Abstract
Cytokines have been used extensively as adjuvants in vaccines. However, practical considerations limit their use; diffusion from antigen, short half-lives and additional production costs. To address these problems we have developed a technology that efficiently produces inactivated, whole-virus influenza vaccine bearing membrane-bound cytokines. To provide “proof of principle,” we chose chicken interleukin-2 (IL-2) and chicken granulocyte-macrophage colony-stimulating factor. Fusion constructs were generated in which their coding regions were linked to the influenza virus transmembrane encoding domains of the neuraminidase and hemagglutinin genes, respectively. These fusion constructs were used to establish stable Madin–Darby Canine Kidney cell lines, constitutively expressing membrane-bound cytokine. Cell surface expression was verified by immunofluorescence and cytokine-specific bioassays. Influenza virus harvested from infected cytokine-bearing cells was purified, inactivated, and confirmed to include membrane-bound cytokine by immunofluorescence, Western blotting and bioassay. Cytokine bioactivity was preserved using several standard virus inactivation protocols. Both cytokine-bearing influenza vaccines are now being tested for immunogenicity in vivo. Initial experiments indicate that chickens injected with IL-2-bearing influenza have elevated antiviral antibody levels, compared to chickens given conventional vaccine. In conclusion, this technology offers a novel method to utilize cytokines and other immunostimulatory molecules as adjuvants for viral vaccines.
Introduction
Cytokines can serve as potent adjuvants and have been used in DNA vaccines (Kutzler and others 2005; Orson and others 2006) tumor vaccines (Okada and others 2001; Lasek and others 2004) and killed (Ben-Yehuda and others 2003a; Ben-Yehuda and others 2003b) and live(Bukreyev and Belyakov 2002; Bukreyev and others 2002; Kittel and others 2005) viral vaccines. Numerous avian cytokines have been cloned, including interleukin (IL)-1β, IL-2, IL-4, IL-12, IL-15, IL-18, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ and myelomonocytic growth factor (MGF) (Kaiser and others 2005; Giansanti and others 2006). Some avian cytokines have been used as adjuvants for experimental avian vaccines, including IL-2 (Hu and others 2001; Hulse and Romero 2004; Li and others 2004; Zhou and others 2005; Tarpey and others 2008), interferon-γ (Lowenthal and others 1998; Schijns and others 2000; Takehara and others 2003), and MGF (Djeraba and others 2002). While cytokines have been effective as adjuvants in experimental vaccines, some characteristics of soluble cytokines have reduced their utility. These include; a short in vivo half-life, dispersion from the target antigen, and extra production costs. To address the above limitations we have developed a technology to express membrane-bound forms of cytokines on virus particles. The model system we have used is killed influenza vaccines, although our approach is applicable to other enveloped viruses used in killed or live vaccine formulations. Our emphasis on influenza is based on the need to improve adjuvants for avian influenza vaccines for protection of both poultry and humans. Poultry vaccines currently utilize killed whole virus in oil emulsions. These are effective for homologous influenza strains, but may not be effective for heterologous strains and may not prevent infection and virus shedding of homologous virus. Furthermore, oil emulsions may induce local inflammatory reactions.
The novelty in our approach is the development and use of membrane-bound versions of cytokines in viral vaccine design. We expect this technique to prove superior to the use of soluble cytokines as adjuvants. For example, a recent study reported that geese injected with soluble goose IL-2 plus an oil adjuvanted influenza vaccine exhibited a modest (one dilution step) enhancement of the hemagglutination-inhibition (HI) antibody titer (Zhou and others 2005). Other studies, reported above, indicate that chicken IL-2, administered as a protein or plasmid DNA, enhance antibody responses and/or protection against infectious bursal disease virus (Hulse and Romero 2004; Li and others 2004). A recent collaborative study indicates that chIL-2 expressed by a vaccine strain of Marek's disease virus augments neutralizing antibody titers, although it doesn't improve protection from challenge with Marek's virus (Tarpey and others 2007a). However, coadministration of the Marek's vaccine vector encoding chIL-2 with vaccine viruses for infectious bursal disease or infectious bronchitis disease enhanced protection to both bursal disease and bronchitis(Tarpey and others 2007b). Because several groups have used avian IL-2 as a vaccine adjuvant, and in view of our expertise in the cloning and characterization of chicken IL-2, we chose IL-2 as one of the cytokines in this study. The other cytokine chosen for study was chicken GM-CSF, recently cloned by Avery and others (2004). Chicken GM-CSF was selected since mammalian GM-CSF has shown great efficacy in boosting both humoral and cellular immunity and, attached to tumor cells, was highly effective as an adjuvant (Soo Hoo and others 1999; Yei and others 2002; Chang and others 2004).
The use of membrane-bound cytokines derives from recent tumor vaccine studies in which cytokines bound to tumor cell lines were found to retain their bioactivity and augment immunity to the tumor cell lines. Membrane-bound versions of mammalian IL-2 (Nizard and others 2003), IL-4 (Kim and others 2000), IL-12 (Nagarajan and Selvaraj 2002), and GM-CSF (Poloso and others 2002) have been utilized. Our technique involves construction of a fusion gene, whereby the cytokine-encoding region is fused to the region encoding the transmembrane and cytoplasmic tail domains (and proximal stalk regions) of either of two viral membrane glycoproteins (hemagglutinin, HA, and neuraminidase, NA). The fusion construct is then inserted into a eukaryotic plasmid vector under control of the cytomegalovirus promoter, which is subsequently used to establish stably transfected Madin–Darby Canine Kidney (MDCK) cells. We postulated that the viral transmembrane and cytoplasmic tail domains would direct the fusion protein to lipid raft domains at the apical surface of the MDCK cells, the site of influenza virus budding. Virus infection in these producer cell lines would result in incorporation of membrane-bound cytokine as the virus assembles and buds from the apical surface of the cells. The specific questions addressed in these “proof of concept” studies are: (1). Is the membrane-bound form of the cytokine expressed at the cell surface in a bioactive form? (2) Are both the HA and NA transmembrane proteins, which are type I and II membrane proteins, respectively, suitable for cytokine fusion constructs? (3). Does virus readily incorporate the membrane-bound cytokine on budding? (4). Is cytokine bioactivity preserved after inactivation of the virus and propagation of virus in the presence of TPCK-trypsin (which is required for growth of virus in vitro), and (5). Does the membrane-bound cytokine serve as an adjuvant to augment immune responses?
Materials and Methods
Construction of expression plasmids
The cytosolic and transmembrane domains of influenza NA were fused to chIL-2 mature segment using standard polymerase chain reactin (PCR)-based cloning techniques, resulting in pcDNA3.1/NA-chIL-2, coding for NA-chIL-2 fusion protein. Briefly, the N-terminal 51 amino acids of influenza A/WSN/33 NA segment (Genbank L25817) corresponding to cytoplasmic tail (6 aa), transmembrane (29 aa), and stalk (17 aa) regions and full length coding sequences of mature chIL-2 (Genbank AF000631) were PCR amplified using specific primer pairs containing appropriate restriction endonuclease sites (restriction sites underlined) (NA forward 5′ GACTGGATCCCTGCCATGAATCCAAAC 3′, NA reverse 5′ ACTGCCTTGGTTGCA TAT 3′, chIL-2 Forward 5′ GCATCCAAGGCGCATCTCTATCA 3′, and chIL-2 reverse primer 5′ GCTAGAATTCTTATTTTT GCA 3′).
Chicken GM-CSF was fused to the transmembrane domain of the influenza HA. PCR was used to synthesize the GM-CSF gene, using a technique developed by Dillon and Rosen (1990) that obviated the need for full-length template in the PCR reaction. While the gene itself was not available at the time, its sequence was published (GenBank # NM_001007078.1). Based on the sequence, six oligos (each containing 80–100bp including 25-bp overhangs) were synthesized that spanned an optimized Kozak sequence and the entire coding region (primer sequences listed in Table 1). These six oligos served as both primers and templates in PCR, using conditions as described by Dillon and Rosen (1990), which produced a mixture of PCR products of varying lengths. This mixture was then used as a template for the second PCR reaction using short primers (Table 1) that encoded the 5′ and 3′ ends of the gene, respectively. Following cloning into pcDNA3.1 (pcDNA3.1/chGM-CSF), the expression plasmid (verified by the WSU sequencing core) was transfected into COS7 cells using Lipofectamine2000 (Invitrogen Corp.) as described by the manufacturer. Culture supernatant was collected at 48 h and used as a positive control in the bio-assay for GM-CSF.
Table 1.
Primers Utilized to Synthesize Chicken GM-CSF
| Primers for the first PCR reaction: |
| Forward primer #1 (HindIII-Kozak-ORF-nucleotides #1–80): 5′-GCAT AAGCTT CCACG ATGCTGGCCC AGCTCACTAT TCTGCTTGCC CTCGGGGTGC TCTGCAGCCC TGCGCCCACC ACAACATACT CCTGCTGCTA-3′ |
| Reverse primer #2 (nucleotides # 155 to 156): 5′-ACCGA GGACAGACCT GCTGTGGCCG CTGTGCTCTC CAAGTGACTC GTTATTTCTT CCAGGATGGT GTACACTTTG TAGCAGCAGG AGTATGTTGT GGTGG-3′ |
| Forward primer #3 (nucleotides # 131–230): 5′- CGGCCACAGC AGGTCTGTCC TCGGTACCCA TGGACATCAG GGATAAAACC TGTCTGCGTA ACAACCTGAA AACATTCATA GAGTCCTTGA AAACAAATGG-3′ |
| Reverse primer #4 (nucleotides # 305–206): 5′-GTTAT GTTCGAGAAG AGGCGTTCAC ACTCGTGAAC TCTGTTCAGC TGAAAGACGA TTCCGCTTTC TTCCTCTGTC CCATTTGTTT TCAAGGACTC TATGA-3′ |
| Forward primer #5 (nucleotides # 281–380): 5′-GTGAACGCCT CTTCTCGAAC ATAACTCCCA CCCCGCAGGT TCCTGATAAG GAATGTAGAA CTGCACAAGT ATCGAGGGAA AAATTCAAAG AGGCATTAAA-3′ |
| Reverse primer #6 (nucleotides # 435–356): 5′-GCAT GAATTC TTAGA TGCAGTCTTT CTCCTCTGGG AGCACATCAG AGAGGTAAAT AAAGAAAGTT TTTAATGCCT CTTTGAATTT TTCCC-3′ |
| Primers for the second PCR reactions to make soluble and membrane-bound GM-CSF: |
| Primer #7 (used for soluble and membrane-bound GM-CSF) (forward primer with HindIII restriction site): 5′-GCAT AAGCTT CCACG ATGCTGGCCC AGCTC-3′ |
| Primer #8 (used for soluble GM-CSF) (reverse primer with EcoR1 restriction site): 5′-GCAT GAATTC TTAGA TGCAGTCTTT CTCCT-3′ |
| Primer #9 (used for membrane-bound GM-CSF) (reverse primer with BamH1 restriction site): 5′-GCATAAGCTTCCACGATGCTGGCCCAGCTC-3′ |
For construction of membrane bound chicken GM-CSF-HA the original forward primer and a new reverse primer (Table 1) were used to remove the stop codon (TAA) and replace the EcoR1 site with a BamH1 restriction site. This allowed for its ligation in frame with HA derived from the influenza virus A/WSN/33 strain in the plasmid vector pcDNA3.1+. The HA fragment was amplified by PCR using primers (forward primer) 5′-CCGGATCCAATGGGACTTATGATTATCC-3′ and (reverse primer) 5′-CCGAATTCTCAGATGCATATTCTGCACTGC-3′. It includes HA nucleotides 1521 to 1730 (70 amino acids) and is denoted HA1521. These nucleotides encode for the cytoplasmic tail, transmembrane region, and a portion of the stalk region 26 amino acids in length. HA was ligated into pcDNA3.1+ via restriction sites BamH1 (5′) and EcoR1 (3′). The HA DNA sequence was confirmed by DNA sequencing.
Transfection and selection of MDCK cells
MDCK cells were trypsinized and seeded at 60% confluency immediately before transfection. Transfection was performed using Lipofectamine2000 as recommended by the manufacturer's protocol (Invitrogen) using 3 μg of plasmid DNA in 6-well plates. Stable transfectants were subsequently cultured in growth media supplemented with Geneticin (1.5 mg/mL) for at least 1 week. Geneticin-resistant cells were then subcloned by limiting dilution in 96-well plates in media supplemented with Geneticin (1 mg/mL) and screened for membrane-bound chIL-2 or chicken GM-CSF (chGM-CSF) by bioassay.
Bioassay for chIL-2 using Concanavalin A–activated chicken spleen cells
Activated T cells for use as indicator cells were prepared as described by Kolodsick and others (2001). Briefly, spleen cells from an exsanguinated market chicken were cultured at 107 cells/mL in Iscoves' medium with bovine serum albumin (BSA), 2 mg/mL, Concanavalin A (ConA),10 μg/mL, and pen and strep at 40°C. At 24 h ConA was neutralized with 0.05 M α-methyl mannopyrannoside, the media diluted 2-fold and supplemented with autologous serum, final concentration of 2%, and the culture was incubated for 2 additional days. Viable cells were isolated following separation on Histopaque, washed, counted, and used as indicator cells. To assay chIL-2 bioactivity on stably transfected MDCK sub-clones, 90% confluent MDCK/NA-chIL-2 cells (and MDCK/vector control cells) in flat bottom 96-well plates were treated with mitomycin C at 50 μg/mL for 1 h at 37°C. Mitomycin C was then removed by washing (3×) and chicken T blasts were added (5 × 104 cells/well) in Iscoves' medium supplemented with 2% autologous chicken serum, 2 mg/mL bovine serum albumin (Sigma) and penicillin/streptomycin. Cultures were incubated overnight at 40°C in 5% CO2 and pulsed for the last 6 h with 1 μCi of [3H] thymidine, with or without 10−6 M fluorodeoxyuridine as described. (Fluorodeoxyuridine reduces the endogenous synthesis of thymidine). Cells were harvested 6 h later on glass fiber filters using an automated harvester and counted in a liquid scintillation counter. Controls included blast cells alone and blast cells stimulated with soluble recombinant chIL-2 (prepared as described by Kolodsick and others (others 2001). To assay chIL-2 on killed influenza virus particles, dilutions of virus were added to round bottom 96 well plates. Then chicken blast cells were added (2 × 104/well), incubated overnight, pulsed with 3H-thymidine and harvested 6 h later.
Bioassay for chGM-CSF using chicken bone marrow cells
Bone marrow (BM) was collected from the femurs and tibias of a freshly exsanguinated market chicken by flushing with cold Iscoves medium. BM cells were passed through a 100-mm mesh nylon gauze, and collected by centrifugation. Red blood cells were removed by centrifugation at 50g for 5 min. BM cells were subsequently washed 3× with cold Iscoves medium and resuspended at 3 × 105 cells/150 μL in complete media (Iscoves medium supplemented with 5% FCS, 2% autologous chicken serum, 2 mM l-glutamine, 1 mM pyruvate,100 mg/mL streptomycin and 100 U/mL penicillin,). For the cell-based bioassay, GM-CSF-transfected MDCK cells grown to 90% confluency in 96-well flat-bottom plates, were pretreated with mitomycin C, 50 mg/mL for 1 h, washed three times and then incubated with 3 × 105 BM cells. For the viral-based assay, BM cells (3 × 105 cells/well in 150 μL) were incubated for 3 days at 40°C with dilutions of inactivated influenza A/PR/8/34 virus or A/PR/8/34 virus-bearing membrane-bound chGM-CSF in 96-well round bottom plates. Wells were pulsed with 3H-thymidine during the last 20 h. The plate was harvested and counted using a scintillation counter.
IL-2 neutralization assay
Rabbit anti-chIL-2 antiserum with neutralizing activity (Stepaniak and others 1999) was used to verify the bioactivity of membrane-bound IL-2. In addition, two monoclonal anti-chIL-2 antibodies (clones O and G; both IgG1k) developed by Kolodsick, Stepaniak, and Sundick (unpublished data) which bind to specific sites of IL-2 were also used in neutralization assays. Monoclonal O binds to the C-terminus of IL-2, as evidenced by its reactivity in Western blots with full-length IL-2, but not with IL-2 deletion mutants in which two or more of the nine C-terminal amino acids are deleted. Monoclonal G reacts with a site on IL-2 distinct from the COOH end, since it reacts equally well in Western blots with full-length IL-2 and an IL-2 mutant in which the nine C-terminal amino acids are deleted. MDCK cells transfected with NA-IL-2 were treated with 15 mM β-propiolactone for 20 min at room temperature (RT). β-Propiolactone was then washed away, and the cells were incubated with diluted rabbit anti-chIL-2 serum, monoclonal antibody O or G for 1 h at 37°C. As a negative control, cells were incubated with diluted normal rabbit serum or Dulbecco's modified Eagle's medium (DMEM) medium. Chicken T-cell blasts (5 × 104/well), prepared as above, were added to the cells and cultures were incubated overnight at 40°C, pulsed for 6 h with 3H-thymidine+ fluorodeoxyuridine, and counted. To determine the ability of anti-chIL-2 antibodies to inhibit influenza virus membrane-bound chIL-2 induced proliferation, 300 hemagglutinating units (HAU) of virus bearing chIL-2 (virus inactivated by 56°C for 20 min) were incubated with diluted rabbit anti-chIL-2 antiserum, or monoclonal antibody O or G, for 1 h at 37°C. Then chicken T blasts were added as described above, pulsed with 3H-thymidine and counted.
Determination of the effects of TPCK-trypsin on IL-2 bioactivity
To enable multiple rounds of influenza virus replication and to maximize yields of virus grown in MDCK cells, it is necessary to add TPCK-trypsin during virus propagation. Since this enzyme has the potential to cleave IL-2, IL-2-bearing virus grown in the presence of TPCK-trypsin was monitored for retention of its IL-2 bioactivity. Monolayers of stably transfected MDCK/NA-chIL-2 cells or vector control MDCK cells were infected with influenza A/UDORN/72 (H3N2) at a multiplicity of infection of 1.5. Following an adsorption period of 1 h at 37°C, the cells were washed twice with PBS and then incubated in 5 mL DMEM supplemented with various dilutions of TPCK-trypsin (Sigma Chemical Company) for 24 h. Supernatants of infected cells were harvested at a time in which the MDCK cells were killed. Cellular debris was removed by centrifugation at 800g for 20 min at 4°C. Virus was then pelleted through a 14% OptiPrep™ (Axis-Schield) cushion at 88,000g for 1 h at RT and inactivated at 56°C for 20 min. Pelleted virus was resuspended in PBS with Mg2+, Ca2+, quantitated and standardized by hemagglutination assay prior to bioactivity assessment.
The hemagglutination titers were determined as described previously (Webster and others 2002a). Briefly, 50 μL of virus supernatants were serially diluted 2-fold in PBS in V-bottom 96-well plates. Then 50 μL of 0.5% chicken red blood cells were added to each well. The plates were incubated for 1 h at RT and the HA patterns were read, and the results expressed as HAU/50 μL.
Virus purification and inactivation
Supernatants harvested from influenza infected MDCK control cells or membrane-bound cytokine-expressing MDCK cells were precleared by centrifugation at 800g for 10 min at 4°C. The virions were pelleted through a 14% OptiPrep™ cushion at 88,000g for 60 min at 10°C, followed by banding over a 14–26% OptiPrep™ gradient at 55,000g for 45 min at 10°C. Banded virus was collected and concentrated by ultracentrifugation at 75,000g for 45 min at 10°C followed by resuspension in PBS. Protein content of purified virions was determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology).
Virus inactivation was carried out on purified virions using one of three inactivation protocols: (1) For β-propiolactone inactivation, virus was treated with 0.1% β-propiolactone in PBS PH 7.4 for 20 min at RT. The reaction was stopped by the addition of sodium thiosulfate (final concentration 40 mM); (2) For heat treatment, virus was exposed to 56°C for 20, 40, or 60 min. (3) For ultraviolet (UV)-inactivation of virus, virus was irradiated at a distance of 6 inches with 1500 μW/s/cm2 UV for 3, 6, or 12 min. After virus inactivation, virus was pelleted a second time by centrifugation to remove residual contaminants. To confirm virus inactivation, MDCK cells were incubated with inactivated virus (5 μg total viral protein) for a period of 5 days at 37°C in the presence of 1.5 μg/mL TPCK-trypsin. Absence of cytopathic effect and lack of hemagglutination activity in the supernatants of exposed cells were criteria used to confirm complete inactivation of the virus.
Western blot analysis
Purified virions lysed in Laemmli sample buffer (Biorad) were separated by sodium dodecyl sulfate (SDS)-12% poly-acrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride membrane (Immun-blot-PVDF; Bio-Rad). After transfer, the membrane was blocked for 1 h at RT in 5% nonfat dry milk in TTBS buffer (0.5% Tween 20, 200 mM NaCl, and 50mM Tris, pH 7.5). Blots were incubated with either mouse anti-chIL-2 monoclonal antibody G, and/or goat antiviral NA (N2) diluted 1:1000 at 4°C overnight. The blot was washed three times with TTBS and subsequently incubated with rabbit antimouse, or antigoat horseradish peroxidase–conjugated secondary antibody at 1:3000 dilution (Sigma) at RT for 1 h. After washing, the proteins were visualized by enhanced chemiluminescence (ECL, Amersham).
Vaccination studies in chicks
On the day of immunization, vaccine administered without adjuvant was diluted in 200 μL PBS. Vaccine emulsified in incomplete Freund's adjuvant (ICFA) (Sigma Chemical Co.) was first diluted in 250 μL PBS and then slowly emulsified with 250 μL ICFA by passage between two syringes. The viscosity of the emulsion was checked by its integrity after dropping onto H2O.
White Leghorn male chicks were purchased locally (Townline Farms, Zeeland, MI), and maintained in the division of Laboratory Animal Resources at Wayne State University. Sera of 1-week-old chicks were tested for maternally transferred antiinfluenza antibodies; all were negative. A total of 40 chickens were used for immunization studies and divided into 5 experimental groups: saline control (n = 10), wt-virus in PBS (n = 7), wt-virus in oil (n = 8), virus-IL-2 in PBS (n = 7), and virus-IL-2 in oil (n = 8). Briefly, 1-week-old chickens were vaccinated subcutaneously at the base of the neck with the inactivated influenza A/Udorn/72 (H3N2) vaccines described above. Vaccines administered without oil adjuvant contained 10 μg of total viral protein (ca. 3 μg HA protein), while vaccines emulsified in ICFA contained 3 μg (ca. 1 μg HA protein). Immunized chickens were boosted subcutaneously 21 days later with the same dosage of vaccine. Two weeks after the booster injection all chickens were sacrificed and 1-mL blood was drawn from the jugular vein. Sera were tested for antiviral antibody responses by enzyme-linked immunosorbent assay (ELISA) and HI assays.
ELISA and HI assays
Individual wells of 96-well ELISA plates (Immulon II, Dynatech Laboratories Inc.) were coated overnight at 4°C with purified influenza virus (A/Udorn/72;10 HAU/well) in carbonate buffer, pH 9.6. Excess virus was discarded, and the coated wells were washed five times with PBS containing 0.05% Tween 20 (PBST). Coated wells were then blocked (2% BSA in PBST) overnight at 4°C, and then incubated with serial 2-fold dilutions of chicken sera in blocking buffer overnight at 4°C. Following extensive washing, wells were incubated with alkaline phosphatase-conjugated rabbit anti-chicken IgY (1/2000 dilution; Sigma; whole molecule) for 3 h at RT. Following washing, the amount of alkaline phosphatase activity in each well was determined by incubation with substrate (2,2′-Azino-bis(3-ethylbenothiazoline-6-sulfonic acid) (Sigma) for 30 min at RT. The reaction was stopped by addition of 1% SDS, and the absorbance at 405 nm was determined. The HI assay was performed as described (Webster and others 2002a) using heat-inactivated and periodate-treated sera.
Statistical analysis
To determine whether ELISA titers among the groups of chickens were significantly different from each other, analysis of variance and Bonferoni's Multiple Comparison Test (Prism, Graphpad) were performed and a value of p < 0.05 was considered significant. To determine significance in the HI assays a χ2 analysis was performed.
Results
Development and characterization of a stable MDCK cell clone that expresses membrane-bound, bioactive chIL-2 on its surface
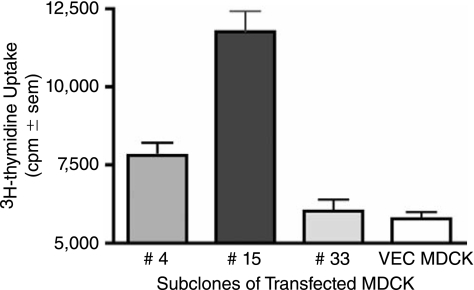
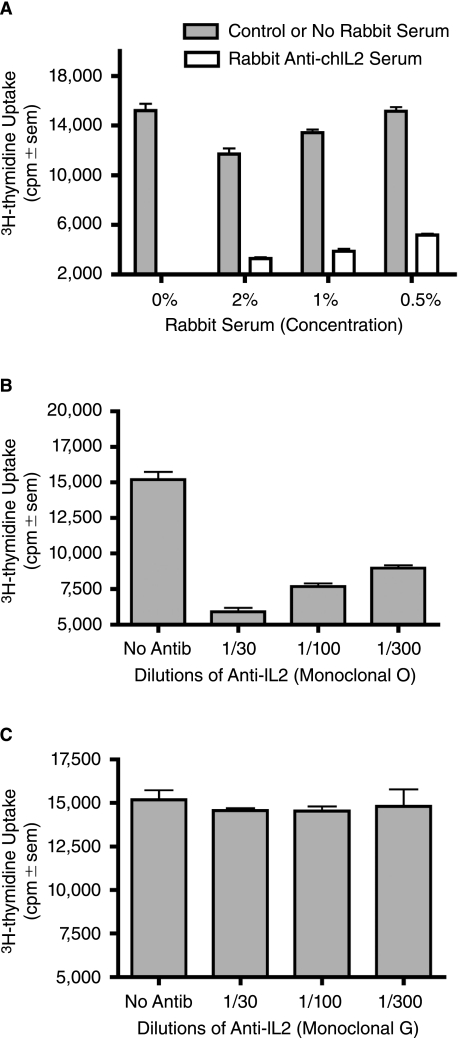
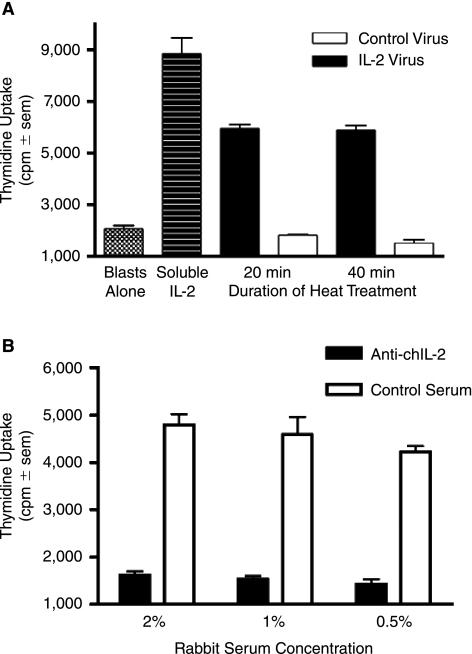
These studies were designed to test whether chicken specific cytokines could be incorporated into influenza virus particles in a membrane-bound form to serve as immuno-modulatory adjuvants. To achieve this aim, we generated an expression plasmid, pcDNA3.1/NA-chIL-2, in which the coding region of mature chicken IL-2 was fused in frame to the N-terminus of the influenza A/WSN NA gene. The N-terminal portion of NA encodes for the 17-membrane proximal amino acids of the NA stalk as well as for the transmembrane and cytoplasmic tail regions of the NA, and thereby, anchors chIL-2 to the membrane. Following transfection of MDCK cells, selection with G418 and limited dilution subcloning, individual MDCK subclones were screened directly for chIL-2 bioactivity. As shown in Figure 1, subclone 15 cells induced the greatest uptake of 3H-thymidine compared to control MDCK cells and two other MDCK/NA-chIL-2 expressing subclones. To confirm that the elevated thymidine uptake was due to biological activity of surface expressed chIL-2, neutralization studies were performed using rabbit anti-chIL-2 antiserum and two mouse anti-chIL-2 monoclonal antibodies. The rabbit antiserum and the monoclonal clone O anti-chIL-2 antibody, but not clone G, were neutralizing (Fig. 2). The O clone antibody recognizes the C-terminus of chIL-2, which contains a bioactive site, while clone G reacts with an unknown site distal to the C-terminus (Kolodsick and Sundick, unpublished data). Based on the data presented here clone G recognizes a non-neutralizing epitope. It should be noted that both rabbit anti-chIL-2 and clone G recognize the membrane bound chIL-2 fusion construct on the surface of MDCK cells as demonstrated by surface immunofluorescence microscopy (data not shown). Collectively, these data demonstrate that the isolated subclone 15 of MDCK/NA-chIL-2 cells expresses bioactive chIL-2 on its cell surface. These cells were then used for all subsequent studies using membrane-bound chIL-2.
FIG. 1.
Membrane-bound NA-chIL-2 expressed on the surface of MDCK subclones is bioactive. Subclones 4, 15, and 33 of MDCK cells stably transfected with the NA-chIL-2 construct or wild-type (wt) MDCK cells were assayed for chicken IL-2 bioactivity by co-culturing mitomycin C pre-treated cells with activated chicken T blasts and measuring 3H-thymidine uptake (quadruplicate samples).
FIG. 2.
Anti-chIL-2 antibodies neutralize membrane-bound chIL-2 bioactivity on MDCK/NA-chIL-2 cells. Three concentrations of rabbit anti-chIL-2 antiserum (A), monoclonal mouse anti-chIL-2 antibody O (specific for the C-terminus of chIL-2) (B) and monoclonal mouse anti-chIL-2 G (C) were evaluated for their neutralizing activity in an IL-2 bioassay. β-propiolactone treated MDCK/NA-chIL-2 cells seeded in 96-well plates were incubated with chicken T-cell blasts for 24 h in the presence of chIL-2 specific antibodies or nonspecific control antisera. Proliferation was measured by 3H-thymidine incorporation (quadruplicate samples), which was present during the final 6 h.
NA-chIL-2 is incorporated into released virus and retains bioactivity following viral inactivation
A critical part of our hypothesis is that membrane-bound chIL-2 expressed at the cell surface of MDCK cells will be readily incorporated into budding influenza particles following influenza infection. A further prerequisite is that these purified virus particles bearing chIL-2 will retain bioactivity following virus inactivation.
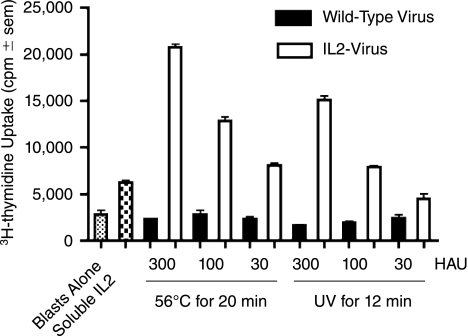
Ultraviolet irradiation has been used to prepare killed vaccines by inactivating nucleic acid. Wang and others (2004) studied the effect of UV treatment on both virus inactivation and protein recovery. In their study, α1-proteinase inhibitor was selected as the target protein because of the sensitivity of this enzyme to UV-irradiation (presence of UV-absorbing amino acids near the enzyme's reactive center). They demonstrated that UV treatment could inactivate viruses without causing significant protein damage. Therefore, UV irradiation seems suitable for the preparation of inactivated antiviral vaccines bearing bioactive cytokine on their surface. To test this hypothesis, purified influenza viruses were UV irradiated for 3, 6, and 12 min under a UV lamp at 1500 μW s/cm2. To confirm viral inactivation, MDCK cells were incubated with UV-treated viruses at 37°C for 3–5 days in the presence of TPCK-treated trypsin. Growth of virus was monitored by its cytopathic effect and by determining HAU in cell culture supernatants. Treatment for 12 min was required to completely abolish influenza virus infectivity in a cell culture assay. To evaluate retention of cytokine bioactivity following viral inactivation, dilutions of virus (expressed as HAU), were evaluated for their ability to stimulate chicken T blast proliferation. As shown in Figure 3, UV-inactivated virus-bearing membrane-bound chIL-2 was stimulatory at all tested concentrations, whereas wild-type virus had no proliferative inducing activity. In parallel studies, heat inactivation of influenza virus was also evaluated in view of its simplicity and safety. Virus was subjected to 56°C for 20, 40, and 60 min. NA-chIL-2 bioactivity was preserved (Fig. 3) and virus infectivity was abolished (data not shown) following heat treatment for 20 min. Virion-bound NA-chIL-2 also tolerated 56°C for 40 and 60 min without loss of bioactivity (data not shown).
FIG. 3.
NA-chIL-2 bioactivity on influenza A/Udorn/72 (H3N2) viral particles is preserved following viral inactivation with UV or heat treatment. Influenza A/Udorn/72 virus harvested from infected MDCK/NA-chIL-2 cells or from vector control MDCK cells was purified by sedimentation through a 14% OptiPrep™ cushion and inactivated by UV treatment for 12 min or heat at 56°C for 20 min. Chicken T blasts were incubated with 30–300 hemagglutinating units (HAU) of inactivated virus for 24 h, the last 6 h in the presence of 3H-thymidine. Proliferation was measured by 3H-thymidine incorporation. Controls included wells with blast cells alone or blast cells with recombinant soluble chIL-2 (2 × 10−7 mM).
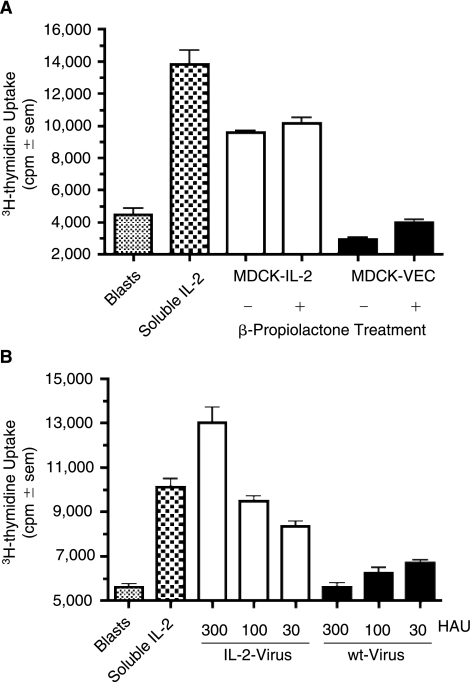
β-Propiolactone is a widely used agent for the inactivation of influenza virus and studies were, therefore, performed to test the resistance of IL-2 bioactivity to β-propiolactone treatment. Soluble recombinant IL-2 was treated with 0.1 (15 mM), 0.033 (5 mM) or 0% β-propiolactone and tested for IL-2 bioactivity; β-propiolactone at 15 mM induced only a minor reduction of bioactivity, 18%, and at 5 mM had no effect on IL-2 bioactivity (data not shown). Subsequently, MDCK cells expressing vector control or NA-IL-2 were treated with 0.1% β-propiolactone and compared with the same cells treated with mitomycin C alone; β-propiolactone had no adverse effect on membrane-bound IL-2 bioactivity expressed at the cell surface (Fig. 4A). Finally, purified influenza A/Udorn/72 propagated in NachIL-2-expressing MDCK cells, was treated with 0.1% β-propiolactone and tested for IL-2 bioactivity. As depicted in Figure 4B, chIL-2 bioactivity was retained on virus particles following viral inactivation. Note that live virus-bearing IL-2 was not compared with inactivated virus-bearing IL-2 out of concern that live virus might adversely affect the bioassay. Nevertheless, the comparisons of soluble IL-2 and MDCK-bearing IL-2 with and without β-propiolactone suggest that β-propiolactone treatment of virus-IL-2, had minimal, if any, effect on the bioactivity of IL-2.
FIG. 4.
Membrane-bound NA-chIL-2 bioactivity on MDCK cells and virions is preserved following inactivation with β-propiolactone. (A) Mitomycin C treated NA-chIL-2-MDCK and wild-type MDCK cells were exposed for 20 min to either β-propiolactone or solvent alone prior to testing for IL-2 using T blasts. Samples were run in quadruplicate and soluble recombinant IL-2 (2 × 10−5 mM) served as a positive control. (B) Influenza A/Udorn/72 virus propagated in MDCK/NA-chIL-2 or MDCK/Vec cells was tested for chIL-2 bioactivity after purification, quantitation, and inactivation with β-propiolactone. Samples were run in quadruplicate and soluble recombinant chIL-2 at 10−6 mM served as a positive control.
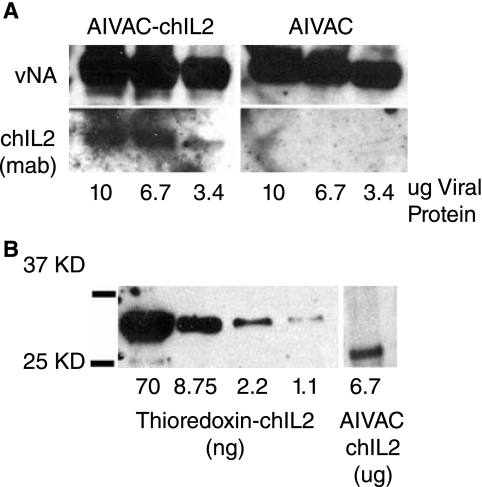
Western blot analysis and surface immunofluorescence microscopy of filamentous influenza budding from the cell surface (data not shown) were also routinely used to confirm direct incorporation of membrane-bound chIL-2 into budding virions. As demonstrated in Figure 5, NA-chIL-2 could readily be detected in immunoblots of highly purified virion preparations that had been propagated in MDCK/NA-chIL-2 cells compared to virus grown in wild-type MDCK cells. Further, it is apparent that full-length viral NA (vNA in Fig. 5A) incorporation is not significantly reduced due to packaging of the NA-chIL-2. This is important, because inactivated influenza vaccines should be capable of inducing antibody responses to both major viral glycoproteins, the HA and NA. Figure 5B demonstrates how NA-chIL-2 incorporation can be quantitated based on nanogram of chIL-2/μg of total viral protein. Here, using Western blot analysis and recombinant thioredoxin-conjugated chIL-2, a standard curve was established revealing that incorporation of NA-chIL-2 was on the order of 2 ng/μg of total viral protein.
FIG. 5.
Western blot analysis of highly purified influenza virus confirms incorporation of membrane-bound NA-chIL-2 into virions. (A) Gradient-purified influenza A/Udorn/72 propagated in MDCK/NA-chIL-2 (AIVAC-chIL-2) or MDCK/Vec (AIVAC) cells was subjected to SDS-12% PAGE and subsequent transfer to PDVF membrane. Blots were incubated with monoclonal anti-chIL-2 (clone G) and goat antineuraminidase antisera (anti-N2), followed by incubation with HRP-conjugated secondary antibodies. Protein bands were visualized by chemiluminescence. ChIL-2 was detectable in as little as 3.4 μg of total viral protein. (B) To quantitate the levels of virion incorporated NA-chIL-2, a standard curve was derived from a Western blot of purified recombinant Thioredoxin-chIL-2 and mean density of NA-chIL-2 in virions was extrapolated to yield 2 ng chIL-2/μg of total viral protein.
These initial studies were performed with influenza virus A/Udorn/72 virus which is a filamentous strain of the virus (Roberts 1998) and as such is not ideal for vaccine production. To demonstrate the universality of this approach and confirm that membrane-bound NA-chIL-2 incorporation is not restricted to a given subtype of influenza virus, we propagated influenza A/WSN/33, an H1N1 subtype, in MDCK/NA-chIL-2 cells and verified incorporation by bioassay. As demonstrated in Figure 6A, influenza A/WSN/33 virus harvested from infected MDCK/NA-chIL-2 cells also incorporated membrane-bound chIL-2 and induced chicken T-blast proliferation. To further confirm that NA-chIL-2 on the virus surface was responsible for blast cell proliferation, the proliferation assays were routinely performed in the presence of neutralizing anti-chIL-2 antibodies. As shown in Figure 6B, rabbit anti-chIL-2 dramatically inhibited proliferation induced by chIL-2 on the viral surface.
FIG. 6.
NA-chIL-2 is incorporated into H1N1 influenza virions. (A) WSN virus harvested from NA-chIL-bearing MDCK or control MDCK was purified by sedimentation through a 14% Optiprep cushion and subjected to heat treatments at 56°C. Infectivity assays on MDCK cells determined that both heat treatments destroyed viral infectivity. Inactivated virus (300 HAU) was added to cultures of T-cell blasts and uptake of 3H-thymidine was measured. Samples were run in quadruplicate and soluble recombinant IL-2 at 2 × 10−7 mM served as a positive control. (B) Rabbit antichIL-2 was added to WSN virus preparations an hour prior to addition to T blast cultures and completely neutralized virus-induced 3H-thymidine uptake.
Determination of the effects of TPCK-trypsin on IL-2 bioactivity
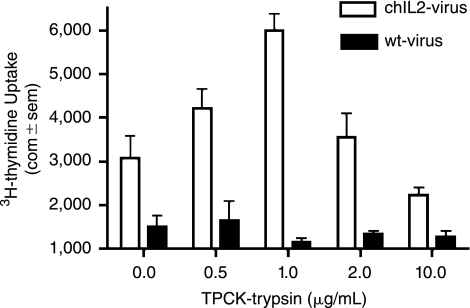
Because exogeneous trypsin is required for cell culture propagation of most human and low pathogenic avian strains of influenza, its effects on membrane-bound IL-2 were also determined. Accordingly, influenza A/Udorn/72 virus was propagated in MDCK/NA-chIL-2 cells in the presence or absence of exogeneous TPCK-trypsin (TPCK inhibits residual chymotrypsin activity). Cultures were maintained until complete cytopathic effect was observed throughout the monolayer. Harvested virus was heat inactivated, purified, standardized by HAU and viral protein content and tested by bioassay. It should be noted that all cultures grown in the presence of TPCK-trypsin yielded similar viral titers, whereas virus grown in the absence of TPCK-trypsin had very low titers. As expected, virus grown with low amounts of TPCK-trypsin required longer propagation times. Highest chIL-2 bioactivity was observed at a TPCK-trypsin concentration of 1 μg/mL (Fig. 7). Higher concentrations reduced, but did not eliminate, bioactivity, and the lowest concentration, 0.5 μg/mL, paradoxically also reduced IL-2 bioactivity. Thus virus can be propagated in MDCK/NA-chIL-2 cells in the presence of TPCK-trypsin without inactivating virion-bound IL-2.
FIG. 7.
Influenza virus can be propagated in vitro in the presence of trypsin without inactivating virion-bound IL-2. Influenza virus (A/Udorn/72) was harvested from NA-chIL-2-bearing MDCK or control MDCK cells in the presence of variable amounts of TPCK-trypsin. Virus was purified by gradient sedimentation, inactivated at 56°C for 20 min, and adjusted to 200 HAU/well. Samples were tested in quadruplicate for chIL-2 bioactivity.
Chicken GM-CSF can be readily incorporated into influenza virus as a membrane-bound HA fusion protein
To test the versatility of this technology, we made an additional cytokine fusion construct whereby the cytokine coding region of GM-CSF was fused in frame to the C-terminus of the HA gene, encoding for a short stalk and the transmembrane and cytoplasmic tail domains of the viral HA. Thus, in contrast to NA fusion constructs, which result in type II membrane fusion proteins, HA fusion constructs result in type I membrane fusion proteins. Thus, either the free N-terminal or C-terminal domains of cytokines can be exposed extracellularly using this approach. We further reasoned that this would allow for adaptation of cytokines in the event of steric hindrance or other structural incompatibilities when the cytokine is only expressed in one particular form.
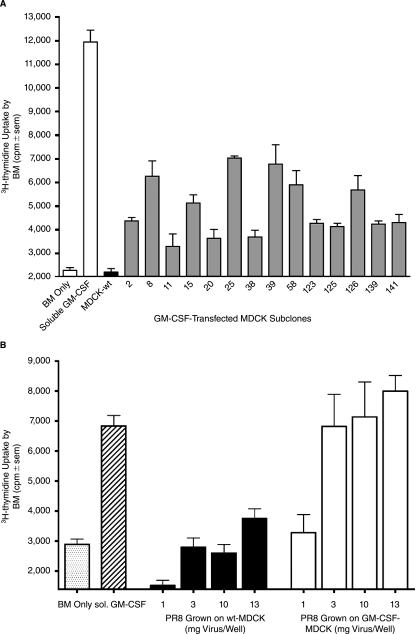
For these studies the full-length chGM-CSF (see Materials and Methods section) was first inserted into the expression plasmid pcDNA3.1 in order to facilitate expression of the soluble form of chGM-CSF upon transfection of COS7 cells. Supernatants from transfected COS7 cells served as a positive control in the GM-CSF-based bioassay evaluating the proliferation of chicken bone marrow cells as indicators. The supernatants from pcDNA3.1/chGM-CSF transfected COS7 cells were stimulatory over a wide dose range, while control supernatants from vector control-transfected COS7 cells displayed no proliferative activity (data not shown). Having established a functional bioassay for chGM-CSF, we next tethered the GM-CSF to the HA C-terminus and inserted the resultant fusion construct into plasmid pcD-NA3.1. The plasmid, pcDNA3.1/chGM-CSF-HA encodes the full-length chGM-CSF fused in frame to the membrane proximal C-terminal portion of the HA, which includes the transmembrane and cytoplasmic tail portions of the protein and 26 amino acids of the extracellular stalk (amino acids 497–565 of the HA). Following transfection of MDCK cells, stable subclones were selected based on resistance to G418 and the presence of GM-CSF bioactivity. As observed in Figure 8A, a number of GM-CSF-transfected MDCK sub-clones tested positive for proliferative activity of chicken bone marrow cells.
FIG. 8.
Membrane-bound chicken-derived GM-CSF fused to the viral HA is readily expressed in a bioactive form and is packaged into influenza virions. (A) Chicken-derived GM-CSF was fused inframe to the carboxy-terminal domain encoding for a short stalk, the transmembrane and cytoplasmic tail regions of the viral HA and inserted into pcDNA3.1 expression plasmid (pcDNA3.1-chGM-CSF/HA). Transfected MDCK cells were selected with Geneticin and cloned by limiting dilution. Each subclone was tested for GM-CSF bioactivity by coculturing mitomycin C-pretreated cells with chicken bone-marrow cells for 3 days at 40°C, the last 20 h with 3H-thymidine and measuring thymidine incorporation (triplicates). Those subclones inducing the greatest uptake of thymidine are compared with nontransfected MDCK (MDCK-wt) and soluble chicken GM-CSF (1/100 dil of supernatant from transfected COS-7 cells). (B) Influenza virus A/PR/8 (H1N1) was harvested from infected (moi = 1) chGM-CSF/HA expressing MDCK (subclone 39) or wild-type MDCK, concentrated and inactivated with β-propiolactone. Dilutions of the virus were evaluated for GM-CSF bioactivity in a chicken bone marrow proliferation assay. Soluble chicken GM-CSF served as a positive control. Samples were tested in triplicate for 3H-thymidine uptake.
MDCK subclone 39, which remained amongst the highest subclones in repeat testing for GM-CSF bioactivity, was used to propagate influenza virus. Influenza virus A/PR/8/34 was propagated in either subclone 39 or wild-type MDCK cells, gradient purified and inactivated with β-propiolactone. Influenza virus bearing membrane-bound chGM-CSF/HA induced proliferation of chicken bone marrow cells in a dose dependent fashion (3–13 μg of total viral protein), while wild-type PR8 virus was only marginally stimulatory at the highest concentration and exhibited no proliferative activity at lower concentrations (Fig. 8B). In summary, chicken-derived GM-CSF as a membrane-bound HA fusion construct, was incorporated into virus during viral budding and retained bioactivity after viral inactivation.
Vaccination of chicks with influenza vaccine bearing chIL-2
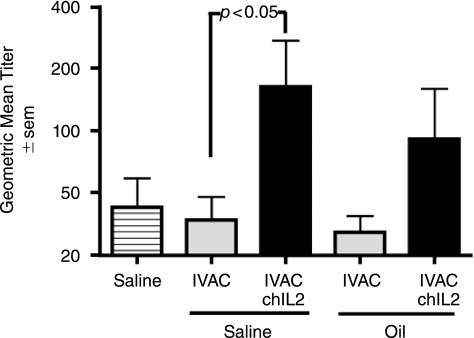
To determine whether IL-2 on the influenza virus surface has immunostimulatory properties, we compared elicited immune responses in 1-week-old white Leghorn male chicks that were immunized subcutaneously with UV-inactivated, conventional avian influenza vaccine (AIVAC) or chIL-2 bearing avian vaccine (AIVAC-chIL-2) in either PBS or in an oil emulsion. Conventional vaccine was defined as wild-type, whole virus–inactivated vaccine without membrane-bound cytokine. Chickens were boosted 2 weeks later and final bleeds were obtained at day 14 post-boost. Antibodies against influenza virus in serum were analyzed by ELISA and HI. As shown in Figure 9, chicks inoculated with AIVAC-chIL-2 in saline had significantly (p < 0.05) higher antiviral antibody titers than chicks injected with conventional IVAC. AIVAC-chIL-2 administered in oil induced a higher mean titer than conventional AIVAC in oil, but the differences were not statistically significant (p > 0.05). A comparison of the same sera by HI assay indicated that seven of seven chicks injected with AIVAC-chIL-2 in saline had titers of 20 or more, but only three of seven chicks injected with conventional AIVAC in saline had titers of 20 (χ2 value of p = 0.018). In summary, this is the first report describing the immunostimulatory potential of membrane-bound chicken IL-2 when presented directly on a virus particle.
FIG. 9.
Virion-bound chIL-2 augments antibody responses in chickens. Chicks were injected twice with UV-inactivated filamentous influenza, A/Udorn, bearing chicken IL-2 (IVAC-chIL-2) or with wild-type influenza vaccine (IVAC); 10 μg viral protein per dose for chicks receiving an aqueous vaccine and 3 μg per dose for chicks receiving an oil emulsion vaccine. Sera were tested for virus-specific antibody 2 weeks after the boost by ELISA (7–9 chicks per group). Chicks injected with IVAC-chIL-2 (in a saline formulation) had significantly higher geometric mean titers than chicks injected with wild-type IVAC (ANOVA and Bonferoni's multiple comparison test; p < 0.05). Chicks receiving IVAC-chIL-2 in oil also had elevated antibody responses, but the difference was not statistically significant (p > 0.05).
Discussion
Our strategy of directly incorporating membrane-bound cytokines into influenza virus particles by establishment of stably transfected producer cell lines presents a novel system to incorporate numerous cytokines, chemokines, costimulatory molecules, and Toll-like receptor ligands directly into viral vaccines. In contrast to the coadministration of soluble adjuvants, this technology maintains the adjuvant in close proximity to the viral antigen and very little, if any, becomes systemic. Thus, the adjuvant effects are highly localized helping to augment local immune cascades. Another advantage is its cost effectiveness, since cell lines, once transfected, continue to produce the combined adjuvanted virus with no additional processing. Further, this technique is ideal for cytokines that are difficult to produce in Escherichia coli; e.g., cytokines that have multiple intrachain disulfide bonds and/or are stabilized by glycosylation. By choosing between the two transmembrane proteins of influenza, HA and NA, type I and type II membrane glycoproteins, respectively, one may tether a cytokine at either its C- or N-terminus. Further, this technology is applicable to any enveloped virus or virus-like particle that buds from cellular membranes. Other potential viruses include Herpes, HIV, Rabies as well as the chicken pathogens Newcastle Disease virus and Marek's disease virus.
This report provides the “proof of concept” of our cytokine-bearing influenza virus vaccine approach (CYT-IVAC), yet further studies are required to develop this technology. First, challenge experiments are needed to verify the efficacy of the whole virus vaccines bearing membrane-bound immunomodulators. Second, incorporation of other cytokines and immune modulators should be evaluated to determine the scope and limitations of this technology. The cytokines that appear most promising are IL-2, IL-4, GM-CSF, and IL-12, because they have been used as membrane-bound adjuvants on tumor cell vaccines (Soo Hoo and others 1999; Kim and others 2000; Faulkner and others 2001; Yei and others 2002; Nizard and others 2003; Chakrabarti and others 2004). Third, the effects of various cytokine adjuvants on T-cell responses need to be evaluated, since cellular immunity plays a role in viral clearance during influenza and other viral infections. In this regard, IL-15 should also be considered, as it is known to increase the numbers of memory CD8 T cells and it activates NK cells (Rubinstein and others 2002; Villinger and others 2004; Kutzler and others 2005).
The studies described here validated several key steps in the use of membrane-bound cytokines on whole virus vaccines. First, cell surface expression and retention of bioactivity was demonstrated for two diverse cytokines, chicken IL-2 and GM-CSF. In addition, use of both type I and II transmembrane components to tether the cytokines was demonstrated. Importantly, we were able to demonstrate that lengthening of the stalk domain of the HA protein by at least 26 amino acids does not have adverse effects on cell surface expression, cytokine bioactivity, or its incorporation into virus. This is important in considering the design of other immunostimulatory fusion constructs, because accessibility may be critical for preservation of bioactivity. Extension of the stalk domain may prevent the membrane-bound cytokines from being masked by neighboring HA and NA molecules. Current studies are being conducted to evaluate whether flexibility in the NA stalk region is advantageous for preservation of cytokine bioactivity in virus particles.
A second milestone validated in our studies was the incorporation of membrane-bound cytokines directly into budding virus particles. Incorporation was confirmed by Western blot analysis, indirect immunofluorescence and specific bioassay on purified virus particles. In the case of chIL-2, neutralizing antibodies inhibited bioactivity on virus particles. We also validated and confirmed that cytokine bioactivity was preserved by three different viral inactivation protocols: UV, β-propiolactone and heat. Thus, diverse inactivation conditions can be used depending upon the sensitivity of individual immunostimulatory molecules. In addition, incorporation was not strain or morphology specific, because infection with both a filamentous strain, A/Udorn/72 (H3N2) and 3 spherical strains A/WSN/34 (H1N1), A/PR/8 (H1N1) and A/chick/California (H6N2) (data not shown) of influenza led to incorporation of membrane-bound cytokine. This is likely due to the fact that the cytoplasmic tail and transmembrane domains of the HA and NA are responsible for their accumulation in lipid rafts in producer cells (Zhang and others 2000). Importantly, the cytoplasmic tail regions of NA and HA are highly conserved amongst all influenza type A viruses, so the choice of viral strain providing the HA or NA fusion construct is not critical.
It should be noted that this technology is designed for enhancing the immunogenicity of killed influenza virus. Modifications of the technology would be needed for application to live viral vaccines, since the cytokine would be lost after the first replicative cycle in vivo. Nevertheless, to produce the killed vaccine it is important to consider the possible effects of these cytokine-fusion constructs on viral replication in vitro. They might reduce the amount of wild-type NA or HA expressed on virus, although Western blots of wild-type NA on virus expressing NA-IL-2 (Fig. 5) failed to detect any loss of wild-type NA. Alternatively, the addition of cytokine to virus might enable viral replication in nonpermissive cell lines expressing the cognate receptor. This would be a novel approach to the development of cell lines for the production of hard to grow virus and merits further study.
Finally, in vivo vaccination studies in chickens using inactivated, influenza vaccine-bearing chicken IL-2, demonstrated a significant augmentation of humoral anti-viral antibody responses. Virus bearing chIL-2 injected in saline induced elevated levels of anti-viral antibody as confirmed by ELISA and HI assay. It should be noted that we intentionally used a low dose of virus antigen to discern adjuvant activity of the membrane-bound cytokine; the vaccine dose was further reduced 3-fold in the oil formulation. Low dose vaccine formulations have particular relevance pertaining to H5N1 vaccines for humans since high doses with a boost are required to generate sufficient HI titers (Luke and Subbarao 2006). Our CYT-IVAC technology may enable the use of lower and/or single vaccine doses to achieve protection. Together these studies warrant further evaluation of our CYT-IVAC technology.
The technology has application for protection of swine, poultry, and humans against influenza viruses. Killed poultry influenza vaccines are currently in use in China, Mexico, Vietnam, Egypt, Indonesia, and elsewhere. However, current vaccines are in need of improvement, since they fail to eliminate viral shedding and they provide limited protection against heterotypic strains. There is also concern that current vaccines promote the generation of escape variants (Smith and others 2006). Chicken IL-2 appears to be beneficial, but other chicken cytokines are also currently being evaluated in our laboratory: GM-CSF, IL-4, IL-15 and IL-12.
Novel and improved vaccines are urgently needed to protect humans against pandemic influenza strains. Because rapid scale-up of production is a necessity, utilization of cell culture based systems is rapidly gaining acceptance. As cell culture based vaccine production improves, cell lines could be transformed with a variety of human cytokines and other costimulatory factors, like C3d and CD40L and stored frozen until needed. At such time the cell line could be rapidly expanded and used for viral production.
Since current vaccination only induces protective immunity in ∼50% of the elderly, tailored influenza vaccine employing different immunomodulators may prove more beneficial and stimulate waning immunity in that population (Ben-Yehuda and others 2003a). IL-2, IL-4, GM-CSF, and IL-12 are cytokines that have been effective as membrane-bound constructs in tumor vaccines (Soo Hoo and others 1999; Kim and others 2000; Faulkner and others 2001; Yei and others 2002; Nizard and others 2003; Chakrabarti and others 2004) and therefore warrant initial consideration in the design of viral vaccines. Of course, a multitude of other cytokines and chemokines might also be considered. Licensing of cytokine-bearing influenza particles could be achieved using current human influenza vaccine strains, including H5N1.
At the time our experiments were concluding, Kueng and others (2007) described an alternative approach to target cytokines to lipid rafts of virus producer cell lines. They fused the carboxyl-terminal ends of IL-2, IL-4, and GM-CSF to the glycosylphosphatidylinositol (GPI) acceptor sequence of the human Fc-γ receptor III (CD16b), and transfected these genes into producer cells. Virus-like particles were induced by transfection with Moloney virus Gag-Pol resulting in incorporation of cytokines into viral-like particles. The cytokines retained bioactivity comparable on a molar basis to soluble cytokines. In principal, the Kueng technique and ours should yield similar results, because both target cytokines to lipid rafts. However, there are differences in methodology (e.g., intact influenza virus versus gag viral-like particles) and comparative vaccine studies are needed to determine which technique is superior for specific virus-cytokine combinations. For example, the NA tail domain allows fusion to the amino terminus of cytokines, which might enhance the bioactivity of some cytokines or immunostimulators. This type of linkage may not be conformationally stable or active using a GPI acceptor sequence. Also, influenza and other enveloped viruses might have a preference for incorporating self-protein segments rather than GPI acceptor sequences. Nevertheless, both techniques have the potential to efficiently incorporate immunostimulators onto enveloped viruses and thereby enhance the efficacy of killed or noninfectious vaccines.
Acknowledgments
This study was supported in part by Public Health Service grants (AI47783, AI065591) from the National Institute of Allergy and Infectious Diseases and in part through a grant from the Childrens Research Hospital of Michigan (to P.C.R.).
References
- Avery S. Rothwell L. Degen WD. Schijns VE. Young J. Kaufman J. Kaiser P. Characterization of the first nonmammalian T2 cytokine gene cluster: the cluster contains functional single-copy genes for IL-3, IL-4, IL-13, and GM-CSF, a gene for IL-5 that appears to be a pseudogene, and a gene encoding another cytokinelike transcript, KK34. J Interferon Cytokine Res. 2004;24(10):600–610. doi: 10.1089/jir.2004.24.600. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda A. Joseph A. Barenholz Y. Zeira E. Even-Chen S. Louria-Hayon I. Babai I. Zakay-Rones Z. Greenbaum E. Galprin I. Immunogenicity and safety of a novel IL-2-supplemented liposomal influenza vaccine (INFLUSOME-VAC) in nursing-home residents. Vaccine. 2003a;21(23):3169–3178. doi: 10.1016/s0264-410x(03)00251-2. [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda A. Joseph A. Zeira E. Even-Chen S. Louria-Hayon I. Babai I. Zakay-Rones Z. Greenbaum E. Barenholz Y. Kedar E. Immunogenicity and safety of a novel liposomal influenza subunit vaccine (INFLUSOME-VAC) in young adults. J Med Virol. 2003b;69(4):560–567. doi: 10.1002/jmv.10345. [DOI] [PubMed] [Google Scholar]
- Bukreyev A. Belyakov IM. Expression of immunomodulating molecules by recombinant viruses: can the immunogenicity of live virus vaccines be improved? Expert Rev Vaccines. 2002;1(2):233–245. doi: 10.1586/14760584.1.2.233. [DOI] [PubMed] [Google Scholar]
- Bukreyev A. Skiadopoulos MH. McAuliffe J. Murphy BR. Collins PL. Schmidt AC. More antibody with less antigen: can immunogenicity of attenuated live virus vaccines be improved? Proc Natl Acad Sci USA. 2002;99(26):16987–16991. doi: 10.1073/pnas.252649299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R. Chang Y. Song K. Prud'homme GJ. Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA) Vaccine. 2004;22(9–10):1199–1205. doi: 10.1016/j.vaccine.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Chang DZ. Lomazow W. Joy Somberg C. Stan R. Perales MA. Granulocyte-macrophage colony stimulating factor: an adjuvant for cancer vaccines. Hematology. 2004;9(3):207–215. doi: 10.1080/10245330410001701549. [DOI] [PubMed] [Google Scholar]
- Dillon PJ. Rosen CA. A rapid method for the construction of synthetic genes using the polymerase chain reaction. Biotechniques. 1990;9(3):298–300. [PubMed] [Google Scholar]
- Djeraba A. Musset E. Lowenthal JW. Boyle DB. Chausse AM. Peloille M. Quere P. Protective effect of avian myelomonocytic growth factor in infection with Marek's disease virus. J Virol. 2002;76(3):1062–1070. doi: 10.1128/JVI.76.3.1062-1070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner L. Buchan G. Lockhart E. Slobbe L. Wilson M. Baird M. IL-2 linked to a peptide from influenza hemagglutinin enhances T cell activation by affecting the antigen-presentation function of bone marrow-derived dendritic cells. Int Immunol. 2001;13(6):713–721. doi: 10.1093/intimm/13.6.713. [DOI] [PubMed] [Google Scholar]
- Giansanti F. Giardi MF. Botti D. Avian cytokines—an overview. Curr Pharm Des. 2006;12(24):3083–3099. doi: 10.2174/138161206777947542. [DOI] [PubMed] [Google Scholar]
- Hu W, editor; Kolodsick JE, editor; Stepaniak JA, editor; Sundick RS, editor. Enhanced immune reponses to Marek's disease virus glycoprotein B by co-injection with recombinant chicken IL-2. Kennett Square: American Association Avian Pathologists; 2001. pp. 269–274. [Google Scholar]
- Hulse DJ. Romero CH. Partial protection against infectious bursal disease virus through DNA-mediated vaccination with the VP2 capsid protein and chicken IL-2 genes. Vaccine. 2004;22(9–10):1249–1259. doi: 10.1016/j.vaccine.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Kaiser P. Poh TY. Rothwell L. Avery S. Balu S. Pathania US. Hughes S. Goodchild M. Morrell S. Watson M. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res. 2005;25(8):467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- Kim YS. Sonn CH. Paik SG. Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000;7(10):837–843. doi: 10.1038/sj.gt.3301175. [DOI] [PubMed] [Google Scholar]
- Kittel C. Ferko B. Kurz M. Voglauer R. Sereinig S. Romanova J. Stiegler G. Katinger H. Egorov A. Generation of an influenza A virus vector expressing biologically active human interleukin-2 from the NS gene segment. J Virol. 2005;79(16):10672–10677. doi: 10.1128/JVI.79.16.10672-10677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodsick JE. Stepaniak JA. Hu W. Sundick RS. Mutational analysis of chicken interleukin 2. Cytokine. 2001;13(6):317–324. doi: 10.1006/cyto.2001.0846. [DOI] [PubMed] [Google Scholar]
- Kueng HJ. Leb VM. Haiderer D. Raposo G. Thery C. Derdak SV. Schmetterer KG. Neunkirchner A. Sillaber C. Seed B. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J Virol. 2007;81(16):8666–8676. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA. Robinson TM. Chattergoon MA. Choo DK. Choo AY. Choe PY. Ramanathan MP. Parkinson R. Kudchodkar S. Tamura Y. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175(1):112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- Lasek W. Basak G. Switaj T. Jakubowska AB. Wysocki PJ. Mackiewicz A. Drela N. Jalili A. Kaminski R. Kozar K. Complete tumour regressions induced by vaccination with IL-12 gene-transduced tumour cells in combination with IL-15 in a melanoma model in mice. Cancer Immunol Immunother. 2004;53(4):363–372. doi: 10.1007/s00262-003-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Liang X. Huang Y. Meng S. Xie R. Deng R. Yu L. Enhancement of the immunogenicity of DNA vaccine against infectious bursal disease virus by co-delivery with plasmid encoding chicken interleukin 2. Virology. 2004;329(1):89–100. doi: 10.1016/j.virol.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Lowenthal JW. York JJ. O'Neil TE. Steven RA. Strom DG. Digby MR. Potential use of cytokine therapy in poultry. Vet Immunol Immunopathol. 1998;63(1–2):191–198. doi: 10.1016/s0165-2427(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Luke CJ. Subbarao K. Vaccines for pandemic influenza. Emerg Infect Dis. 2006;12(1):66–72. doi: 10.3201/eid1201.051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S. Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res. 2002;62(10):2869–2874. [PubMed] [Google Scholar]
- Nizard P. Gross DA. Babon A. Chenal A. Beaumelle B. Kosmatopoulos K. Gillet D. Anchoring cytokines to tumor cells for the preparation of anticancer vaccines without gene transfection in mice. J Immunother. 2003;26(1):63–71. doi: 10.1097/00002371-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Okada H. Villa L. Attanucci J. Erff M. Fellows WK. Lotze MT. Pollack IF. Chambers WH. Cytokine gene therapy of gliomas: effective induction of therapeutic immunity to intracranial tumors by peripheral immunization with interleukin-4 transduced glioma cells. Gene Ther. 2001;8(15):1157–1166. doi: 10.1038/sj.gt.3301496. [DOI] [PubMed] [Google Scholar]
- Orson FM. Kinsey BM. Densmore CL. Nguyen T. Wu Y. Mbawuike IN. Wyde PR. Protection against influenza infection by cytokine-enhanced aerosol genetic immunization. J Gene Med. 2006;8(4):488–497. doi: 10.1002/jgm.864. [DOI] [PubMed] [Google Scholar]
- Poloso NJ. Nagarajan S. Mejia-Oneta JM. Selvaraj P. GPI-anchoring of GM-CSF results in active membrane-bound and partially shed cytokine. Mol Immunol. 2002;38(11):803–816. doi: 10.1016/s0161-5890(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Roberts PC. Lamb Robert A. Compans Richard W. The M1 and M2 proteins of influenza a virus are important determinants in filamentous particle formation. Virology. 1998;240:127–137. doi: 10.1006/viro.1997.8916. [DOI] [PubMed] [Google Scholar]
- Rubinstein MP. Kadima AN. Salem ML. Nguyen CL. Gillanders WE. Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169(9):4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- Schijns VE. Weining KC. Nuijten P. Rijke EO. Staeheli P. Immunoadjuvant activities of E. coli- and plasmid-expressed recombinant chicken IFN-alpha/beta, IFN-gamma and IL-1beta in 1-day- and 3-week-old chickens. Vaccine. 2000;18(20):2147–2154. doi: 10.1016/s0264-410x(99)00537-x. [DOI] [PubMed] [Google Scholar]
- Smith NM. Bresee JS. Shay DK. Uyeki TM. Cox NJ. Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-10):1–42. [PubMed] [Google Scholar]
- Soo Hoo W. Lundeen KA. Kohrumel JR. Pham NL. Brostoff SW. Bartholomew RM. Carlo DJ. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J Immunol. 1999;162(12):7343–7349. [PubMed] [Google Scholar]
- Stepaniak JA. Shuster JE. Hu W. Sundick RS. Production and in vitro characterization of recombinant chicken interleukin-2. J Interferon Cytokine Res. 1999;19(5):515–526. doi: 10.1089/107999099313965. [DOI] [PubMed] [Google Scholar]
- Takehara K. Kobayashi K. Ruttanapumma R. Kamikawa M. Nagata T. Yokomizo Y. Nakamura M. Adjuvant effect of chicken interferon-gamma for inactivated Salmonella Enteritidis antigen. J Vet Med Sci. 2003;65(12):1337–1341. doi: 10.1292/jvms.65.1337. [DOI] [PubMed] [Google Scholar]
- Tarpey I. Davis PJ. Sondermeijer P. van Geffen C. Verstegen I. Schijns VE. Kolodsick J. Sundick R. Expression of chicken interleukin-2 by turkey herpesvirus increases the immune reponse against Marek's disease virus but fails to increase protection against virulent challenge. Avian Pathol. 2007a;36(1):69–74. doi: 10.1080/03079450601113159. [DOI] [PubMed] [Google Scholar]
- Tarpey I. van Loon AA. de Haas N. Davis PJ. Orbell S. Cavanagh D. Britton P. Casais R. Sondermeijer P. Sundick R. A recombinant turkey herpesvirus expressing chicken interleukin-2 increases the protection provided by in-ovo vaccination with infectious bursal disease and infectious bronchitis virus. Vaccine. 2007b;25:8529–8535. doi: 10.1016/j.vaccine.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Villinger F. Miller R. Mori K. Mayne AE. Bostik P. Sundstrom JB. Sugimoto C. Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22(25–26):3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Wang J. Mauser A. Chao SF. Remington K. Treckmann R. Kaiser K. Pifat D. Hotta J. Virus inactivation and protein recovery in a novel ultraviolet-C reactor. Vox Sang. 2004;86(4):230–238. doi: 10.1111/j.0042-9007.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- Webster RG. Cox N. Stohr K. WHO Manual on animal influenza diagnosis and surveillance. 2002a.
- Yei S. Bartholomew RM. Pezzoli P. Gutierrez A. Gouveia E. Bassett D. Soo Hoo W. Carlo DJ. Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine. Gene Ther. 2002;9(19):1302–1311. doi: 10.1038/sj.gt.3301803. [DOI] [PubMed] [Google Scholar]
- Zhang J. Pekosz A. Lamb RA. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J Virol. 2000;74(10):4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JY. Chen JG. Wang JY. Wu JX. Gong H. cDNA cloning and functional analysis of goose interleukin-2. Cytokine. 2005;30(6):328–338. doi: 10.1016/j.cyto.2004.12.015. [DOI] [PubMed] [Google Scholar]