Abstract
Background
Few studies have focused on the outcomes of nonambulatory patients diagnosed with spinal cord compression from metastatic cancer. The purpose of this study was to review the morbidity and mortality suffered by these patients.
Methods
Over a 10-year period (1996–2006), a retrospective review was undertaken to assess the outcomes of 39 nonambulatory patients diagnosed with spinal cord compression from metastatic cancer.
Results
Treatment for cord compression included corticosteroids (n = 33), radiation (n = 25), and surgical decompression (n = 13). Nonetheless, 23 patients (59%) required bowel and/or bladder catheterization, and 33 (85%) required pain medications. Twenty-five (64%) did not regain ambulation. Only 13 patients (33%) went home without assistance. In contrast, 10 (26%) were transferred to a nursing home, 6 (15%) were sent home with hospice, 5 (13%) went home with home health care, and 1 (3%) was moved to a hospice inpatient facility. At the time of this report, all patients had died with a median survival of 76 days (range, 4–1975 days). Long-term survivors who lived beyond a year were primarily patients who had regained ambulation.
Conclusion
Metastatic cord compression causes severe morbidity and compromised survival in patients who become nonambulatory. Future palliative care efforts should focus on further characterizing and addressing these needs.
Introduction
Spinal cord compression from metastatic cancer is a devastating event that can result in permanent neurologic damage. Although this event occurs in only 2%–6% of patients with metastatic cancer, the medical literature is replete with descriptions of clinical diagnostic and therapeutic strategies, which consistently underscore the importance of rapid diagnosis and treatment of this cancer complication.1,2
However, to our knowledge, few studies have focused on metastatic cord compression among patients who have already suffered major neurologic compromise. These nonambulatory patients comprise 21%–48% of all patients with cancer who develop cord compression with rates varying markedly from study to study and often not reported.2,3 What happens to patients with cancer once they become nonambulatory after a cord compression? Do they ever regain their ability to walk? Are they ever able to function at home again? Do they ever receive cancer therapy after such an event? Do they suffer a rapid demise? Admittedly, these questions pertain to only a very small group of patients with cancer, but in view of the potentially devastating nature of cord compression, it remains important to explore the potential needs of these patients from a palliative care standpoint.
To answer the questions posed above, we undertook this descriptive study to assess the needs and outcomes of patients with solid tumors who had become nonambulatory as a result of cord compression.
Methods
Overview
Given the relative rarity of metastatic cord compression with resulting nonambulation, we decided that a retrospective review from a major tertiary medical center would provide the most efficient study design to answer the questions posed above. The Mayo Clinic Institutional Review Board approved this study prior to its initiation. Thereafter, the Mayo Clinic Tumor Registry was contacted for acquisition of medical records between 1996 through 2006. A deliberate decision was made to acquire and review records available only after 1995, as the widespread diagnostic use of magnetic resonance imaging (MRI) for cord compression had been implemented by this time and a decade's experience would be available.4–6
Patient eligibility
The present study focused on patients who met the following criteria: (1) diagnosis of a solid tumor malignancy; (2) radiographic confirmation of metastatic cord compression; (3) greater than or equal to 18 years of age at the time of cord compression; (4) nonambulatory status after the cord compression with the former defined as medical record evidence of being wheel chair- or bed-bound and/or unable to lift both legs against gravity; and (5) documentation of follow up in the medical record beyond an initial visit to the Mayo Clinic. This latter point was required in order that the study team be able to report meaningful descriptive data on patients.
Review of medical records
All medical records from patients who met the above criteria were reviewed in depth. Efforts were made to retrieve the following information: (1) level of cord compression with respect to the spinal cord; (2) dates of hospitalization when appropriate; (3) use of bowel and/or bladder catheterization and, if possible, the duration of catheterization; (4) whether or not a psychiatric consultation had been pursued; (5) use of antidepressants; and (6) destination after discharge from the hospital, such as a step-down nursing facility or the patient's home.
A deliberate decision was made not to evaluate the circumstances surrounding the diagnosis of cord compression because many patients were referred to our institution, a tertiary medical center, after the cord compression had occurred. It was clearly not the intention of this study to reexplore the obvious but critical role of the prompt diagnosis and treatment of this entity.
Data analyses
All data are presented descriptively, and numeric data are presented as the median values with an accompanying range. Kaplan-Meier survival curves were constructed with JMP, version 7.0.1 (SAS Institute, Cary, NC).
Results
Demographics
A total of 39 patients met this study's eligibility criteria and are the focus of this report. The median age was 63 years (range, 24–87), and 24 (62%) were men.
Malignant diagnoses included cancer of the lung and prostate as well as sarcoma, the former being the most common but with several other cancer types also represented (Table 1). Prior to the cord compression, 64%, 56%, and 41% of patients had been treated with surgery, chemotherapy, and radiation, respectively, with several patients having received more than one treatment modality.
Table 1.
Demographicsa
| Age, median in years (range) | 63 (24–87) |
| Gender | |
| Male | 24 (62) |
| Female | 15 (38) |
| Cancer | |
| Lung | 7 (18) |
| Prostate | 7 (18) |
| Sarcoma | 4 (10) |
| Colorectal | 3 (7) |
| Kidney | 3 (7) |
| Breast | 2 (5) |
| Endometrial | 1 (3) |
| Esophageal | 1 (3) |
| Melanoma | 1 (3) |
| Other | 10 (26) |
| Prior cancer therapyb | |
| Surgery | 25 (64) |
| Radiation | 22 (56) |
| Chemotherapy | 16 (41) |
| Hormonal | 9 (23) |
| Otherc | 4 (10) |
| None | 6 (15) |
Numbers in parentheses denote percentages unless otherwise noted.
Numbers do not add to 100% because some patients received various treatments.
Other includes primarily experimental therapies.
Cord compression characteristics
The diagnosis of spinal cord compression was confirmed by MRI in 35 (89%) patients, while 3 (8%) were diagnosed by computed tomography (CT) myelogram and 1 (3%) by chest CT. One (3%) patient presented with cord compression at the cervical level, 5 (13%) at the lumbar level, and 33 (84%) at the thoracic level. Notably, 4 patients suffered from one or more subsequent episodes of cord compression with 2 developing it at the same site as before.
In terms of treatment of cord compression, 33 patients (85%) received corticosteroids, 25 (64%) received radiation, and 13 (33%) underwent surgical decompression.
Morbidity
After the cord compression, 23 patients (59%) required bowel and/or bladder catheterization. We were unable to determine from the medical record how many regained continence. Thirty-three (85%) patients had pain that was treated with pain medications (Table 2). While hospitalized, no patients received a psychiatric consultation. However, 7 were receiving an antidepressant in the hospital, and it appeared that the use of this medication preceded the diagnosis of cord compression.
Table 2.
Interventions Post-Cord Compressiona
| Bowel or bladder catheterization | |
|---|---|
| Yes | 23 (59) |
| No | 14 (36) |
| Unknown | 2 (5) |
| Pain medications | |
| Yes | 33 (85) |
| No | 6 (15) |
| Cancer therapyb | |
| None | 23 (59) |
| Radiation | 8 (21) |
| Chemotherapy | 4 (10) |
| Hormonal therapy | 3 (8) |
| Surgery | 1 (3) |
| Otherc | 2 (5) |
Numbers in parentheses denote percentages.
Numbers do not add to 100% because some patients received various treatments, although “none” denotes absolutely no treatment after the cord compression.
Other includes experimental therapies.
Four patients (10%) died in the hospital, and 2 were never hospitalized. For all other patients, the median time from diagnosis of cord compression to discharge from the hospital was 10 days (range, 1–41). At the time of hospital or clinic discharge, only 13 (33%) patients went home without assistance. In contrast, 10 (26%) were transferred to a nursing inpatient facility, 6 (15%) were sent home with hospice, 5 (13%) went home with nursing home health care, and 1 (3%) was moved to a hospice inpatient facility (Table 3).
Table 3.
Destination after Hospitalizationa
| Destination | n (%) |
|---|---|
| Home without assistance | 13 (33) |
| Nursing facility | 10 (26) |
| Home with hospice | 6 (15) |
| Home with home health care | 5 (13) |
| Hospice inpatient facility | 1 (3) |
| Died in hospital | 4 (10) |
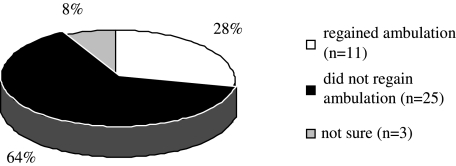
Twenty-five patients (64%) did not regain their ability to ambulate, 11 (28%) did, and 3 (8%) had insufficient records to allow adequate assessment of ambulatory status (Fig. 1). Twenty-three patients (59%) received no further cancer therapy (Table 2), particularly if they had not regained ambulation. Among patients who never regained ambulation, 1 received radioactive iodine, and 2 others received hormonal therapy.
FIG. 1.
Ambulatory outcomes among patients after metastatic spinal cord compression.
Mortality
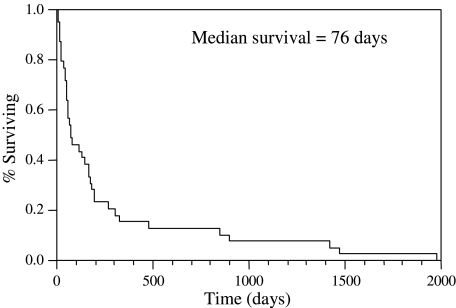
All patients had died by the time of this report. The median survival after diagnosis of malignant spinal cord compression was 76 days (range: 4-1975 days) (Fig. 2). The median survival among the 25 patients who did not regain ambulation was 51 days (range, 4–898 days), and the median survival among those who did was 266 days (range, 56–1975 days). Long-term survivors, who lived beyond a year, included 4 patients who had regained ambulation. Two other patients did not regain ambulation but nonetheless went on to live 475 and 898 days.
FIG. 2.
Median survival for the cohort was 76 days (range, 4–1975 days).
Discussion
This study found that most patients with cancer who become nonambulatory after cord compression do poorly. Within our case series, more than half required bowel and/or bladder catheterization after the cord compression, most needed pain medications, many required inpatient care or assisted care at home, only a few went on to ever receive further cancer therapy (beyond that provided as treatment for the cord compression itself), and nearly all died within days to months of the complication. Only a small handful of long-term survivors lived beyond a year, and 4 of 6 of these patients had regained ambulation. Taken together, such observations illustrate the extreme morbidity and mortality associated with this untoward event, and they also point out the importance of focusing on palliative care efforts to address these patients' end-of-life needs.
Indeed, the vast majority of studies on cord compression ceased follow-up or further description of outcomes once patients reached an irreversible nonambulatory state. To date, only a small number have focused on nonambulatory patients with cancer’ subsequent morbidity.3,7,8 Most notable is a study from Conway and others8 in which a subgroup of 319 patients were prospectively evaluated. Although most of these patients regained ambulation, 54 were completely nonambulatory at 1 month after diagnosis. In this study, the median survival of patients unable to walk was 35 days, and the majority required further hospital or hospice care after discharge from the hospital as well as extended bowel/bladder catheterization. Although the results of our study are very similar to those reported by Conway et al.,8 our study is unique and particularly relevant as it appears to be the first of its kind undertaken in a group of patients within the United States. Our findings underscore that, even within a different healthcare system, the complications faced by these patients are extreme and, in fact, the challenges entailed in meeting these patients' palliative care needs appear to be universal.
Two other points about our study findings are noteworthy. First, we had focused on patients who had records beyond a first visit to our institution. Conceivably, many nonambulatory patients in poor condition may have come to our institution for a second opinion only to learn that they had limited therapeutic options and only to suffer a rapid demise shortly thereafter. In effect, the findings reported here are perhaps more favorable than what might have been observed in a consecutive series of patients; and, in reality, the outcomes following a cord compression may be even more bleak.
Second, the current study has the same limitations inherent in any retrospective study, including limited availability of data. For example, details on the resulting emotional status of patients and the extent of their depression are difficult to capture with the current study design, even though we found that no patient appeared to have depression that was severe enough to warrant a psychiatric consultation or the initiation of an antidepressant. Nonetheless, patients may have had milder depression, which cannot be readily or accurately captured by means of a retrospective review of the medical record.
Despite the foregoing, this study clearly makes the point that patients who become nonambulatory after metastatic cord compression suffer notable morbidity and mortality, perhaps even more severe and extensive than that reported here. Our findings emphasize the importance of palliative care efforts in addressing these patients' end-of-life needs. Specifically, patients who never regained ambulation appear to have greater needs and shorter survival. These patients may benefit from a relatively early discussion of hospice resources.
Acknowledgment
This study was funded in part by K24CA131099.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Loblaw DA. Perry J. Chambers A. Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23:2028–2037. doi: 10.1200/JCO.2005.00.067. [DOI] [PubMed] [Google Scholar]
- 2.Helweg-Larsen S. Sorensen PS. Kreiner S. Prognostic factors in metastatic spinal cord compression: A prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163–1169. doi: 10.1016/s0360-3016(99)00333-8. [DOI] [PubMed] [Google Scholar]
- 3.Cowap J. Hardy JR. A'Hern R. Outcome of malignant spinal cord compression at a cancer center: Implications for palliative care services. J Pain Symptom Manage. 2000;19:25–264. doi: 10.1016/s0885-3924(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 4.Smoker WR. Godersky JC. Knutzon RK. Keyes WD. Norman D. Bergman W. The role of MR imaging in evaluating metastatic spinal disease. AJR Am J Roentgenol. 1987;149:1241–1248. doi: 10.2214/ajr.149.6.1241. [DOI] [PubMed] [Google Scholar]
- 5.Norman D. Mills CM. Brant-Zawadzki M. Yeates A. Crooks LE. Kaufman L. Magnetic resonance imaging of the spinal cord and canal: Potentials and limitations. AJR Am J Roentgenol. 1983;141:1147–1152. doi: 10.2214/ajr.141.6.1147. [DOI] [PubMed] [Google Scholar]
- 6.Williams MP. Cherryman GR. Husband JE. Magnetic resonance imaging in suspected metastatic spinal cord compression. Clin Radiol. 1989;40:286–290. doi: 10.1016/s0009-9260(89)80205-3. [DOI] [PubMed] [Google Scholar]
- 7.Turner S. Marosszeky B. Timms I. Boyages J. Malignant spinal cord compression: a prospective evaluation. Int J Radiat Oncol Biol Phys. 1993;26:141–146. doi: 10.1016/0360-3016(93)90185-x. [DOI] [PubMed] [Google Scholar]
- 8.Conway R. Graham J. Kidd J. Levack P Scottish Cord Compression Group. What happens to people after malignant cord compression? Survival, function, quality of life, emotional well-being and place of care 1 month after diagnosis. Clin Oncol (R Coll Radiol) 2007;19:56–62. doi: 10.1016/j.clon.2006.11.010. [DOI] [PubMed] [Google Scholar]