Abstract
Activation of the orphan nuclear receptor TR3/Nur77 (NR4A1) promotes apoptosis and inhibits pancreatic tumor growth, but its endogenous function and the effects of its inactivation have yet to be determined. TR3 was overexpressed in human pancreatic tumors compared to non-tumor tissue. siRNA-mediated knockdown of TR3 or cell treatment with the TR3 antagonist 1, 1-bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) decreased proliferation, induced apoptosis, and decreased expression of anti-apoptotic genes including Bcl-2 and survivin in pancreatic cancer cells. Survivin suppression was mediated by formation of a TR3-Sp1-p300 DNA binding complex on the proximal GC-rich region of the survivin promoter. When administered in vivo DIM-C-pPhOH induced apoptosis and inhibited tumor growth in an orthotopic model of pancreatic cancer, associated with inhibition of the same anti-apoptotic markers observed in vitro. Our results offer preclinical validation of TR3 as a drug target for pancreatic cancer chemotherapy, based on the ability of TR3 inhibitors to block the growth of pancreatic tumors.
Keywords: TR3, Nur77, drug target, pancreatic cancer
INTRODUCTION
The NR4A family members were first characterized as immediate-early genes induced in PC-12 cells by nerve growth factor (1). The three major family members NR4A1 (TR3, Nur77), NR4A2 (Nurr1) and NR4A3 (Nor1) are orphan nuclear receptors and, like other members of the nuclear receptor superfamily of transcription factors, NR4A family genes share common structural features (2, 3). This includes N- and C-terminal domains which may contain activation functions (AFs) and a DNA-binding and variable hinge domains located between the N- and C-terminal regions. Overexpression of Nur77 in transgenic mice results in apoptosis in thymocytes (4), and transgenic mice in which Nur77 and the related Nor-1 genes have been simultaneously knocked out develop lethal acute myeloid leukemia (5). The endogenous functions of Nur77 are variable and tissue-specific, and there are reports showing that TR3 regulates muscle lipolysis and glucose metabolism (6, 7) and plays a protective role in arthritis (8) and atherogenesis (9).
TR3 plays an important role in mediating cancer and tumor cell death by several agents including tetradecanoylphorbol-13-acetate (TPA) and the retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) (10–14). Apoptosis-inducing agents usually enhance expression and nuclear export of Nur77 which is necessary for induction of apoptosis. There is evidence in some cell lines that compound-induced nuclear translocation of Nur77 results in formation of a mitochondrial proapoptotic Nur77-bcl-2 complex which initiates cell death (12). A recent report shows that a Nur77-derived nanopeptide could be used to convert bcl-2 into a proapoptotic moiety which initiates apoptosis (15). In colon cancer cells, apoptosis-inducing agents also induce Nur77 export and induction of apoptosis is associated with cytoplasmic and not mitochondrial location of TR3 (13). In contrast, TR3 plays a role as a survival and an anti-apoptotic factor in certain cancer cells in which TR3 was not exported from nucleus (16, 17). This suggests that TR3 can induce both cell growth and apoptosis in the same cell type depending on the stimuli and its subcellular localization.
Studies in this laboratory have demonstrated the structure-dependent activation of nuclear TR3 by a series of diindolylmethane (DIM) derivatives and 1,1-bis(3′-indolyl)-1-(p-anisyl)methane (DIM-C-pPhOCH3) has been identified as the prototype activator of TR3 (18, 19). DIM-C-pPhOCH3 induces apoptosis in pancreatic and colon cancer cells, and RNA interference using a small inhibitory RNA for TR3 (siTR3) shows that activation of apoptosis by DIM-C-pPhOCH3 is dependent on nuclear TR3 (18, 19) and is associated with induction of several proapoptotic genes including tumor necrosis factor-related apoptosis inducing ligand (TRAIL) and p21 (19, 20). 1,1-Bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) was identified as a TR3 antagonist that blocked activation of the receptor by DIM-C-pPhOCH3; however, DIM-C-pPhOH alone was also cytotoxic to pancreatic cancer cells (18). In this study, we show that both siTR3 and DIM-C-pPhOH block activation and function of TR3 and demonstrate for the first time that endogenous nuclear TR3 enhances pancreatic cancer cell proliferation and survival and exhibits pro-oncogenic activity. Thus, compounds such as DIM-C-pPhOH represent a novel class of anticancer drugs that inactivate nuclear TR3, and using survivin as a model this study describes a novel molecular mechanism of TR3 inactivation in vitro and the inhibition of pancreatic tumor growth by DIM-C-pPhOH.
MATERIALS AND METHODS
Cell lines and plasmids
Panc1, MiaPaCa-2, and L3.6pl human pancreatic cancer cell lines were obtained and maintained as previously described (18, 20). The Flag-tagged and YFP-tagged full-length TR3 were constructed by inserting PCR-amplified full-length TR3 fragments into the EcoRI/BamHI site of p3XFLAG-CMV-10 expression vector (Sigma-Aldrich) and pEYFP-C1 expression vector (Clontech), respectively. The Gal4-TR3 chimeras Gal4-TR3-WT (amino acid 1 to 598), Gal4-TR3-AB (amino acid 1 to 266), and Gal4-TR3-CF (amino acid 267~598) were constructed by inserting PCR-amplified each fragment into the EcoRI/HindIII site of pM vector (Clontech). All other reporter constructs have been previously described (18–23).
Antibodies, reagents, quantitative real-time PCR, Western blot analysis, and immunoprecipitation
TR3 and Sp1 antibodies were purchased from Imgenex (San Diego, CA) and Upstate (Temecula, CA), respectively. Flag and β-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Bcl-2 and p300 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and all remaining antibodies were purchased from Cell Signaling Technology (Beverly, MA). DIM-C-pPhOH was synthesized and purified in this laboratory as previously described (18). Reporter lysis buffer, luciferase reagent, and β-Galactosidase (β-Gal) reagent were supplied by Promega (Madison, WI). Quantitative real-time PCR, western blot analysis and immunoprecipitation were undertaken as previously described (20). The sequences of the survivin primers used for real-time PCR were as follows: sense 5′-CAG ATT TGA ATC GCG GGA CCC-3′, antisense 5′-CCA AGT CTG GCT CGT TCT CAG-3′.
Transfection, siRNA oligonucleotides, cell proliferation assay, and reporter gene assay
Cells were transfected with 100 nM of each siRNA duplex for 7 hr using LipofectAMINE 2000 reagent (Invitrogen) following the manufacturer’s protocol. The sequences of siRNA oligonucleotides used were as follows: TR3 5′-CAG UCC AGC CAU GCU CCU C dTdT-3′; Sp1 5′-AUC ACU CCA UGG AUG AAA UGA dTdT-3′; and p300 5′-AAC CCC UCC UCU UCA GCA CCA dTdT-3′. As a negative control, a nonspecific scrambled small inhibitory RNA (siScr) oligonucleotide was used (Qiagen). Cell proliferation and reporter gene assays were performed as previously described (18–20).
Annexin V staining and subcellular localization assay
Vybrant apoptosis assay kit #2 was purchased from Molecular Probes (Eugene, OR), and annexin V staining was performed as previously described (18–20). For subcellular localization assay, cells were seeded on cover glass and transfected with YFP-TR3 for 6 hr. After incubation for 24 hr, cells were treated with either DMSO or DIM-C-pPhOH for 6 hr and mounted in mounting medium including DAPI (Vector Laboratory, CA). Fluorescent images were collected and analyzed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss, Jena, Germany).
DNA-binding assay and chromatin immunoprecipitation (ChIP) assay
GC-rich DNA binding of Sp1 and p300 was measured using an Universal EZ-TFA transcription factor assay Chemiluminescent kit (Upstate Biotechnology, Inc) according to the manufacturer’s protocol. A biotinylated double-stranded oligouncleotide containing Sp1 consensus sequence was used as a capture probe and an unlabeled oligonucleotide containing the identical consensus sequence as the capture probe was used as a competitor. A negative control without the capture probe was also used in each assay. The ChIP assay was performed using ChiP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif) as previously described (20). The survivin primers which contains several GC-rich sites were 5′-TCC AGG ACT CAA GTG ATG CTC-3′ (sense), and 5′-TCA AAT CTG GCG GTT AAT-3′ (antisense).
Orthotopic implantation of human pancreatic tumor cells in the pancreas of nude mice and treatment
Male athymic nude mice (NCr-nu/nu) were purchased and maintained as previously described (24). To produce tumors, L3.6pl cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Only suspensions consisting of single cells with >90% viability were used for the injections. Injection of cells into the pancreas was performed as described previously (24). Seven days after implantation of tumor cells, tumor-bearing mice were randomized and treated by oral gavage with either DIM-C-pPhOH in corn oil at a dose of 30 mg/kg/day (7 mice) or corn oil (5 mice) for4 weeks. The size and weight of the primary pancreatic tumors and body weight were recorded. Tumor volumes were calculated by using the following formula: 0.5 × (length) × (width)2.
TUNEL assay and immunohistochemical analysis
Tissue sections were deparaffinized and rehydrated, and TUNEL assay was performed using a DeadEnd Fluorometric TUNEL system (Promega) according to the manufacturer’s protocol. Fluorescent images were collected and analyzed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss). Immunohistochemical staining for TR3 was performed on paraffin-embedded specimens by using the standard avidin-biotin complex (ABC) method. Immunostaining intensity was scored as absent, low, or high. There was no specific staining when secondary antibody was used alone as a negative control. Human pancreatic tumor specimens were obtained from a selection of tumor and non-tumor tissues obtained at the University of Texas M.D. Anderson Cancer Center (Houston, TX).
Statistical Analysis
Statistical significance of differences in protein levels, luciferase activity, cell and tumor growth between groups were analyzed using either Student’s t-test or analysis of variance (ANOVA) with Scheffe’s test. The results are expressed as means with error bars representing 95% confidence intervals for three experiments for each group unless otherwise indicated, and a p value of less than 0.05 was considered statistically significant. All statistical tests were two-sided.
RESULTS
TR3 knockdown and the TR3 antagonist DIM-C-pPhOH inhibit cell growth and induce apoptosis in pancreatic cancer cells
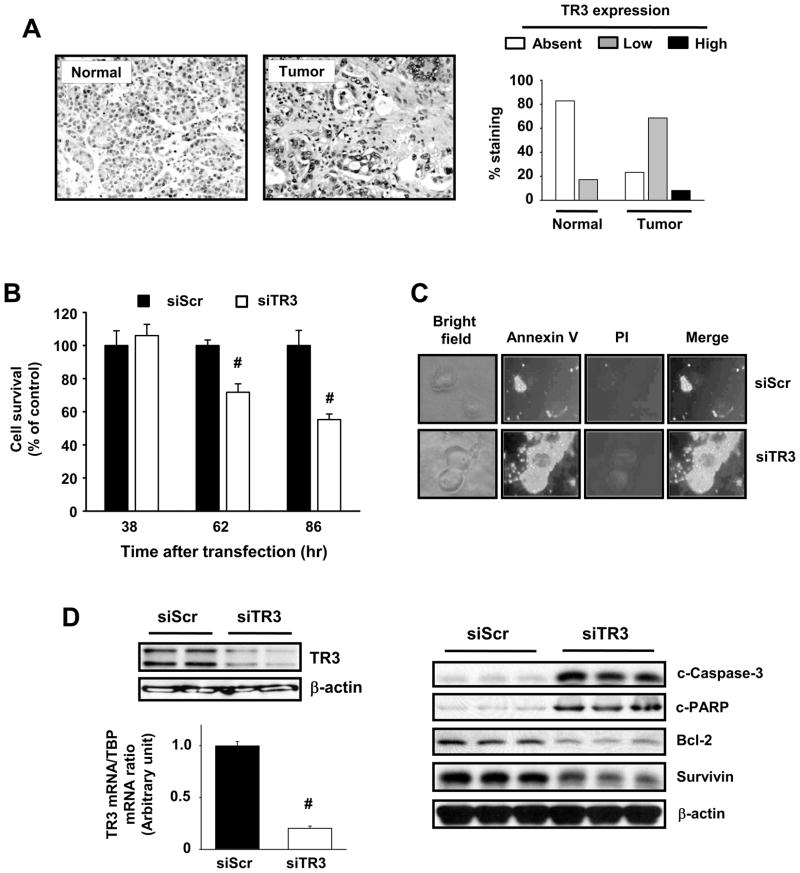
TR3 is primarily expressed as a nuclear protein in pancreatic and other cancer cell lines and this receptor is overexpressed in human colon (19). TR3 was also overexpressed in a panel (89) of human pancreatic tumors (77%), whereas 83% of non-tumor pancreatic tissues did not express TR3, and the receptor was primarily expressed in the nucleus of human pancreatic tumors (Fig. 1A). The endogenous function of nuclear TR3 in cancer cell lines and tumors is unknown and in this study, we used RNA interference in pancreatic cancer cells to investigate the effects of TR3 knockdown on cell proliferation and apoptosis. Figure 1B illustrates that transfection of Panc1 cells with siTR3 significantly decreased cell proliferation and induced Annexin V staining, demonstrating that endogenous TR3 not only facilitates cell growth but also cell survival by repressing apoptosis. Figure 1C confirms that siTR3 decreases TR3 mRNA and protein and this was accompanied by decreased expression of bcl-2 and survivin and induction of cleaved caspase-3 and PARP cleavage (Fig. 1D), confirming activation of apoptosis. Knockdown of TR3 also inhibited cell growth and induced apoptosis in L3.6pL and MiaPaCa-2 human pancreatic cancer cells (Suppl. Fig. 1). Thus, endogenous nuclear TR3 exhibits pro-oncogenic activity in pancreatic cancers and is thereby a potential drug target for pancreatic cancer chemotherapy.
Figure 1.
TR3 expression in human pancreatic tumors and cells and effects of knockdown by RNAi. (A) TR3 protein staining in pancreatic tumor and non-tumor tissue. TR3 was immunostained in pancreatic tumor and non-tumor tissue from 89 patients, and histograms representing non-detectable, low and high staining were derived as outlined in the Materials and Methods. (B) Panc1 cell growth. After transfection with siScr or siTR3 for the indicated times, the number of cells in each well was counted (* significant growth inhibition; p<0.05). (C) Annexin V staining. Panc1 cells were transfected with siScr or siTR3 for 72 hr and stained for Annexin V and propidium iodide. Knockdown of TR3 induced the translocation of phosphatidylserine from the inner to outer leaflet of the plasma membrane, which in combination with negative propidium iodide staining, as seen in the merged images, indicates the induction of apoptosis. (D) siTR3 decreases TR3 and affects TR3-regulated gene products. Panc1 cells were transfected with either siScr or siTR3 for 72 hr and whole cell lysates were analyzed by Western blot analysis for TR3 or TR3-regulated genes and TR3 mRNA levels were determined (in triplicate) by real-time PCR (* significant decrease; p<0.05).
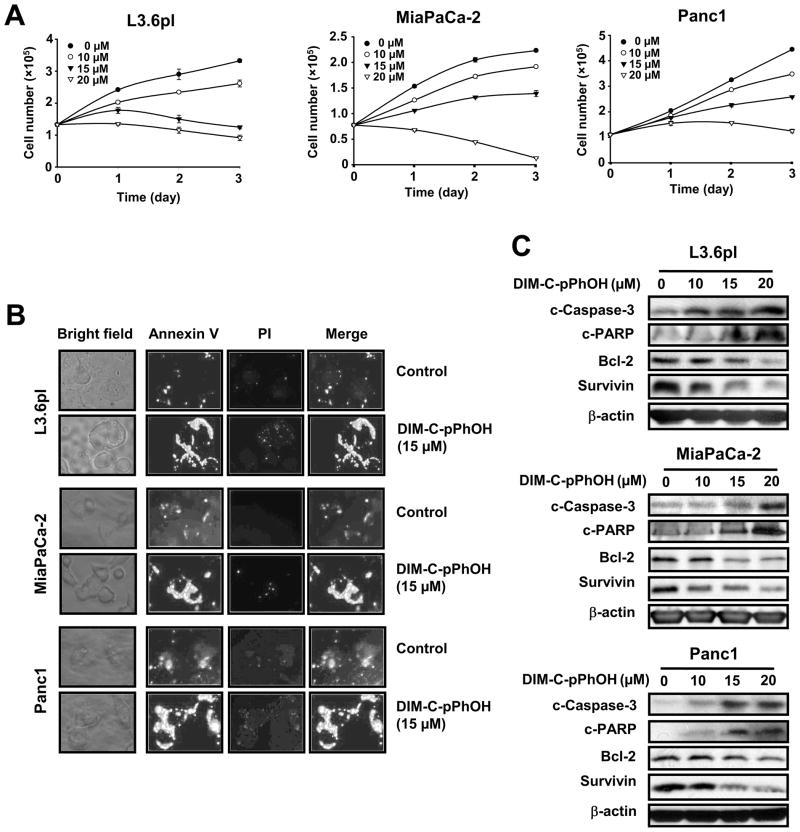
Previous studies show that DIM-C-pPhOH inhibits activation of nuclear TR3 by other C-DIM analogs (18, 19) and, DIM-C-pPhOH-dependent inactivation of TR3 was investigated in L3.6pL, MiaPaCa-2 and Panc1 pancreatic cancer cells. DIM-C-pPhOH significantly inhibited proliferation of all three cell lines (Figs. 2A), and growth inhibitory IC50 (48 hr) values were 11.35, 13.87 and 15.61μM, respectively. Similar results were observed in Panc28 cells; however, the anti-proliferative effects were somewhat delayed and not observed until after treatment for > 48 hr (data not shown). DIM-C-pPhOH also induced Annexin V staining in the three pancreatic cancer cell lines (Fig. 2B) and Western blot analysis of lysates from cells after treatment with DIM-C-pPhOH showed that expression of bcl-2 and survivin was decreased and caspase-3 and PARP cleavage were induced (Fig. 2C). Thus, DIM-C-pPhOH decreased proliferation and induced apoptosis in pancreatic cancer cells and the effects of this compound overlapped with those observed after TR3 knockdown (Fig. 1).
Figure 2.
DIM-C-pPhOH inhibits cell growth and induces apoptosis in pancreatic cancer cells. (A) Cell growth inhibition. L3.6pL, MiaPaCa-2, and Panc1 cells were treated with either various concentrations of DIM-C-pPhOH or DMSO (control) for 3 days, and the number of cells in each well was counted on days 1, 2, and 3. (B) Annexin V staining. L3.6pL, MiaPaCa-2, and Panc1 cells were treated with either DMSO or 15 μM of DIM-C-pPhOH for 18 hr and stained for Annexin V and propidium iodide. (C) Western blot analysis. L3.6pL, MiaPaCa-2, and Panc1 cells were treated with either DMSO or various concentrations of DIM-C-pPhOH for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods.
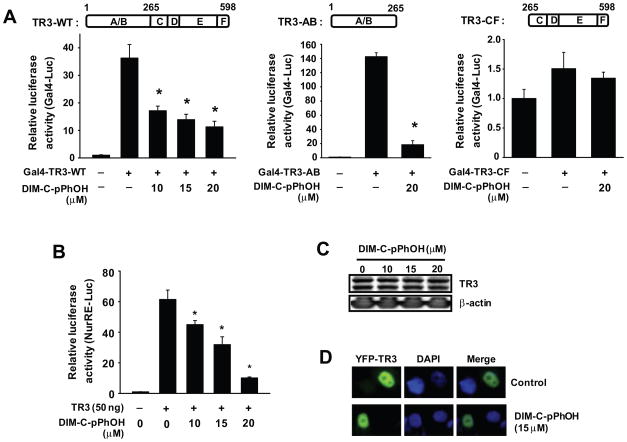
DIM-C-pPhOH inhibits nuclear TR3 transactivation via its N-terminal region
Inactivation of TR3 by DIM-C-pPhOH was further investigated using wild-type (GAL4-TR3-WT), C-terminal deletion (GAL4-TR3-AB), and N-terminal deletion (GAL4-TR3-CF) TR3-GAL4 chimeras transfected into Panc1 cells along with a GAL4-luc reporter gene (containing five tandem GAL4 response elements). In Panc1 cells transfected with GAL4-luc and GAL4-TR3-WT or GAL4-TR3-AB, treatment with DIM-C-pPhOH significantly decreased activity (Fig. 3A). In contrast, luciferase activity in Panc1 cells transfected with GAL4-luc and EV (empty vector) or GAL4-TR3-CF was low (Fig. 3D) and DIM-C-pPhOH did not affect luciferase activity, demonstrating that transactivation was primarily associated with the N-terminal region of TR3 and DIM-C-pPhOH specifically decreased this activity. In addition, we also observed that DIM-C-pPhOH decreased luciferase activity in Panc1 cells transfected with a construct (NuRE-luc) containing a TR3 response element linked to a luciferase reporter gene (Fig 3B), and DIM-C-pPhOH inhibited activity of phorbol ester-induced activation of NuRE-luc (Suppl. Fig. 2). Western blot analysis of lysates from Panc1 cells treated with DIM-C-pPhOH did not exhibit changes in levels of TR3 protein (Fig. 3C); moreover, in cells transfected with YFP-TR3, light microscopy showed that treatment with DIM-C-pPhOH did not induce nuclear export of TR3 since YFP-GFP co-localized with DAPI staining (Fig. 3D) and this was consistent with results of a previous study in Panc28 cells in which treatment with DIM-C-pPhOH did not change levels or subcellular location (nucleus) of TR3 (18).
Figure 3.
DIM-C-pPhOH inhibits TR3 transactivation via its N-terminal A/B domain. (A) Deactivation of Gal4-TR3 chimeras. Gal4-Luc (0.1 μg) was cotransfected with 10 ng of each Gal4-TR3-WT, Gal4-TR3-AB, or Gal4-TR3-CF into Panc1 cells for 5 hr and treated with various concentrations of DIM-C-pPhOH for 18 hr. Luciferase activity was determined as described in the Materials and Methods (* significantly decreased activity; p<0.05). (B) NuRE-luc deactivation. Panc1 cells were cotransfected with NurRE-Luc (0.1 μg) and 50 ng of Flag-TR3 for 5 hr, and treated with various concentrations of DIM-C-pPhOH for 18 hr. Luciferase activity (relative to β-galactosidase activity) was determined as described in the Materials and Methods (* significantly decreased activity; p<0.05). (C) Effects of DIM-C-pPhOH on TR3 expression. Panc1 cells were treated with various concentrations of DIM-C-pPhOH for 24 hr, and whole cell lysates were analyzed by Western blot analysis as described in the Materials and Methods. (D) Subcellular localization of TR3. Panc1 cells were transfected with YFP-TR3 (0.5 μg) and treated with either DMSO or 15 μM of DIM-C-pPhOH for 12 hr. Images were obtained as described in the Materials and Methods.
DIM-C-pPhOH-mediated inhibition of cell proliferation and induction of apoptosis are TR3-dependent
Further confirmation that DIM-C-pPhOH acts by inactivating TR3 was observed in rescue experiments where overexpression of TR3 alone increased proliferation of Panc1 cells and partially-reversed DIM-C-pPhOH-mediated inhibition of cell proliferation (Suppl. Fig. 3A). In addition, overexpression of TR3 rescued cells from DIM-C-pPhOH-induced caspase-3 and PARP cleavage and increased bcl-2 and survivin expression (Suppl. Fig. 3B), and quantitation of these responses are illustrated in Supplemental Figure 3C. Thus, DIM-C-pPhOH-induced proapoptotic/growth inhibitory activity is rescued by overexpression of TR3, further confirming that DIM-C-pPhOH inhibits the endogenous pro-oncogenic activity of TR3.
DIM-C-pPhOH and TR3 knockdown decrease survivin expression through inhibition of Sp1-dependent transactivation
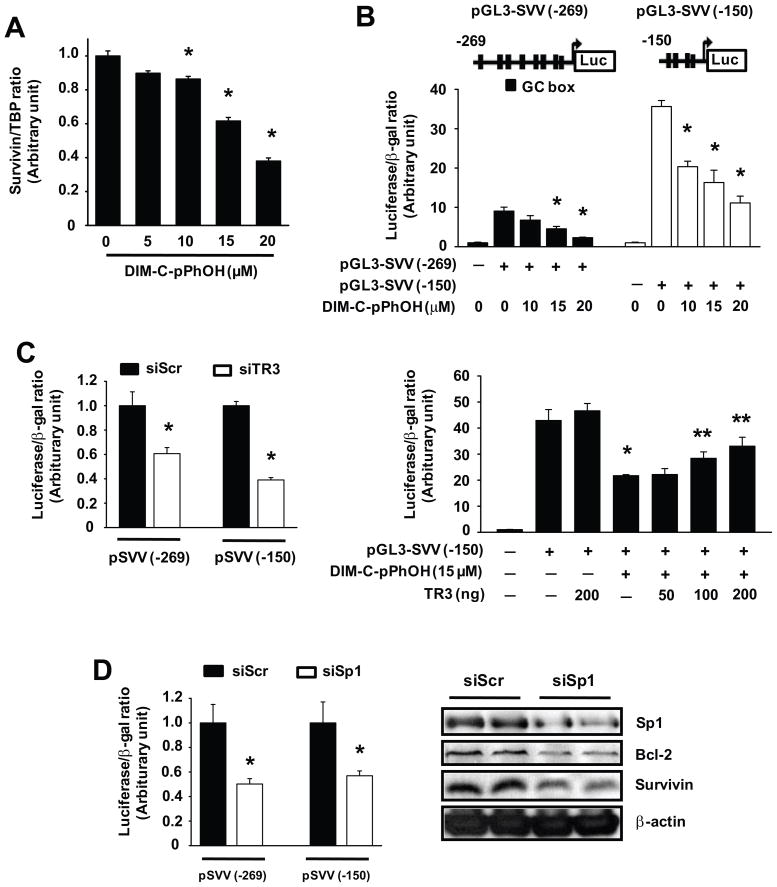
One of the major targets of TR3 in pancreatic cancer cells is survivin (Figs. 1D and 2C) which is overexpressed in pancreatic tumors (25, 26) and may be a drug target for cancer chemotherapy (25–27). Therefore, we used survivin as a model gene for investigating the molecular mechanism of gene regulation by endogenous TR3. Figure 4A shows that DIM-C-pPhOH decreased survivin mRNA levels and this corresponded to the effects of this compound on survivin protein expression (Fig. 2C). DIM-C-pPhOH also decreased luciferase activity in Panc1 cells transfected with the pGL3-SVV(−269) and the pGL3-SVV(−150) constructs (Fig. 4B) which contain the GC-rich −269 to +49 and −150 to +49 regions of the survivin promoter, respectively. Luciferase activity in Panc1 cells transfected with pGL3-SVV(−269) and pGL3-SVV(−150) was also decreased after cotransfection with siTR3 (compared to non-specific siScr), confirming comparable effects by DIM-C-pPhOH (TR3 inactivation) and siTR3 (TR3 knockdown) on these constructs (Fig. 4C). Furthermore, DIM-C-pPhOH-dependent downregulation of luciferase activity in cells transfected with pGL3-SVV(−150) was partially rescued by overexpression of wild-type TR3 (Fig. 4C). Thus, DIM-C-pPhOH-mediated inactivation of survivin can be partially reversed by TR3 overexpression, confirming the role of this receptor in regulating endogenous levels of survivin. The survivin gene is regulated by Sp transcription factors (28, 29), and results in Figure 4D show that, in Panc1 cells transfected with the pSVV constructs and siSp1 (Sp1 knockdown), luciferase activity was decreased. siSp1 also decreased survivin and bcl-2 protein expression and this is consistent with previous reports showing that survivin and bcl-2 are Sp regulated genes (28, 29).
Figure 4.
DIM-C-pPhOH and TR3 knockdown inhibit survivin expression via down-regulation of its transcription in pancreatic cancer cells. (A) Decreased survivin mRNa. Panc1 cells were treated with various concentrations of DIM-C-pPhOH for 18 hr, and TR3 mRNA levels were determined by real-time PCR (* significantly decreased activity; p<0.05). (B) Decreased luciferase activity. Panc1 cells were transfected with 0.1 μg of either pGL3-SVV (−269) or pGL3-SVV (−150) and treated with various concentrations of DIM-C-pPhOH for 18 hr. Luciferase activity was determined as outlined in the Materials and Methods (* significantly decreased activity; p<0.05). (C) Inhibition by siTR3 or DIM-C-pPhOH. Panc1 cells were cotransfected with each siRNA and pSVV(−269) or pSVV(−150) as indicated and/or cotransfected with Flag-TR3 and treated with 15 μM DIM-C-pPhOH. Luciferase activity was determined as described in the Materials and Methods (* significantly decreased activity by siTR3 or DIM-C-pPhOH alone; ** significant reversal of the effects by TR3 overexpression; p<0.05). (D) Effects of Sp1 knockdown. Panc1 cells were cotransfected with each siRNA and pGL3-SVV as indicated. Luciferase activity or western blot analysis of whole cell lysates were determined as described in the Materials and Methods (* significantly decreased activity; p<0.05).
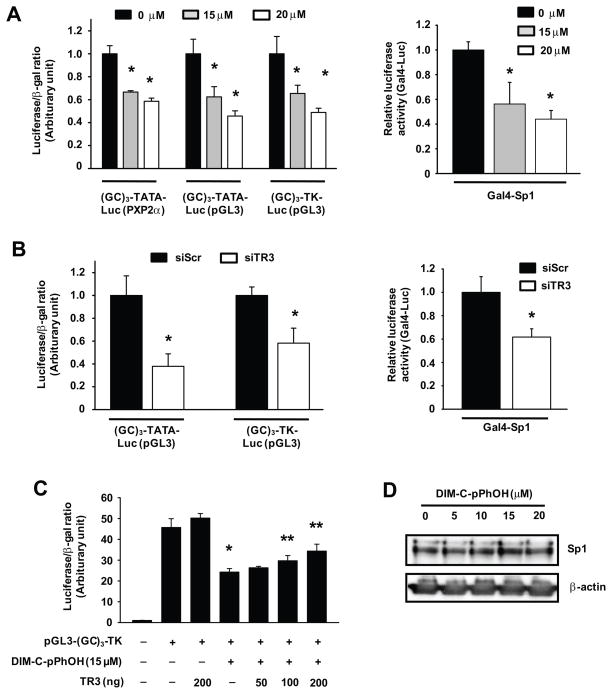
Since TR3 directly interacts with Sp1 bound to GC-rich promoter sites (20), the role of DNA-bound Sp1 in mediating the effects of TR3 was further investigated in Panc1 cells transfected with constructs containing three tandem GC-rich sites or a GAL4-Sp1 chimera and a GAL4-luc reporter gene. Treatment of cells with DIM-C-pPhOH (Fig. 5A) or transfection with siTR3 (Fig. 5B) decreased transactivation with all Sp1-regulated constructs and, in Panc1 cells transfected with pGL3-GC3-TK, the suppressive effects of DIM-C-pPhOH were partially reversed by TR3 overexpression (Fig. 5C) and this was not accompanied by Sp1 downregulation (Fig. 5D).
Figure 5.
DIM-C-pPhOH and TR3 knockdown decrease Sp1-dependent transactivation in pancreatic cancer cells. (A) Transfection with GC-rich constructs and Gal4-Sp1. Panc1 cells were transfected with GC-rich constructs or Gal4-Sp1/Gal4-luc as indicated and treated with various concentrations of DIM-C-pPhOH for 18 hr. Luciferase activity was determined as described in the Materials and Methods (*significantly decreased activity; p<0.05). (B) Effects of TR3 knockdown. Panc1 cells were cotransfected with each siRNA and (GC)3-Luc or Gal4-Sp1/Gal4-luc. Luciferase activity was determined as described in the Materials and Methods (* significantly decreased activity; p<0.05). (C) TR3 rescue experiment. Panc1 cells were cotransfected with Flag-TR3 (or empty vector) and pGL3-(GC)3-TK-Luc, and treated with 15 μM of DIM-C-pPhOH for 18 hr. Luciferase activity was determined as described in the Materials and Methods (* significantly decreased activity; ** significant rescue by TR3 overexpression; p<0.05). (D) Sp1 expression, Panc1 cells were treated with DIM-C-pPhOH for 24 hr and nuclear extracts were analyzed for Sp1 protein by western immunoblots as described in the Materials and Methods.
TR3-dependent regulation of survivin through interactions with Sp1 is coregulated by p300
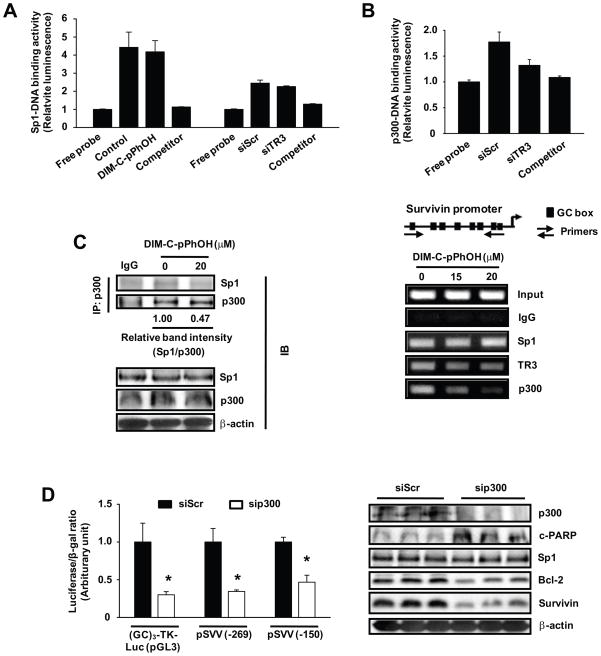
Since DIM-C-pPhOH or TR3 knockdown does not decrease Sp1 expression (20) (Fig. 5D), we investigated the effects of TR3 inactivation on Sp1-DNA binding using a non-radioactive EZ-transcription factor DNA binding assay. The results (Fig. 6A) show that neither DIM-C-pPhOH nor siTR3 decreased Sp1-DNA binding compared to control or non-specific siScr. Direct interaction of Sp1 and TR3 has previously been reported (20) and was confirmed in co-immunoprecipitation experiments with lysates from Panc1 cells transfected with Flag-TR3 (Suppl. Fig. 4). Since the nuclear coregulator p300 interacts with both Sp1 and TR3 (30, 31), we investigated the role of p300 in regulating survivin expression and Figure 6B shows that in a ChIP assay, Sp1, TR3 and p300 were constitutively bound to the proximal GC-rich survivin promoter. Moreover, after treatment with DIM-C-pPhOH, there was a marked decrease in p300 binding to the promoter, a slight loss of TR3, and Sp1 binding was unchanged, and this was consistent with results of the Sp1-DNA binding assay in Figure 6A. Using this same assay and the GC-rich probe, the levels of p300-DNA binding were decreased by siTR3 (TR3 knockdown) (Fig. 6B), and similar results were observed after treatment with DIM-C-pPhOH (data not shown). Moreover, in coimmunoprecipitation studies using the p300 antibody, DIM-C-pPhOH decreased the Sp1/p300 ratio that coimmunoprecipited (Fig. 6C), whereas expression of Sp1 and p300 proteins was unchanged. The contributions of p300 as a regulator of TR3/Sp1-dependent survivin gene expression was confirmed in Panc1 cells cotransfected with siScr or sip300 and consensus GC-rich (GC3-TK-luc) or GC-rich survivin [pSVV(−269) or SVV(−150)] constructs (Fig. 6D). Knockdown of p300 decreased luciferase activity in cells transfected with the three constructs. Moreover, knockdown of p300 in Panc1 cells also decreased levels of survivin and bcl-2 proteins, increased PARP cleavage, but did not affect expression of Sp1 protein (Fig. 6D). Thus, the TR3-dependent regulation of survivin through interaction with Sp1 is also coregulated by p300.
Figure 6.
TR3-dependent regulation of survivin through interactions with Sp1 are coregulated by p300. (A) Effect of DIM-C-pPhOH and siTR3 on Sp1 binding to GC-rich Sp1 consensus sequence. Panc1 cells were treated with 20 μM of DIM-C-pPhOH for 6 hr or transfected with either siScr or siTR3, and nuclear extracts were tested for Sp1-DNA binding activity as described in the Materials and Methods. (B) ChIP and DNA binding assays. Panc1 cells were treated with either DMSO or various concentrations of DIM-C-pPhOH for 6 hr, and interactions of Sp1, TR3, and p300 with the survivin promoter were determined as described in the Materials and Methods. Panc1 cells were transfected with either siScr or siTR3, and nuclear extracts were tested for p300-DNA binding activity as described in the Materials and Methods. (C) Coimmunoprecipitation of p300 and Sp1 proteins. Panc1 cells were treated with 20 μM of DIM-C-pPhOH for 6 hr, and nuclear extracts were prepared. Endogenous p300 was immunoprecipitated with anti-p300 antibodies and the immunoprecipitates were analyzed by Western blot analysis for Sp1 and p300 as described in the Materials and Methods. (D) Effects of p300 knockdown. Panc1 cells were cotransfected with GC-rich constructs and siRNAs (siScr or sip300), and luciferase activity or analysis of whole cell lysates were determined as described in the Materials and Methods (* significantly decreased activity; p<0.05).
DIM-C-pPhOH induces apoptosis and inhibits tumor growth in an orthotopic mouse model of human pancreatic cancer
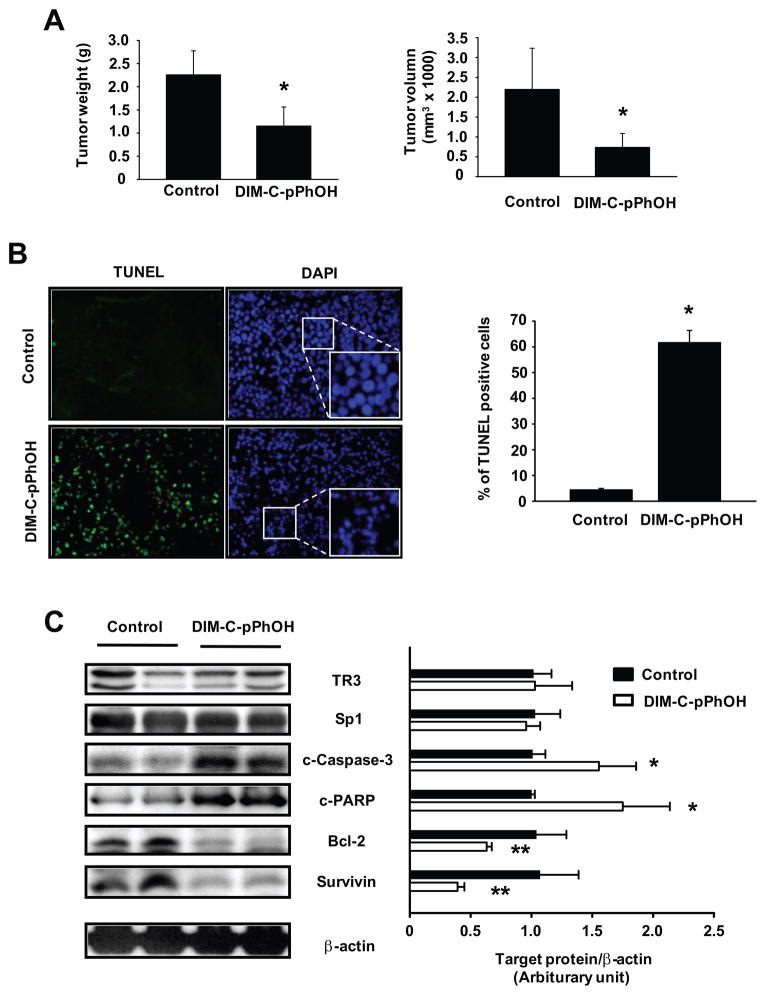
Since inhibition of TR3 by RNA interference or DIM-C-pPhOH induces apoptosis and inhibits growth of pancreatic cancer cells, we investigated the anticancer activity of DIM-C-pPhOH in a murine orthotopic model for pancreatic cancer using L3.6pL cells. Cells were injected directly into the pancreas and after 7 days, mice were treated with DIM-C-pPhOH (30 mg/kg/day) for 28 days and the effects on tumor growth and weight were then determined. The results in Figures 7A demonstrate that DIM-C-pPhOH significantly inhibited tumor volumes and weights compared to the corn oil control tumors. At this dose, changes in body or organ weights or histopathological changes were not observed in the treated animals, suggesting that DIM-C-pPhOH had no discernible toxicity to the mice (data not shown). TUNEL staining of pancreatic tumors demonstrated that DIM-C-pPhOH significantly induced apoptosis (Fig. 7B), and DAPI nuclear staining also verified that the number of apoptotic cells which showed irregular edges around the nucleus, nuclear shrinkage, and an increased number of nuclear body fragments was increased in the tumor sections obtained from DIM-C-pPhOH-treated mice compared with control mice (Fig. 7B). Moreover, western blot analysis of tumor lysates showed that DIM-C-pPhOH decreased levels of bcl-2 and survivin proteins, induced caspase 3 and PARP cleavage and did not affect Sp1 or TR3 expression (Fig. 7C). The comparable effects of DIM-C-pPhOH observed in vitro and in vivo demonstrated that drugs such as DIM-C-pPhOH that inactivate TR3, a pro-oncogenic protein overexpressed in pancreatic tumors, represent a new class of mechanism-based drugs for treating pancreatic cancer.
Figure 7.
Effects of DIM-C-pPhOH on growth and apoptosis in an orthotopic mouse model of human pancreatic cancer. (A) Effects on tumor weights and volume. L3.6pL human pancreatic cancer cells were orthotopically implanted into athymic nude mice, and each mouse was dosed by oral gavage with either corn oil (control) or DIM-C-pPhOH (30 mg/kg/day) for 4 weeks starting 7 days after implantation. Median tumor weights and volumes were calculated as described in the Materials and Methods (* significantly decreased effects; p<0.05). (B) TUNEL assay. The TUNEL assay was performed on tumor sections as described in the Materials and Methods, and the number of TUNEL positive cells was counted from randomly selected microscopic fields. DAPI stains both apoptotic and nonapoptotic cells blue and fluorescein-12-dUTP incorporation results in localized green fluorescence within the nucleus of apoptotic cells. Fluorescent images were collected at high (x400) magnification (* significantly increased TUNEL staining; p<0.05). (C) Protein expressions in tumor lysates. Tumor lysates from tumor samples were further analyzed by Western blot analysis as described in the Materials and Methods, and each lane represents a different tumor sample [* significantly increased caspase-3 and PARP cleavage; **significantly decreased bcl-2 and survivin protein levels (relative to β-actin loading control); p<0.05].
DISCUSSION
We previously identified DIM-C-pPhOH as a potential TR3 inactivator or antagonist since DIM-C-pPhOCH3-mediated induction of TRAIL is inhibited by both DIM-C-pPhOH and siTR3 (18). A previous report showed that antisense TR3 enhanced tumor necrosis factor α (TNFα )-mediated apoptosis in fibroblasts in which either RelA or TRAF2 have been abrogated (32). Moreover, in these TNFα-sensitive cells, TR3 was not exported from the nucleus and it was concluded that for TNFα-mediated responses, TR3 is a survival effector protein. These observations are consistent with our previous report showing that although DIM-C-pPhOH blocked activation of TR3 by DIM-C-pPhOCH3, the former compound exhibits delayed cytotoxicity in Panc28 cells (18), and this was confirmed in MiaPaCa-2, L3.6pL and Panc1 cells where DIM-C-pPhOH inhibited cell proliferation (Fig. 2A and Suppl. Fig. 1). Therefore, we hypothesized that endogenous TR3 which is overexpressed in human colon (19) and pancreatic tumors (Fig. 1) may contribute to the growth and survival of pancreatic cancer and thereby was a pro-oncogenic factor. This was investigated by both RNA interference and DIM-C-pPhOH-dependent inactivation of TR3, and the results obtained using siTR3 and DIM-C-pPhOH were complementary since both reagents inhibited cell proliferation, induced markers of apoptosis, and decreased expression of the anti-apoptotic genes bcl-2 and survivin (Figs. 1 and 2). The only major difference between siTR3 and DIM-C-pPhOH was that the oligonucleotide decreased TR3 expression (Fig. 1D), whereas DIM-C-pPhOH inhibited TR3-mediated responses but did not affect levels of TR3 (Fig. 3D) which remained in the nucleus.
These results suggest that DIM-C-pPhOH inactivates TR3 and this was further investigated using Panc1 cells transfected with a luciferase construct containing TR3-binding response elements (NuRE-luc) or various Gal4-TR3 fusion constructs that activate a Gal4 reporter construct (Gal4-luc) containing five tandem Gal4 response elements (Fig. 3). DIM-C-pPhOH decreases transactivation in Panc1 cells transfected with NuRE-luc, demonstrating that DIM-C-pPhOH inactivates TR3. It was also apparent from studies with the GAL4-TR3 constructs that the major transactivation region of TR3 in Panc1 cells was associated with the N-terminal A/B domain, whereas minimal to non-detectable activity was observed using the Gal4-TR3-CF construct which contains the C-terminal region of TR3. These results are consistent with previous studies demonstrating the high activity of the N-terminal A/B domain of TR3 which contains activation function-1 (33, 34), and we are currently investigating the mechanisms associated with DIM-C-pPhOH-dependent deactivation of TR3 through its A/B domain.
The intriguing proapoptotic and growth inhibitory effects of DIM-C-pPhOH and siTR3 were accompanied by downregulation of survivin and bcl-2 genes/protein (Figs. 1D and 2C), and among the most pronounced effects was the downregulation of survivin. Survivin plays a role in cell cycle progression and is a member of the inhibitor of apoptosis protein family that inhibits apoptosis through interactions with caspases (25–27). Survivin is overexpressed in multiple tumor types including pancreatic cancer (25, 26), and survivin expression is increased during pancreatic intraepithelial neoplasia progression into pancreatic ductal adenocarcinoma (26). These observations coupled with increasing interest in survivin as a target for cancer therapy (35) prompted us to use survivin as a model to further investigate the mechanisms of TR3-dependent repression of gene expression. DIM-C-pPhOH not only decreased survivin protein expression (Fig. 2C) but also decreased survivin mRNA levels (Fig. 4A) and luciferase activity in Panc1 cells transfected with survivin constructs (Fig. 4B). The role of TR3 inactivation in mediating downregulation of survivin was confirmed in TR3 rescue experiments and RNA interference studies which demonstrated that loss of TR3 results in decreased luciferase activity in Panc1 cells transfected with survivin promoter constructs (Fig. 4C). Previous studies show that survivin expression is regulated by Sp1 and other Sp proteins that are overexpressed in cancer cells and tumors, and Sp1 knockdown or inhibition decreases survivin levels (28, 29). Recent studies in this laboratory show that TR3 directly interacts with Sp1 (20), suggesting that one possible mechanism of TR3-dependent downregulation of survivin by DIM-C-pPhOH may be due to inactivation of Sp1-mediated transactivation or by loss of Sp1. Results summarized in Figure 5 demonstrate that both DIM-C-pPhOH and siTR3 decreased Sp1-dependent transactivation using several Sp1-responsive (GC-rich) promoter-reporter or Gal4-Sp1 chimeric constructs; however, these effects were not associated with changes in Sp1 protein expression (Fig. 5D). These data show that loss of TR3 or inactivation of this receptor by DIM-C-pPhOH decreased Sp1-dependent transactivation, suggesting that endogenous TR3 enhances Sp1-dependent transactivation and exhibits “coactivator-like” activity for expression of survivin in pancreatic cancer cells.
Previous reports show that p300 interacts with both TR3 and Sp1 (30, 31) and p300 interacts with the A/B domain of TR3 and plays a role in coactivator recruitment (34). Interestingly, one study showed that binding of TR3 to the histone acetyltransferase domain of p300 also decreases transcription (30). In this study, p300 acted as a coregulatory protein that contributed to the endogenous activity of TR3 and knockdown of p300 decreased Sp1-dependent transactivation (Fig. 6D). Moreover, loss of p300 by RNA interference in Panc1 cells (Fig. 6D) resulted in the same alterations of genes/responses observed after treatment of cells with DIM-C-pPhOH (Fig. 2C) or knockdown of TR3 (Fig. 1D), namely, downregulation of survivin and bcl-2, induction of caspase-3 and PARP cleavage. Thus, the pro-oncogenic activity of TR3 is coregulated by p300 and using the survivin gene as a model, we show that this involves Sp1-TR3-p300 interactions at the proximal GC-rich survivin promoter (Fig. 6).
The novel mechanistic observations discovered in this study suggest that compounds such as DIM-C-pPhOH that inactivate TR3 may represent a new class of anticancer drugs, and our in vivo studies using an orthotopic pancreatic tumor model show that DIM-C-pPhOH inhibited pancreatic tumor growth. Moreover, the effects of DIM-C-pPhOH on apoptosis, survivin and bcl-2 observed in vitro were also observed in tumors from animals treated with this agent (Fig. 7). This study demonstrates for the first time that TR3 is a pro-oncogenic factor in pancreatic cancer and contributes to the proliferation and survival of cancer cell lines and tumors and inactivation of TR3 by DIM-C-pPhOH resulted in growth inhibition and apoptosis in pancreatic cancer cells and tumors. These results are unique among nuclear receptors since we have shown that activation of TR3 by DIM-C-pPhOCH3 (18) and inactivation by DIM-C-pPhOH (this study) inhibited pancreatic cancer cell and tumor growth and induced apoptosis through different pathways. C-DIMs including the TR3 activator DIM-C-pPhOCH3 and the inactivator DIM-C-pPhOH also induce the extrinsic apoptosis pathway through activating ER stress and this represents a TR3-independent pathway induced by DIM-C-pPhOH (22, 23). However, preliminary microarray studies [(19) and unpublished results] have identified some of the different proapoptotic genes induced by these compounds, and their TR3-dependent apoptotic pathways are currently being investigated. These data suggest that TR3 is a unique target for development of new mechanism-based chemotherapies for pancreatic and colon cancer and other tumor types that overexpress this orphan receptor.
Supplementary Material
Acknowledgments
Funding: This work was supported by National Institutes of Health (R01CA124998) and the Texas A&M AgriLife.
Footnotes
Conflict of Interest: All authors have no conflicts of interest to disclose.
References
- 1.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–8. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 2.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 3.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–74. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 4.Winoto A. Genes involved in T-cell receptor-mediated apoptosis of thymocytes and T-cell hybridomas. Semin Immunol. 1997;9:51–8. doi: 10.1006/smim.1996.0053. [DOI] [PubMed] [Google Scholar]
- 5.Mullican SE, Zhang S, Konopleva M, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–5. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem. 2005;280:12573–84. doi: 10.1074/jbc.M409580200. [DOI] [PubMed] [Google Scholar]
- 7.Chao LC, Zhang Z, Pei L, Saito T, Tontonoz P, Pilch PF. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol. 2007;21:2152–63. doi: 10.1210/me.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Silva S, Han S, Zhang X, Huston DP, Winoto A, Zheng B. Reduction of the incidence and severity of collagen-induced arthritis by constitutive Nur77 expression in the T cell lineage. Arthritis Rheum. 2005;52:333–8. doi: 10.1002/art.20736. [DOI] [PubMed] [Google Scholar]
- 9.Pires NM, Pols TW, de Vries MR, et al. Activation of nuclear receptor Nur77 by 6-mercaptopurine protects against neointima formation. Circulation. 2007;115:493–500. doi: 10.1161/CIRCULATIONAHA.106.626838. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Lin B, Agadir A, et al. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol. 1998;18:4719–31. doi: 10.1128/mcb.18.8.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu X, Chang C. TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J Biol Chem. 2003;278:42840–5. doi: 10.1074/jbc.M305594200. [DOI] [PubMed] [Google Scholar]
- 12.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–40. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–7. [PubMed] [Google Scholar]
- 14.Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–92. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 15.Kolluri SK, Zhu X, Zhou X, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–98. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bras A, Albar JP, Leonardo E, de Buitrago GG, Martinez A. Ceramide-induced cell death is independent of the Fas/Fas ligand pathway and is prevented by Nur77 overexpression in A20 B cells. Cell Death Differ. 2000;7:262–71. doi: 10.1038/sj.cdd.4400653. [DOI] [PubMed] [Google Scholar]
- 17.Kolluri SK, Bruey-Sedano N, Cao X, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. 2003;23:8651–67. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–14. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 19.Cho SD, Yoon K, Chintharlapalli S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–83. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 20.Lee SO, Chintharlapalli S, Liu S, et al. p21 Expression is induced by activation of nuclear nerve growth factor-induced Bα (NGFI-Bα, Nur77) in pancreatic cancer cells. Mol Cancer Res. 2009;7:1169–78. doi: 10.1158/1541-7786.MCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–23. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 22.Lei P, Abdelrahim M, Cho SD, Liu X, Safe S. Structure-dependent activation of endoplasmic reticulum stress-mediated apoptosis in pancreatic cancer by 1,1-bis(3′-indoly)-1-(p-substituted phenyl)methanes. Mol Cancer Ther. 2008;7:3363–72. doi: 10.1158/1535-7163.MCT-08-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis. 2008;29:1139–47. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–68. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 25.Lee MA, Park GS, Lee HJ, et al. Survivin expression and its clinical significance in pancreatic cancer. BMC Cancer. 2005;5:127. doi: 10.1186/1471-2407-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhanot U, Heydrich R, Moller P, Hasel C. Survivin expression in pancreatic intraepithelial neoplasia (PanIN): steady increase along the developmental stages of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2006;30:754–9. doi: 10.1097/00000478-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Ling X, Pan D, et al. Molecular mechanism of inhibition of survivin transcription by the GC-rich sequence-selective DNA binding antitumor agent, hedamycin: evidence of survivin down-regulation associated with drug sensitivity. J Biol Chem. 2005;280:9745–51. doi: 10.1074/jbc.M409350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li GD, Fang JX, Chen HZ, et al. Negative regulation of transcription coactivator p300 by orphan receptor TR3. Nucleic Acids Res. 2007;35:7348–59. doi: 10.1093/nar/gkm870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Pan L, Feng Y, et al. P300 plays a role in p16INK4a expression and cell cycle arrest. Oncogene. 2008;27:1894–904. doi: 10.1038/sj.onc.1210821. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, Suzuki N, Mirtsos C, et al. Nur77 as a survival factor in tumor necrosis factor signaling. Proc Natl Acad Sci U S A. 2003;100:8276–80. doi: 10.1073/pnas.0932598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maira M, Martens C, Batsche E, Gauthier Y, Drouin J. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23:763–76. doi: 10.1128/MCB.23.3.763-776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277:33001–11. doi: 10.1074/jbc.M203572200. [DOI] [PubMed] [Google Scholar]
- 35.Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2005;9:360–72. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.