Abstract
Purpose
Alcoholism is a devastating disease that can cause patient and family suffering and is frequently underdiagnosed. Preliminary studies suggest that it is associated with increased symptom expression and opioid dose escalation. The CAGE questionnaire is a widely used tool for alcoholism screening. The purpose of this study was to determine the frequency and characteristics of patients who screen positive for alcoholism in a palliative care outpatient clinic (PCOC).
Methods
We reviewed 665 consecutive charts of patients referred to the PCOC and collected data regarding age, gender, and type of cancer. For the first 100 consecutive CAGE positive (CAGE+) and 100 consecutive CAGE negative (CAGE−) patients, time from advanced cancer diagnosis (AC) to PCOC was calculated, and symptoms (Edmonton Symptom Assessment Scale, ESAS) and Morphine Equivalent Daily Dose (MEDD) were collected.
Results
CAGE was available for 598 of 665 (90%) patients. Of 598 patients, 100 (17%) were CAGE+. CAGE+ patients were younger (58 versus 60 years, p < 0.05), predominantly male (68% versus 47%, p < 0.0001), and with head/neck malignancies (24% versus 9%, p < 0.05). CAGE+ patients were referred earlier (5 ± 19 versus 13 ± 27 months after AC, p < 0.0001). At baseline, pain, sleep, dyspnea, well-being, and total symptom distress were significantly worse among CAGE+ patients. Both groups showed similar improvement in symptoms. CAGE+ patients were more frequently on opioids upon referral (47/100 versus 29/100, p < 0.05) and follow-up (27/65 versus 16/68, p < 0.05). At follow-up, opioid doses did not show significant changes.
Conclusion
Seventeen percent of the patients were CAGE+. These patients were referred earlier to palliative care, had more symptom expression, and were more frequently on opioids. The palliative care team successfully improved symptom control in both groups without opioid dose escalation.
Introduction
Alcoholism is a devastating disease which can cause patient and family suffering.1,2 It occurs in approximately 8% of the general population,3 being more frequent among hospitalized patients (approximately 20%).4 Alcoholism is related to other addictive substances such as tobacco and illicit drugs,5 and the use of all these substances to help coping with life stressors is defined as chemical coping.6 some reports suggest that patients with a tendency to cope chemically express higher symptom distress.7–9
Substance abuse including alcoholism are frequently underdiagnosed among cancer and palliative care patients.10,11 its detection is important because it has been described as a poor prognostic factor for cancer pain control.12,13 More than 80% of patients with advanced cancer will receive opioids during the course of their disease, frequently in high doses.14 It is not possible to effectively manage cancer-related pain without using these potentially addictive medications. Identifying patients at risk for chemical coping could lead to increased awareness about the potential risks involved in opioid prescribing, and ultimately more effective pain management by including counseling and preventing opioid dose escalation in patients at increased risk. In our center, all patients are routinely screened for alcoholism using the CAGE questionnaire, a simple, four-item screening survey.15
The purposes of this study were to determine the frequency and characteristics of CAGE positive (CAGE+) patients with regards to demographics, symptom burden, and opioid use.
Methods
We reviewed the electronic charts of 665 consecutive patients seen for the first time at the Palliative Care Clinic (PCC) at the University of Texas M.D. Anderson Cancer Center before January 2007 to find the first 100 consecutive CAGE + patients. Information regarding demographics was collected for all patients. For the first 100 consecutive CAGE negative (CAGE−) and 100 consecutive CAGE+ patients, date of advanced cancer diagnosis, symptom scores, and opioid doses were also collected.
The CAGE questionnaire is a simple, four-item screening survey for alcoholism (Table 1).15 Two positive answers (CAGE+) yield a sensitivity of approximately 90% and specificity of more than 95% to detect alcoholism.16 For the purpose of this study, a patient was considered CAGE + when he/she answered “yes” to at least 2 questions.
Table 1.
The CAGE Questionnaire
| 1 Have you ever felt you should Cut down on your drinking? |
| 2 Have people Annoyed you by criticizing your drinking? |
| 3 Have you ever felt bad or Guilty about your drinking? |
| 4 Have you ever had a drink first thing in the morning or to get rid of a hangover (Eye-opener)? |
Symptoms were recorded for the first visit to the PCC and first follow-up using the Edmonton symptom Assessment System (ESAS), a widely used and validated tool to assess nine symptoms (pain, nausea, drowsiness, dyspnea, anxiety, depression, anorexia, sleep, and fatigue) and general feeling of well-being in a 0–10 scale.17,18 Patients' ratings are recorded and graphed, and a total symptom distress score (0–90) is calculated as the sum of the first nine symptoms' scores.
The total daily opioid dosage was calculated for the first visit to the PCC and first follow-up by converting the total opioid dosage during 24 hours to an equivalent dose of oral morphine (Morphine Equivalent Daily Dose, MEDD), following standard equianalgesic conversion tables.19
Descriptive statistics were used to summarize demographical data and symptoms and opioid dose at baseline and follow up. χ2 tests were used to determine associations between categorical variables. Differences between continuous variables were analyzed using t tests for normally distributed data and Wilcoxon rank-sum tests for non-normally distributed data. Analyses of before and after symptom scores were made including only those patients with individual symptom scores 1 or greater at baseline. Significance levels less than 0.05 were considered significant.
Results
CAGE information was available for 598 of 665 patients (90%): of 598 patients, 100 were CAGE+ (17%). Patients' characteristics are summarized in Table 2. CAGE+ patients were significantly younger, predominantly males, and with a diagnosis of head and neck malignancies (Table 2).
Table 2.
Patient Demographics
| |
CAGE |
|
|---|---|---|
| Negative n (%) | Positive n (%) | |
| Male gender | 280/498 (47%) | 68/100 (68%)a |
| Race | ||
| White | 375 (75%) | 74 (74%) |
| African American | 55 (11%) | 17 (17%) |
| Hispanic | 44 (9%) | 8 (8%) |
| Asian | 15 (3%) | 1 (1%) |
| other | 9 (2%) | 0 0 |
| Total | 498 (100%) | 100 (100%) |
| Type of cancer | ||
| Gastrointestinal | 116 (23%) | 23 (23%) |
| Lung | 109 (22%) | 19 (19%) |
| Urologic | 59 (12%) | 8 (8%) |
| Head/neck | 44 (9%) | 24 (24%)b |
| Breast | 47 (9%) | 5 (5%) |
| Gynecologic | 42 (8%) | 6 (6%) |
| Hematologic | 17 (3%) | 3 (3%) |
| other | 64 (13%) | 12 (12%) |
| Total | 498 (100%) | 100 (100%) |
| Median age (range) | 60 (16–91) | 58 (28–87)b |
p < 0.0001.
p < 0.05.
CAGE+ patients were referred to palliative care 5 ± 19 months (median, standard deviation) after the diagnosis of advanced cancer, versus 13 ± 27 months for CAGE− patients (p < 0.0001).
Symptom scores at baseline are summarized in Table 3. CAGE+ patients presented with significantly worse scores for pain, sleep, dyspnea, and total symptom distress. Sensation of well-being scores were significantly greater in the CAGE+ group. Anxiety, depression, and fatigue showed a not significant trend to be higher among CAGE+ patients.
Table 3.
Median Baseline Symptom Distress According to CAGE Status
| Symptom | CAGE negative (n = 100) Median score (interquartile range) | CAGE positive (n = 100) Median score (interquartile range) |
|---|---|---|
| Pain | 4 (2–7) | 6 (4–8)a |
| Fatigue | 6 (4–8) | 7 (5–8) |
| Nausea | 0 (0–3) | 0 (0–3) |
| Depression | 2 (0–5) | 3 (0–6) |
| Anxiety | 3 (0–5) | 3.5 (0–6) |
| Drowsiness | 4 (1–6) | 5 (1–8) |
| Dyspnea | 2 (0–4) | 3 (0–6)b |
| Appetite | 5 (2–7) | 5 (3–8) |
| sleep | 4 (2–6) | 5 (3–7)b |
| Well-being | 5 (3–7) | 6 (4–8)b |
| Total symptom distress | 31 (21–42) | 39 (29–51)b |
p < 0.005.
p < 0.05.
Of 200 patients, 67 evaluated for symptoms and opioid dose did not return for a follow-up visit (32 in the CAGE-group and 35 in the CAGE+ group, p = 0.65). Both groups showed symptom improvement at follow up (Fig. 1). Statistically significant improvements were found in both groups for fatigue, anxiety, drowsiness, dyspnea, appetite, and sensation of well-being.
FIG. 1.
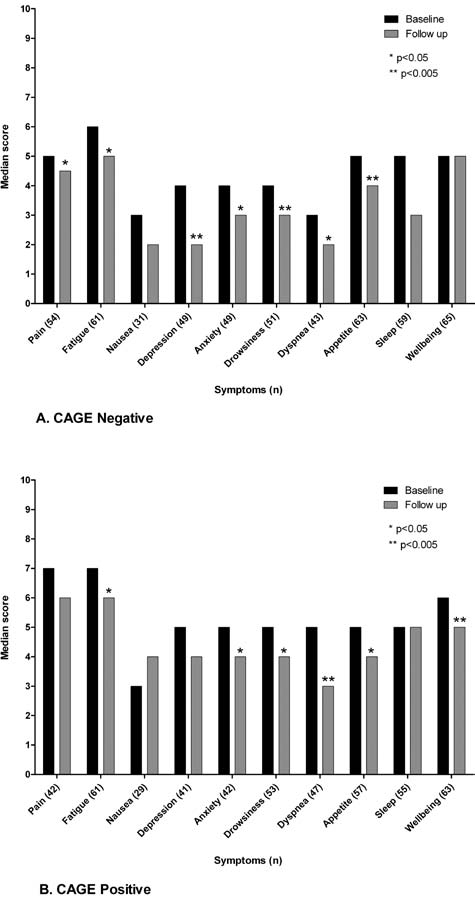
Median baseline and follow up ESAS symptom scores for patients with symptoms at baseline.
Significantly more CAGE+ patients were already receiving opioids at consultation, and the median MEDD for these patients was higher than for the CAGE− patients already on opioids at consultation. Opioid usage decreased both in frequency and dose upon first follow-up in both groups. The opioid dose has also decreased between baseline and follow up in both groups, however, the difference was not statistically significant (Table 4).
Table 4.
Number of Patients Receiving Opioids and Median Opioid Dose
| CAGE negative | CAGE positive | p | |
|---|---|---|---|
| Number of patients receiving opioids | |||
| At consultation | 29/100 (29%) | 47/100 (47%) | <0.05 |
| At follow-up | 16/68 (24%) | 27/65 (41%) | <0.05 |
| Opioid dose (mg/d) | |||
| Median MEDD at consultation—all patients on opioids (interquartile range) | 60 (47.5–200) | 100 (50–150) | 0.6280 |
| Median MEDD at consultation—only patients with follow-up (interquartile range) | 50 (30–200) | 60 (30–135) | 0.9639 |
| MEDD at follow-up (interquartile range) | 30a (0–125) | 60b (0–100) | 0.4250 |
MEDD at consultation X MEDD at follow-up, p = NS.
MEDD at consultation X MEDD at follow-up, p = NS.
MEDD, Morphine Equivalent Daily Dose.
Discussion
We have described the results of the analysis of 665 consecutive patients with advanced cancer seen in our PCC, including detailed exploration of symptoms and opioid usage for the first consecutive 100 CAGE+ and 100 CAGE− patients.
The CAGE questionnaire is routinely administered to all patients seen by our team at the time of consultation. We have found that CAGE was documented in the majority of the medical records (90%). However, the 17% frequency of CAGE+ patients found in this study was slightly lower than expected. other studies in similar populations have shown prevalences such as 27% in an inpatient palliative care unit,11 25% in an outpatient symptom control clinic,20 and 38% among inpatients in a tertiary cancer center being followed by a palliative care consultation team.21 This might be related to the lower frequency of alcohol-related cancers in our ambulatory center as compared to other settings. Head and neck cancers, for example, accounted for 68 of 665 (10%) of our outpatient population, and it was reported elsewhere to account for 21 of 61 (34%) of the cases seen by the inpatient consultation team.21 Underrepresentation of head and neck cancers in PCCs was already reported in another cancer center (8/166; 5% of patients).20 CAGE+ patients had a higher prevalence of head and neck malignancies, as expected.22
CAGE+ patients were more frequently males, as supported by the literature.5 Patients in the CAGE+ group were significantly younger, also confirmed by previous reports of alcoholism in younger patients and the trend that patients with alcoholism to die earlier than the general population.5,23
CAGE+ patients were referred approximately 8 months earlier to palliative care. We hypothesize that this is related to the difficult symptom management and psychological distress in this population. Further research is needed to confirm this observation.
It has been reported that somatic symptoms in cancer patients can have both physical and psychosocial contributors.24 The process by which psychological needs are expressed in physical symptoms is defined as somatization.25 it has been suggested that patients with addictive disorders are at greater risk for somatization and chemical coping.7,12,26,27 Our results showed that CAGE+ patients presented to the PCC with higher symptom expression compared to CAGE− patients. Positive screening for alcoholism may function as a surrogate to detect a tendency to somatization. Both CAGE+ and CAGE− patients had symptom improvement after the palliative care consultation. Our findings suggest that the PCC was capable of providing similar symptom relief independent of the patients' CAGE status. We cannot conclude that symptom relief was equivalent in the different groups due to the small population size and the retrospective nature of the study. CAGE information is regularly used by our multidisciplinary team to assist in the management of patients and families and on the prescription of medications. However, we are not able to determine in this retrospective study if the CAGE results were instrumental in helping the palliative care team to develop strategies to effectively manage symptoms in this population.
CAGE + patients were more frequently referred to palliative care already receiving opioids, probably due to their tendency to express higher symptom distress, leading to earlier opioid therapy initiation.
Patients with alcoholism can suffer stigmatization by health care professionals and this can result in the under-treatment of pain and other symptoms.2 On the other hand, the lack of appropriate diagnosis of alcoholism can result in inappropriate counseling and pharmacologic management, leading to increased patient and family suffering and drug toxicity related to escalation of opioids and other drugs.
The CAGE questionnaire can help in the screening of patients for alcoholism, but it should never be used as a diagnostic tool. Patients who screen positive using the CAGE should undergo formal diagnosis following DSM-IV criteria.
Conclusion
Alcoholism is frequent in the palliative care population, and it is associated with greater symptom expression. A palliative care team aware of patients' risk for alcoholism was able to effectively manage symptoms without opioid dose escalation. Our data suggest that there is a possible association between alcoholism and higher symptom expression, which still needs to be confirmed by prospective studies.
Acknowledgment
Eduardo Bruera is supported in part by National Cancer Institute R01 grants CA122292-01 and CA124481-01, and National Institute of Nursing Research grant NR010162-01A1.
References
- 1.Graham AV. Berolzheimer N. Burge S. Alcohol abuse. A family disease. Prim Care. 1993;1:121–130. [PubMed] [Google Scholar]
- 2.Passik SD. Theobald DE. Managing addiction in advanced cancer patients: Why bother? J Pain Symptom Manage. 2000;3:229–234. doi: 10.1016/s0885-3924(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF. Alcohol consumption, alcohol abuse and alcohol dependence. The United States as an example. Addiction. 1994;11:1357–1365. doi: 10.1111/j.1360-0443.1994.tb03730.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD. Bone LR. Geller G. Mamon JA. Stokes EJ. Levine DM. Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA. 1989;3:403–407. [PubMed] [Google Scholar]
- 5.World Health organization. WHO Expert Committee on Problems Related to Alcohol Consumption. Geneva: World Health organization; 2007. Alcohol availability and consumption in the world; pp. 9–19. [PubMed] [Google Scholar]
- 6.Strasser F. Walker P. Bruera E. Palliative pain management: When both pain and suffering hurt. J Palliat Care. 2005;2:69–79. [PubMed] [Google Scholar]
- 7.Lawlor P. Walker P. Bruera E. Mitchell S. Severe opioid toxicity and somatization of psychosocial distress in a cancer patient with a background of chemical dependence. J Pain Symptom Manage. 1997;6:356–361. doi: 10.1016/s0885-3924(97)00081-x. [DOI] [PubMed] [Google Scholar]
- 8.Elsayem A. Bruera E. Methadone-induced respiratory depression in a patient with a history of alcoholism. J Palliat Med. 2005;5:1062–1066. doi: 10.1089/jpm.2005.8.1062. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales GR. Coyle N. Treatment of cancer pain in a former opioid abuser: Fears of the patient and staff and their influence on care. J Pain Symptom Manage. 1992;4:246–249. doi: 10.1016/0885-3924(92)90081-r. [DOI] [PubMed] [Google Scholar]
- 10.Passik SD. Portenoy RK. Ricketts PL. Substance abuse issues in cancer patients. Part 1: Prevalence and diagnosis. Oncology (Williston Park) 1998;4:517–521. , 524. [PubMed] [Google Scholar]
- 11.Bruera E. Moyano J. Seifert L. Fainsinger RL. Hanson J. Suarez-Almazor M. The frequency of alcoholism among patients with pain due to terminal cancer. J Pain Symptom Manage. 1995;8:599–603. doi: 10.1016/0885-3924(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 12.Nekolaichuk CL. Fainsinger RL. Lawlor PG. A validation study of a pain classification system for advanced cancer patients using content experts: The Edmonton Classification System for Cancer Pain. Palliat Med. 2005;6:466–476. doi: 10.1191/0269216305pm1055oa. [DOI] [PubMed] [Google Scholar]
- 13.Bruera E. MacMillan K. Hanson J. MacDonald RN. The Edmonton staging system for cancer pain: Preliminary report. Pain. 1989;2:203–209. doi: 10.1016/0304-3959(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 14.Hinkka H. Kosunen E. Kellokumpu-Lehtinen P. Lammi UK. Assessment of pain control in cancer patients during the last week of life: Comparison of health centre wards and a hospice. Support Care Cancer. 2001;6:428–434. doi: 10.1007/s005200100245. [DOI] [PubMed] [Google Scholar]
- 15.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;14:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 16.Dhalla S. Kopec JA. The CAGE questionnaire for alcohol misuse: A review of reliability and validity studies. Clin invest Med. 2007;1:33–41. doi: 10.25011/cim.v30i1.447. [DOI] [PubMed] [Google Scholar]
- 17.Bruera E. Kuehn N. Miller MJ. Selmser P. Macmillan K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;2:6–9. [PubMed] [Google Scholar]
- 18.Chang VT. Hwang SS. Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;9:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SK. Pain Management. In: Elsayem A, editor; Driver L, editor; Bruera E, editor. The MD Anderson Symptom Control and Palliative Care Handbook. Houston: M.D. Anderson Cancer Center; 2004. p. 38. [Google Scholar]
- 20.Bruera E. Michaud M. Vigano A. Neumann CM. Watanabe S. Hanson J. Multidisciplinary symptom control clinic in a cancer center: A retrospective study. Support Care Cancer. 2001;3:162–168. doi: 10.1007/s005200000172. [DOI] [PubMed] [Google Scholar]
- 21.Braiteh F. El osta B. Palmer JL. Reddy SK. Bruera E. Characteristics, findings, and outcomes of palliative care inpatient consultations at a comprehensive cancer center. J Palliat Med. 2007;4:948–955. doi: 10.1089/jpm.2006.0257. [DOI] [PubMed] [Google Scholar]
- 22.Boyle P. Macfarlane GJ. Zheng T. Maisonneuve P. Evstifeeva T. Scully C. Recent advances in epidemiology of head and neck cancer. Curr Opin Oncol. 1992;3:471–477. doi: 10.1097/00001622-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Deleyiannis FW. Thomas DB. Vaughan TL. Davis S. Alcoholism: independent predictor of survival in patients with head and neck cancer. J Natl Cancer Inst. 1996;8:542–549. doi: 10.1093/jnci/88.8.542. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi SK. Maguire GP. Persistent somatization in cancer: A controlled follow-up study. J Psychosom Res. 1998;3:249–256. doi: 10.1016/s0022-3999(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 25.Stedman's Medical Dictionary. Philadelphia: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 26.Tien AY. Schlaepfer TE. Fisch HU. Self-reported somatization symptoms associated with risk for extreme alcohol use. Arch Fam Med. 1998;1:33–37. doi: 10.1001/archfami.7.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Bruera E. Neumann C. Brenneis C. Quan H. Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care. 2000;3:16–21. [PubMed] [Google Scholar]


