565. doi: 10.1002/mds.10710. [DOI] [PubMed] [Google Scholar]
- 29.Traynor BJ, Zhang H, Shefner JM, et al. Functional outcome measures as clinical endpoints in ALS. Neurology. 2004;63:1933–1935. doi: 10.1212/01.wnl.0000144345.49510.4e. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto M, Rockenstein E, Crews L, et al. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formaton in a Drosophilia model of Parkinson's disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 32.Jellinger KA. Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain.: incidence and topographic distribution—a pilot study. Acta Neuropathol. 2003;106:191–201. doi: 10.1007/s00401-003-0725-y. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 34.Betarbet R, Greenamyre T. Handbook of clinical neurology, Vol 83, Parkinson's disease and related disorders. Eds. Part 1. Philadelphia: Elsevier; 2007. Parkinson's disease: animal models. [DOI] [PubMed] [Google Scholar]
- 35.Tinsley RB, Bye CR, Parish CL, Tziotis-Vais A, George S, Culvenor JG, Li QX, Masters CL, Finkelstein DI, Horne MK. Dopamine D2 receptor knockout mice develop features of Parkinson disease. Ann Neurol. 2009;66:472–484. doi: 10.1002/ana.21716. [DOI] [PubMed] [Google Scholar]
- 36.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion : Is this the target for Parkinson's disease. Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Langston JW, Forno LS, Tetrud J, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Chen MK, Kuwabara H, Zhou Y, et al. VMAT2 and dopamine neuron loss in a primate model of Parkinson's disease. J Neurochem. 2008;105:78–90. doi: 10.1111/j.1471-4159.2007.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braak H, Del Tredici K, Bratzke H, et al. Staging of the intracerebral inclusion body pathology associate with idiopathic Parkinson's disease (preclinical and clinical stages) J Neurol. 2002;249 Suppl 3:1–5. doi: 10.1007/s00415-002-1301-4. III. [DOI] [PubMed] [Google Scholar]
- 40.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 41.Betarbet R, Sherer TB, MacKenzie, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 42.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 43.McCormack AL, Mak SK, Shenasa M, et al. Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Fan X, Gabbi C, et al. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia. Proc Natl Acad Sci U S A. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansen PJ, Lütjohann D, Abildayeva K, et al. Dietary plant sterols accumulate in the brain. Biochim Biophys Acta. 2006;1761:445–453. doi: 10.1016/j.bbalip.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Tabata RC, Wilson JM, Ly P, et al. Chronic exposure to dietary sterol glucosides is neurotoxic to motor neurons and induces an ALS-PDC phenotype. Neuromolecular Med. 2008;10:24–39. doi: 10.1007/s12017-007-8020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.