Abstract
Interferon-alpha (IFN-α) is employed in the treatment of malignant melanoma; however, it mediates regression of disease in only 10–15% of patients. Currently, its mechanism of action is uncharacterized. Low-dose IFN-α exerts anti-angiogenic effects when used in the treatment of life-threatening hemangiomas of infancy, suggesting anti-angiogenesis as a mechanism of action. IFN-α may exert its anti-tumor effect in the setting of advanced malignancy by inhibiting the secretion of vascular endothelial growth factor (VEGF), a pro-angiogenic substance. We hypothesized that IFN-α would decrease the release of VEGF by melanoma tumors. We studied the effect of IFN-α on VEGF production in nine human melanoma cell lines. We also examined VEGF levels in 49 patients with advanced malignancies who received low-dose IFN-α and interleukin-12 (IL-12) on an NCI-sponsored phase I trial. Human melanoma cell lines produced varying amounts of VEGF in vitro (60–1500 pg/mL at 48 h). Certain melanoma cell lines such as 18105 MEL secreted low levels of VEGF (152 pg/mL) after 48 h of culture, whereas other lines secreted very high levels (FO-1 3,802 pg/mL). Treatment of melanoma cells with IFN-α (2000 U/mL) decreased VEGF secretion by 40–60% in VEGF-high cell lines; however, this effect was not demonstrated in VEGF-low cell lines. In cancer patients, pretreatment VEGF plasma levels varied from 471 to 4200 pg/mL. A decrease in VEGF plasma levels after treatment directly correlated with the number of treatment cycles administered (Pearson correlation, p = 0.04). In summary, IFN-α inhibits VEGF secretion by melanoma cell lines in vitro and may have similar actions in malignancies that respond to IFN-α treatment.
Introduction
Solid tumors must interface and integrate with the circulatory system to grow to a significant size in their host. Tumor cells can stimulate new vessel formation in a paracrine manner via the release of soluble factors with pleiotropic effects. These factors stimulate endothelial cells to proliferate and migrate, thereby resulting in the development of new blood vessels. Multiple groups have suggested a role for vascular endothelial growth factor (VEGF) in the stimulation of tumor angiogenesis (Choueiri and others 2006). Although angiogenesis is a complex process, VEGF is among the most potent angiogenic substances secreted by tumors (Graeven and others 2001). VEGF production has been measured in many tumors; however, the amount produced by different tumor types is highly variable. Of note, tumors that produce high levels of VEGF have been associated with an increased propensity for metastasis (Claffey and Robinson 1996; Gorski and others 2003; Srivastava and others 2003). In addition, inhibition of VEGF-induced angiogenesis has proven to be an effective strategy for the inhibition of tumor growth in vivo (Kabbinavar and others 2003; Johnson and others 2004; Choueiri and others 2006).
IFN-α is a cytokine that demonstrates unique anti-tumor effects and has been used for the treatment of a variety of malignancies including malignant melanoma. The exact mechanism of action remains to be elucidated; however, there exists evidence that interferon-alpha (IFN-α) exerts both a direct anti-tumor effect as well as indirect immunos-timulatory actions (Pyrhonen and others 1992; Tsavaris and others 1996; Fidler 2000; Belardelli and others 2002; Lesinksi and others 2003). The anti-angiogenic effects of IFN-α are less well-characterized. Of note, IFN-α is currently the treatment of choice for life-threatening hemangiomas of infancy due to its ability to inhibit the release of β-FGF, an important pro-angiogenic factor (Folkman 1995).
IFN-α has been administered as a single agent at low doses to patients with advanced cancer or in combination with other immunomodulatory cytokines with anti-angiogenic properties, such as IL-12. However, the effect of these regimens on circulating levels of VEGF remains to be elucidated. In the present study, we have evaluated the effect of IFN-α on the secretion of VEGF by melanoma cells in vitro and in a clinical trial of IL-12 and low-dose IFN-α.
Methods
Cell lines and reagents
Recombinant human IFN-α-2b (specific activity 2 × 108 IU/mg) was obtained from Schering Plough, Inc. (Kenilworth, NJ, USA) and resuspended in phosphate-buffered saline (PBS) supplemented with 0.1% human albumin (Armor Pharmaceutical Co., Kankakee, IL, USA). IL-12 was obtained from Genetics Institute (Cambridge, MA, USA). Human melanoma cell lines, namely FO-1, MEL 39, HT 144, 1074 MEL, 1106 MEL, 1174 MEL, 1259 MEL, 18105 MEL, and SK-MEL-33, were a gift of S. Ferrone (Roswell Park Cancer Institute, Buffalo, NY, USA). Cell lines were cultured in Eagle's Minimal Essential Medium with 2 mM l-glutamine and 10% fetal bovine serum (FBS) supplements.
Assessment of melanoma cell proliferation
Human melanoma cell lines FO-1, MEL 39, and 1259 MEL were suspended in medium alone or medium supplemented with increasing concentrations of IFN-α (102–104 U/mL) and incubated for 72 h in 96-well flat-bottom plates. Proliferation was measured by the MTT Cell Proliferation Assay (American Type Culture Collection, Manassas, VA, USA) according to the manufacturer's specifications.
Assessment of VEGF secretion by melanoma cells in vitro
Melanoma cell lines were suspended in 300 μL of media with or without cytokine and incubated in 96-well flat-bottom plates. After 48 h, basal levels of VEGF in cell culture supernatants were measured using a commercially available ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's recommendations. Optical density of the developed ELISA plate was measured on a Perkin-Elmer bioassay reader using standard correction factors.
Flow cytometry for STAT1 activation and cell proliferation assays
Cell lines were cultured in appropriate media supplemented with PBS or escalating doses of IFN-α for 15 min and assayed for intracellular levels of Tyr(701)-phosphorylated STAT1 (pSTAT1), a downstream signaling intermediate by flow cytometry. Previous studies by our group have shown that lower concentrations of IFN-α (100–1000 U/mL) result in approximately half the induction of pSTAT1 in melanoma cell lines as compared to higher doses (e.g., 10,000 U/mL IFN-α) (Lesinski and others 2007). Cells were harvested from culture media, washed in flow buffer (PBS supplemented with 5% FBS), and fixed with Reagent A, Fix & Perm (Caltag Laboratories, Burlingame, CA, USA), and cold methanol. Cells were then washed and incubated in permeabilization medium (Reagent B) at room temperature for 30 min with 7.5 ng of rabbit anti-human pSTAT1 antibody (BD Transduction Laboratories, San Diego, CA, USA) or an isotype control antibody. The cells were washed again and incubated for 30 min with Alexaflour 488-goat anti-rabbit IgG at room temperature (Molecular Probes, Eugene, OR, USA). Finally, cells were washed, fixed in 1% formalin, and stored at 4°C until analysis. Analyses were done as described previously using a Becton Dickinson FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) equipped with a 488-nm air-cooled argon laser and a 633-nm helium-neon laser (Lesinski and others 2004).
Patients from NCI Phase I clinical trial
Between August 1998 and January 2000, 49 patients with advanced malignancies were enrolled in a NCI Phase I clinical trial of IL-12 and IFN-α (T98-0020) at The Ohio State University Comprehensive Cancer Center (Eisenbeis and others 2005). The primary objective of this trial was to determine the maximum tolerated dose of IFN-α after a single dose of IL-12. A secondary objective was to evaluate the serum levels of VEGF at baseline and during treatment.
Consenting patients meeting the eligibility criteria received IL-12 intravenously (iv) on day 1 followed by subcutaneous (sc) injections of IFN-α-2b (INTRON A®) on days 2, 3, 4, 5, and 6. The entire cycle was repeated every 14 days for a total of 6 months, or until patients exhibited disease progression. IL-12 was given at a dose of 100, 250, 300, or 500 ng/kg. For each dose of IL-12, the IFN-α-2b component was dose escalated within cohorts of three patients (1, 3, 5, 7, or 10 million units [MU]/day). Each patient received the same dose of IL-12 and IFN-α-2b throughout treatment (Eisenbeis and others 2005). Peripheral venous blood samples for VEGF analysis were obtained before and following cytokine administration. Clinical parameters for each patient were recorded, including demographics, cancer type, progression-free survival, overall survival, and the number of cycles received. VEGF levels from the plasma of seven healthy donors were measured as controls. Data from the clinical trial was evaluated using the statistics program SPSS. The Pearson correlation was employed with two-tailed significance. The variables that were evaluated included age, cancer type, IFN-α dosage, IL-12 dosage, IL-12 route of administration, number of treatment cycles, upward/downward trend in plasma VEGF levels, and reduction of plasma VEGF levels over the course of the treatment.
Results
Production of VEGF by melanoma cell lines in vitro
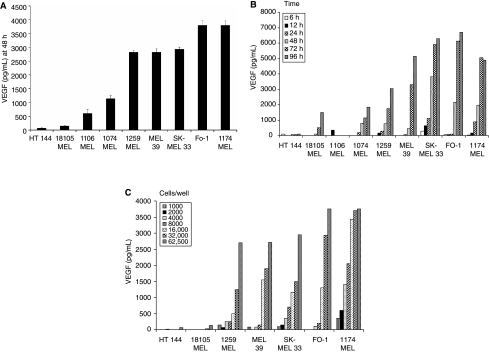
There was significant variability in the baseline secretion of VEGF by melanoma cell lines at the 48 h time point (Fig. 1A). As expected, secretion was time-dependent, with greater levels being observed at the 48–96 h time point as compared to earlier time points (Fig. 1B). Secretion was also dependent on the concentration of cells in culture. As the concentration of cells in culture increased from 1 × 103 cells to 6.25 × 104 cells per well, the secretion of VEGF also increased. For each cell line examined, the greatest secretion of VEGF occurred at a cell concentration of 8 × 104 cells per well (Fig. 1C).
FIG. 1.
Vascular endothelial growth factor (VEGF) secretion by melanoma cell lines. (A) Melanoma cells (HT 144, 18105 MEL, 1106 MEL, 1259 MEL, MEL 39, SK-MEL 33, FO-1, and 1174 MEL; 6.25 × 104 cells/well) were incubated in culture for 48 h and VEGF secretion was measured by ELISA. (B) The same melanoma cell lines were plated at a concentration of 5 × 105 cells/well and incubated for 6–96 h, and VEGF secretion was measured by ELISA. Standard error was calculated from triplicate wells and was <5% in all conditions. (C) Melanoma cells were plated at varying concentrations (1.0 × 103 to 6.25 × 104 cells/well) and incubated for 48 h. VEGF levels in the cell culture supernatants were quantitated by ELISA as in A and B. Standard error was calculated from triplicate wells and was <5%.
Inhibition of VEGF secretion by IFN-α
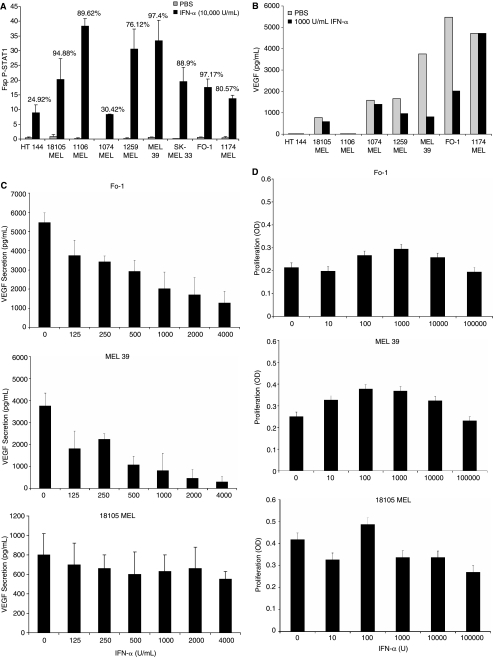
IFN-α activates the Janus-kinase-STAT signaling pathway in cells expressing the IFN-α receptor. To confirm that all melanoma cell lines were sensitive to IFN-α, we measured the activation of STAT1 (phosphorylation at Tyr 701) by flow cytometry. Phosphorylated STAT1 was activated in all tested cell lines following exposure to IFN-α (Fig. 2A). Treatment of melanoma cell lines with 1000 U/mL IFN-α resulted in varying decreases in VEGF secretion after 72 h (Fig. 2B). Published studies by Islam and others indicate that the dosage of IFN-α we employed (103 U/mL) for these experiments is physiologically relevant as the mean Cmax values of IFN-α in the blood of patients after a single high-dose IFN-α-2b infusion (20 MU/m2) were 1575 IU/mL and after four consecutive 5-day cycles of high-dose IFN-α-2b, the mean Cmax value was 2630 IU/mL (Islam and others 2002). Representative cell lines were treated with escalating doses of IFN-α (0–4000 U/mL), which resulted in cell line-specific changes in VEGF secretion (Fig. 2C). The secretion of VEGF by FO-1 and MEL 39 melanoma cells (cell lines that secreted high baseline levels of VEGF) decreased sharply in response to IFN-α. However, VEGF secretion by 18105 MEL cells, a cell line with lower baseline VEGF secretion, did not significantly change when treated with IFN-α. As can be seen in Figure 2D, these doses of IFN-α had minimal anti-proliferative effects on this panel of melanoma cell lines when plated at this high density. Therefore, the decrease in VEGF secretion in response to IFN-α cannot be attributed to a decrease in proliferation.
FIG. 2.
Activation of STAT1, vascular endothelial growth factor (VEGF) secretion, and cellular proliferation in melanoma cell lines after treatment with IFN-α. (A) Melanoma cell lines were stimulated with phosphate-buffered saline (PBS) or 104 U/mL IFN-α for 15 min and levels of p-STAT1 were measured via flow cytometry. Standard error was calculated from triplicate samples. The percentage of cells with a positive response is indicated above each bar. (B) Melanoma cell lines were incubated in culture for 72 h with PBS or 1000 U/mL IFN-α. VEGF secretion was measured by ELISA. Standard error was calculated from triplicate wells and was <5%. (C) FO-1, MEL 39, and melanoma cells were treated with increasing doses of IFN-α, and VEGF secretion at 72 h was assessed by ELISA. (D) FO-1, MEL 39, and melanoma cells were treated with increasing doses of IFN-α and the level of proliferation was assessed by the MTT assay.
VEGF secretion in response to treatment with IFN-α, IL-12, or the combination
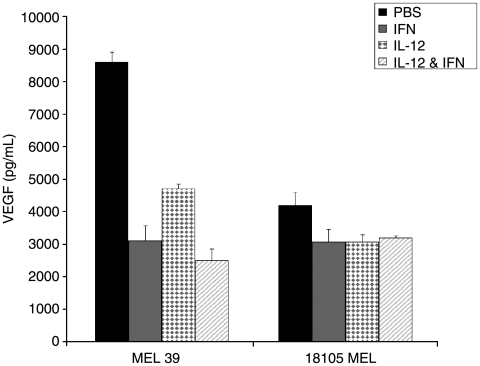
Since we used IFN-α and IL-12 in our clinical trial, we were interested in the effects of these cytokines on melanoma cell line secretion of VEGF. MEL 39, and 18105 MEL cells were treated with PBS, IFN-α (2000 U/mL), IL-12 (10 ng/mL), or the combination, and VEGF levels were measured in the cell culture supernatants (Fig. 3). 18105 MEL cells (low-VEGF cell line) did not display a significant decrease in VEGF secretion upon exposure to these cytokines. Interestingly, MEL 39 demonstrated significantly decreased VEGF secretion following treatment with either IFN-α or IL-12 alone.
FIG. 3.
VEGF secretion in response to in vitro treatment with IFN-α, IL-12, or the combination. MEL 39 and 18105 MEL cells (5 × 105 cells/condition) were treated with PBS, IFN-α (2000 U/mL), IL-12 (10 ng/mL), or IFN-α and IL-12 for 48 h, and VEGF secretion was assessed by ELISA.
VEGF levels in patients undergoing cytokine treatment for advanced malignancy
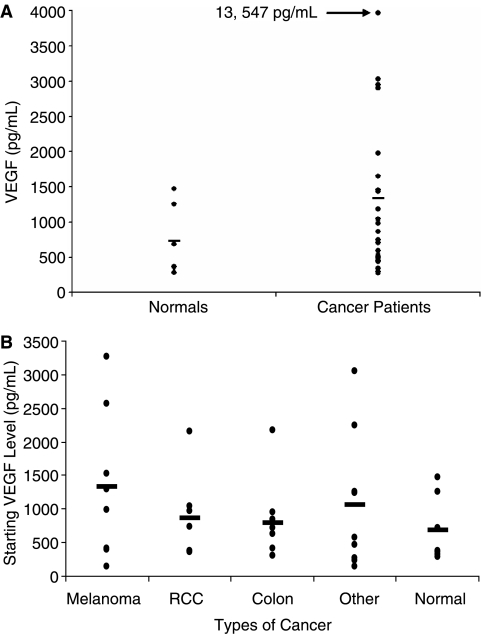
In order to assess the effect of cytokine treatment on VEGF, plasma VEGF levels were measured in patients at baseline and during treatment with IL-12 and IFN-α. The mean pretreatment VEGF level for the 49 cancer patients was 1377 pg/mL (SD ± 2308 pg/mL) (Fig. 4A). VEGF levels ranged from 138 pg/mLina23-year-oldpatientwithangiosarcomato13,547pg/mL in a 54-year-old patient with pancreatic cancer. Eight patients had advanced melanoma and their average pretreatment VEGF level was 1323 pg/mL (SD ± 1107 pg/mL). Seven patients had metastatic renal cell carcinoma and demonstrated an average pretreatment VEGF level of 864 pg/mL (SD ± 642 pg/mL). Nine patients with colorectal cancer had an average pretreatment VEGF level of 797 pg/mL (SD ± 572 pg/mL) (Fig. 4B). In comparison, seven cancer-free healthy controls had a mean baseline VEGF level of 683 pg/mL (S.D. ± 488 pg/mL, range 279–1250 pg/mL) (Fig. 4A and B).
FIG. 4.
Pretreatment VEGF levels of patients receiving therapy with IFN-α and IL-12. (A) Pretreatment levels of VEGF for patients with advanced malignancy (n = 49) as compared to normal donors (n = 7) and (B) for subsets of patients with different types of malignancies are shown. The very high level of VEGF in the patient with pancreatic cancer has been excluded from our statistical analysis to avoid skewing the average of the “Other Cancer” group.
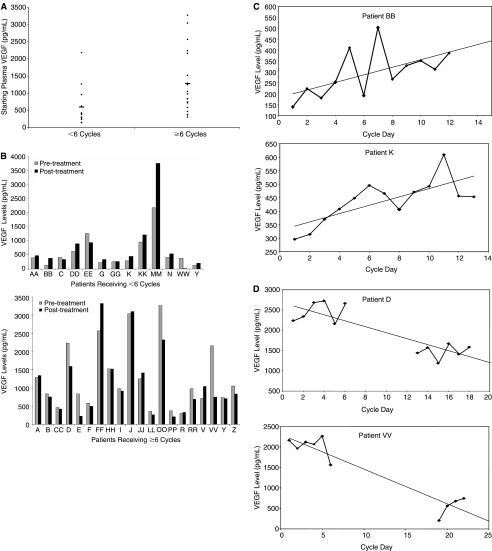
The mean baseline VEGF level was higher among patients receiving greater amounts of therapy (≥6 cycles), as compared to patients who were treated for <6 cycles (Fig. 5A), the mean VEGF level being 1278 ± 903 versus 594 ± 575, respectively. Renal cell carcinoma patients demonstrated the largest decrease in plasma VEGF levels, averaging 864 ± 642 before therapy and 484 ± 323 upon conclusion of therapy. Patients with more rapidly progressive disease generally had a significant rise in VEGF levels over the short course of treatment, whereas those with stable disease exhibited an overall decrease in plasma VEGF (Fig. 5B). In fact, the number of treatment cycles correlated negatively with the percent change in VEGF over the course of treatment. The longer the patient remained on the study, the greater the decrease in VEGF levels over the course of the treatment (Pearson correlation −0.354, p = 0.04).
FIG. 5.
Response of vascular endothelial growth factor (VEGF) levels to treatment correlates with duration of therapy. (A) Initial VEGF levels for patients receiving ≤6 cycles of therapy and patients receiving >6 cycles of therapy are shown. (B) VEGF levels prior to the start of therapy and at the conclusion of therapy are shown for patients receiving <6 cycles of therapy and patients receiving >6 cycles of therapy. Standard error was calculated from triplicate wells and was <5%. (C) Representative VEGF levels in patients with rapidly progressive disease (patient BB, top; patient K, bottom) are shown. Patient BB received 250 ng/kg IL-12 and 3 MU IFN-α and patient K received 100 ng/kg IL-12 and 5 MU IFN-α. (D) Representative VEGF levels in patients with favorable response to therapy (patient D, top; patient VV, bottom) are shown. Patient D received 500 ng/kg IL-12 and 5 MU IFN-α and patient VV received 100 ng/kg IL-12 and 1 MU IFN-α.
Patient K, a 57-year-old colorectal cancer patient, received 100 ng/kg IL-12 and 5 MU IFN-α for three cycles before stopping therapy due to progressive disease. Patient BB, a 43-year-old melanoma patient, received 250 ng/kg IL-12 and 3 MU IFN-α for two cycles before developing progressive disease. The plasma VEGF levels of patient K and BB both exhibited a gradual increase over the course of therapy (Fig. 5C). In contrast, patient VV, a 64-year-old renal cell carcinoma patient receiving 100 ng/kg IL-12 and 1 MU IFN-α, and patient D, a 59-year-old pharyngeal carcinoma patient receiving 500 ng/kg IL-12 and 5 MU IFN-α, demonstrated a significant decrease in plasma VEGF levels over the course of treatment (Fig. 5D). Patient VV and patient K ultimately received 18 and 6 cycles of therapy, respectively, before developing progressive disease.
Discussion
VEGF has been shown to play a critical role in the process of tumor growth and metastasis in several cancers, including melanoma and colorectal carcinoma (Graeven and others 2001; Srivastava and others 2003; Choueiri and others 2006). We hypothesized that IFN-α treatment would decrease the release of VEGF by melanoma cells in vitro and also in cancer patients treated with this cytokine. We first showed that baseline VEGF secretion is highly variable among melanoma cell lines. Treatment of human melanoma cell lines with IFN-α in vitro led to decreased VEGF secretion. Cell lines that secreted higher baseline levels of VEGF were more sensitive to IFN-α inhibition. Other in vitro studies have investigated the effects of IFN-α on VEGF secretion. Von Marschall and others (2003) showed a similar anti-VEGF effect of IFN-α on neuroendocrine tumor cell lines. They concluded that IFN-α confers its anti-tumor activity both in vitro and in vivo, at least in part, via Sp1- and/or Sp3-mediated inhibition of VEGF promoter activity. A variety of factors have been implicated in stimulating VEGF secretion, including hypoxia, the loss of p53 or PTEN, gains of function in Ras or Src, and autocrine stimulation of several tyrosine kinase pathways (Trisciuoglio and others 2005). These factors ultimately stimulate VEGF secretion through PI3 kinase and/or Erk/MAP kinase activation. Bedogni and others (2004) demonstrated that application of a PI3 kinase inhibitor or a MEK1/2 inhibitor to murine melanoma cells decreased secretion of VEGF, indicating that these pathways are active in promoting VEGF secretion in melanoma. Studies by Wu and others (2005) indicated that IFN-α treatment of a hepatocellular carcinoma cell line downregulated multiple genes involved in MAP kinase and PI3 kinase signaling. In addition, they showed that the inhibition of these pathways can decrease tumor cell VEGF secretion. As several different pathways have been shown to stimulate VEGF expression, it is possible that multiple pathways are active in the setting of melanoma. We hypothesize that those cell lines secreting large amounts of VEGF may do so through multiple pathways, one or more of which may be sensitive to the activity of IFN-α. The cell lines that secrete lower levels of VEGF, however, may do so through pathways upon which IFN-α has no action.
Overall, serum VEGF levels in control subjects were significantly lower than those of cancer patients. Analysis of patients grouped by malignancy revealed that renal cell carcinoma patients demonstrated the largest decrease in plasma VEGF level after treatment with IFN-α and IL-12. However, the clinical significance of this decrease in VEGF levels following combination treatment cannot be determined since patients with advanced disease may have cancers that were already highly vascularized. Interestingly, those patients who tolerated >6 cycles of IFN-α and IL-12 showed the greatest decline in VEGF levels. IL-12 has been shown to decrease angiogenesis in several murine tumor models, including melanoma (Sgadari and others 1996; Airoldi and others 2007). The decrease in new blood vessel formation has been partly attributed to decreases in tumor cell and stromal cell secretion of VEGF. This decrease in VEGF production is at least partly dependent on IL-12-induced secretion of IFN-γ but cannot be reproduced by administration of IFN-γ alone (Sgadari and others 1996). The anti-angiogenic effects of IFN-α may be dependent on its ability to inhibit the release of VEGF from tumor cells. With respect to the clinical activity of the IFN-α and IL-12 combination, we have previously published data suggesting that IL-12 induces the endogenous production of IFN-γ which upregulates the level of Jak-STAT signaling intermediates in both tumor cells and immune effector cells, thus sensitizing them to lower doses of IFN-α (Eisenbeis and others 2005). IL-12 may be enhancing the anti-tumor actions of IFN-α through direct anti-angiogenic effects and also through its ability to enhance the effects of IFN-α.
Studies in patients with renal cell carcinoma have demonstrated that combined treatment with IFN-α and SU5416, a selective inhibitor of the tyrosine kinase activity of the VEGF receptor Flk-1/KDR, results in decreased serum levels of VEGF that correlate with clinical response to therapy (Lara and others 2003). In a separate study, treatment of renal cell carcinoma patients with IFN-α and thalidomide produced similar decreases in VEGF levels that were associated with response to therapy (Hernberg and others 2003). The above reports, combined with our clinical findings, suggest a particular sensitivity of renal cell carcinomas to anti-VEGF therapy. In some patients, mutation of the von Hipple-Lindau tumor suppressor protein (pVHL) is associated with the development of renal cell carcinoma, due to the loss of pVHL's inhibitory effects on hypoxia-inducible factors (HIF) responsible for tumor angiogenesis. Overexpression of HIF, HIF-1α, and HIF-2α leads to the upregulation of genes involved in proliferation and angiogenesis (Sun and others 2003). Carroll and Ashcroft (2006) demonstrated in renal cell carcinomas that constitutively express HIF-1α or HIF-2α due to loss of VHL function that high basal levels of VEGF was predominantly dependent on HIF-2α. These data suggest that a variety of factors contribute to the levels of basal VEGF secretion in the tumor microenvironment. Theoretically, the basal level of VEGF or other pro-angiogenic factors could modulate the ability of the host to respond to biologic therapies such as IFN-α and IL-12.
In conclusion, we have demonstrated that VEGF secretion by different melanoma cell lines and among different cancer patients is highly variable and can be inhibited by IFN-α and IL-12 immunotherapy. Patients who respond to IFN-α immunotherapy with a decrease in serum VEGF demonstrated longer disease progression-free survival than non-responders. These findings could lead to improvement in patient selection for anti-angiogenic therapies.
Acknowledgments
This work was supported by National Institutes of Health Grants CA95426, 5 P30 CA16058–28, K24 CA93670 (WEC), and 2-U01 CA-076576–06, The Melanoma Research Foundation, and The Valvano Foundation for Cancer Research Award (GBL).
References
- Airoldi I. Di Carlo E. Cocco C. Taverniti G. D'Antuono T. Ognio E. Watanabe M. Ribatti D. Pistoia V. Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc Natl Acad Sci USA. 2007;104:3996–4001. doi: 10.1073/pnas.0609028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B. O'Neill MS. Welford SM. Bouley DM. Giaccia AJ. Denko NC. Powell MB. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–2560. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- Belardelli F. Ferrantini M. Proietti E. Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Carroll VA. Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Choueiri TK. Bukowski RM. Rini BI. The current role of angiogenesis inhibitors in the treatment of renal cell carcinoma. Semin Oncol. 2006;33:596–606. doi: 10.1053/j.seminoncol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Claffey KP. Robinson GS. Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Metastasis Rev. 1996;15:165–176. doi: 10.1007/BF00437469. [DOI] [PubMed] [Google Scholar]
- Eisenbeis CF. Lesinski GB. Anghelina M. Parihar R. Valentino D. Liu J. Nadella P. Sundaram P. Young DC. Sznol M. Walker MJ. Carson WE. Phase I study of the sequential combination of interleukin-12 and interferon alfa-2b in advanced cancer: evidence for modulation of interferon signaling pathways by interleukin-12. J Clin Oncol. 2005;23:8835–8844. doi: 10.1200/JCO.2005.02.1691. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Angiogenesis and cancer metastasis. Cancer J Sci Am Suppl. 2000;2:134–141. [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Gorski DH. Leal AD. Goydos JS. Differential expression of vascular endothelial growth factor-A isoforms at different stages of melanoma progression. J Am Coll Surg. 2003;197:408–418. doi: 10.1016/S1072-7515(03)00388-0. [DOI] [PubMed] [Google Scholar]
- Graeven U. Rodeck U. Karpinski S. Jost M. Philippou S. Schmiegel W. Modulation of angiogenesis and tumor-genicity of human melanocytic cells by vascular endothelial growth factor and basic fibroblast growth factor. Cancer Res. 2001;61:7282–7290. [PubMed] [Google Scholar]
- Hernberg M. Virkkunen P. Bono P. Ahtinen H. Maenpaa H. Joensuu H. Interferon alfa-2b three times daily and thalidomide in the treatment of metastatic renal cell carcinoma. J Clin Oncol. 2003;21:3770–3776. doi: 10.1200/JCO.2003.01.536. [DOI] [PubMed] [Google Scholar]
- Islam M. Frye RF. Richards TJ. Sbeitan I. Donnelly SS. Glue P. Agarwala SS. Kirkwood JM. Differential effect of IFNα-2b on the cytochrome P450 enzyme system: a potential basis of IFN toxicity and its modulation by other drugs. Clin Cancer Res. 2002;8:2480–2487. [PubMed] [Google Scholar]
- Johnson DH. Fehrenbacher L. Novotny WF. Herbst RS. Nemunaitis JJ. Jablons DM. Langer CJ. DeVore RF. Gaudreault J. Damico LA. Holmgren E. Kabbinavar F. Randomized Phase II trial comparing Bevacizumab plus Carboplatin and Paclitaxel with Carboplatin and Paclitaxel alone in previously untreated locally advanced or metastatic non-small cell cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Kabbinavar F. Hurwitz HI. Fehrenbacher L. Meropol NJ. Novotny WF. Lieberman G. Griffing S. Bergsland E. Phase II, randomized trial comparing Bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Lara PN., Jr Quinn DI. Margolin K. Meyers FJ. Longmate J. Frankel P. Mack PC. Turrell C. Valk P. Rao J. Buckley P. Wun T. Gosselin R. Galvin I. Gumerlock PH. Lenz HJ. Doroshow JH. Gandara DR. SU5416 plus interferon alpha in advanced renal cell carcinoma: a phase II California Cancer Consortium Study with biological and imaging correlates of angiogenesis inhibition. Clin Cancer Res. 2003;9:4772–4781. [PubMed] [Google Scholar]
- Lesinski GB. Anghelina M. Zimmerer J. Bakalakos T. Badgwell B. Parihar R. Hu Y. Becknell B. Abood G. Chaudhury AR. Magro C. Durbin J. Carson WE. The antitumor effects of IFN-alpha are abrogated in a STAT-1 deficient mouse. J Clin Invest. 2003;112:170–180. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesinski GB. Kondadasula SV. Crespin T. Shen L. Kendra K. Walker M. Carson WE. Multiparametric flow cytometric analysis of inter-patient variation in STAT 1 phosphorylation following interferon alpha immunotherapy. J Natl Cancer Inst. 2004;96:1331–1342. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- Lesinski GB. Trefry J. Brasdovich M. Kondadasula SV. Sackey K. Zimmerer JM. Chaudhury AR. Yu L. Zhang X. Crespin TR. Walker MJ. Carson WE. Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13:5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- Pyrhonen S. Kouri M. Holsti LR. Cantell K. Disease stabilization by leukocyte alpha interferon and survival of patients with metastatic melanoma. Oncology. 1992;49:22–26. doi: 10.1159/000227004. [DOI] [PubMed] [Google Scholar]
- Sgadari C. Angiolillo AL. Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- Srivastava A. Ralhan R. Kaur J. Angiogenesis in cutaneous melanoma: pathogenesis and clinical implications. Microsc Res Tech. 2003;60:208–224. doi: 10.1002/jemt.10259. [DOI] [PubMed] [Google Scholar]
- Sun X. Kanwar JR. Leung E. Vale M. Krissansen GW. Regression of solid tumors by engineered overexpression of von Hippel-Lindau tumor suppressor protein and antisense hypoxia-inducible factor-1-alpha. Gene Ther. 2003;10:2081–2089. doi: 10.1038/sj.gt.3302118. [DOI] [PubMed] [Google Scholar]
- Trisciuoglio D. Iervolino A. Zupi G. Del Bufalo Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaris N. Baxevanis C. Kosmidis P. Papamichael M. The prognostic significance of immune changes in patients with renal cancer, melanoma, and colorectal cancer, treated with interferon alpha 2b. Cancer Immunol Immunother. 1996;43:94–102. doi: 10.1007/s002620050308. [DOI] [PubMed] [Google Scholar]
- von Marschall A. Scholz A. Cramer T. Schafer G. Schirner M. Oberg K. Wiedenmann B. Hocker M. Rosewicz S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst. 2003;95:421–437. doi: 10.1093/jnci/95.6.437. [DOI] [PubMed] [Google Scholar]
- Wu WZ. Sun HC. Wu WZ. Sun HC. Shen YF. Chen J. Wang L. Tang ZY. Iliakis G. Liu KD. Interferon alpha 2a down-regulates VEGF expression through PI3 kinase and MAP kinase signaling pathways. J Cancer Res Clin Oncol. 2005;131:169–78. doi: 10.1007/s00432-004-0615-2. [DOI] [PubMed] [Google Scholar]