Abstract
Background
Exaggerated concern for correct performance has been linked to hyperactivity of the medial frontal cortex (MFC) in adult obsessive compulsive disorder (OCD), but the role of the MFC during the early course of illness remains poorly understood. We tested whether hyperactive MFC-based performance monitoring function relates to altered MFC connectivity within task control and default mode networks in pediatric patients.
Methods
Eighteen pairs of OCD and matched healthy youth underwent functional magnetic resonance imaging during performance monitoring and at rest. Task-related hyperactivations in the posterior and ventral MFC were used as seeds for connectivity analyses during task and resting state.
Results
In posterior MFC, patients showed greater activation of dorsal anterior cingulate cortex (dACC) than controls, with greater activation predicting worse performance. In ventral MFC, controls exhibited deactivation, whereas patients activated this region. Compared to controls, patients showed increased dACC - ventral MFC connectivity during task, and decreased dACC - right anterior operculum and ventral MFC- posterior cingulate connectivity during rest.
Conclusions
Excessive activation and increased interactions of posterior and ventral MFC during performance monitoring may combine with reduced resting state connectivity of these regions within networks for task control and default mode to reflect early markers of OCD. Alteration of reciprocal interactions between these networks could potentiate the intrusion of vMFC-based affectively laden, self-referential thoughts while disrupting pMFC-based performance-monitoring function in young patients.
Keywords: pediatric obsessive compulsive disorder, medial frontal cortex, performance monitoring, resting state connectivity, default mode network, task control network
Introduction
Hyperactivity of the medial frontal cortex (MFC) has been posited to underlie repetitive thoughts and behaviors in obsessive compulsive disorder (OCD, 1). One MFC function of relevance for OCD is performance monitoring, since OCD symptoms are often associated with excessive concern for correct performance (2, 3). Performance monitoring involves the detection of interference between competing response options, and the processing of errors, to enable behavioral adjustments. Hypothetically, the repetitive thoughts and behaviors of OCD could stem from failure to resolve interference from pre-potent response sets, or hypersensitivity to errors in “security concern” domains (e.g., contamination/washing, aggression/checking, symmetry/ordering) that make up the typical symptoms of OCD (4). Indeed, interference- and error-processing elicit MFC hyperactivity in adults with OCD (5–9), even when not overtly triggering OCD symptoms. Given the role of the posterior MFC (pMFC) in detecting interference between competing response options (10) and of the ventral MFC (vMFC) in affective response to errors (11), performance-related hyperactivation of these regions in adult OCD has been alternately interpreted to reflect inefficient interference monitoring (pMFC, 5–7), or exaggerated valuation of correct performance (vMFC, 8). Building on these insights from functional neuroimaging studies of adult patients, we sought to determine whether MFC hyperactivity during performance monitoring represents an early marker of illness, and if/how it relates to altered MFC connectivity within the brain in pediatric OCD.
The characterization of MFC-based neural networks in healthy adults provide context for understanding MFC hyperactivity during performance monitoring in OCD. Interference- and error-processing are facilitated by interactions between the pMFC [dorsal anterior cingulate cortex (dACC) into pre-supplementary motor area] and lateral frontal cortex (10, 12) contributing to a “task control network” that is engaged by cognitive effort, but remains connected even at rest (13). The effective regulation of performance by this network depends on concurrent deactivation in the vMFC (14) which, along with the posterior cingulate cortex (PCC), contributes to a “default mode network” (DMN) in which activity increases during rest and decreases during the performance of cognitively demanding tasks (15). In adults with OCD, hyperactivation of both the posterior (5–7) and ventral (6, 8) MFC have been reported during performance monitoring, suggesting a disruption of the normal, reciprocal relationship between these MFC subregions.
To explore the possibility that performance-related abnormalities of the MFC in OCD may be associated with the alteration of task control and default mode networks, we examined performance monitoring function, using the Multisource Interference Task (MSIT, 16), and connectivity of the MFC in pediatric patients compared to healthy youth using functional magnetic resonance imaging (fMRI) and functional connectivity MRI (fcMRI). Based on previous work in adult OCD, we predicted pMFC and vMFC hyperactivation during performance monitoring would define areas with altered function and connectivity in task control (e.g., pMFC - LFC) and default mode (e.g., vMFC - PCC) networks. By studying pediatric OCD, we sought to characterize MFC function and connectivity as early markers of illness.
Methods and Materials
Participants
Subjects ranged in age from 8 to 18 years, and included 18 patients with pediatric OCD and 18 age- and gender-matched healthy youth (Supplement: Table S1). All subjects were evaluated using the Kiddie-Schedule for Affective Disorders-Present and Lifetime Version (17), the Multidimensional Anxiety Scale for Children (MASC, 18) and the Child Depression Inventory (19) and, for patients, the Children’s Yale-Brown Obsessive Compulsive Scale (CYBOCS, 20). Serious medical/neurological illness, head trauma, and mental retardation were not allowed. Among patients, comorbid diagnoses were separation anxiety disorder (n = 5), generalized anxiety disorder (n=1), anxiety NOS (n =3), depression NOS (n=2) and tics (n = 2). Patients with current or past major depressive disorder were excluded, yet, as seen in most clinical samples of pediatric OCD (21), subthreshold depressive symptoms were higher in patients than controls. CYBOCS scores indicated OCD symptom severity to be moderate at the time of scanning, but more severe in the past. As expected, patients reported higher levels of anxiety on the MASC than controls. Twelve patients were taking selective serotonin reuptake inhibitors (8 fluoxetine, 2 sertraline, 1 fluvoxamine, 1 citalopram), and 6 were treatment-naive. After complete description of the study to the subjects and their parents, written informed consent/assent was obtained.
Task
Participants performed the MSIT (16) which requires identification of the ordinal value of the unique number among three digits, “1”, “2”, or “3” (e.g., for “311,” the target is “3”) by pressing a key with one of three fingers (“1” for index finger, “2” for middle finger, “3”for ring finger). Interference was enhanced by presenting the target in a position incongruent with its ordinal value (e.g., “3” presented at the 1st position), and flanked by different numbers (e.g., “11”). In the congruent condition, target placement was compatible with its ordinal value (e.g., “1” presented in the first position) and flanked by zeroes (e.g., “100”). In contrast to the original blocked version of the MSIT (22), the task was adapted for an event-related design to allow for the separation of fMRI BOLD signal associated with correct incongruent, correct congruent and error trials. MSIT stimuli appeared for 500 msec, followed by a 2500 msec ISI (fixation cross), to comprise a trial. A total of 120 incongruent and 120 congruent trials were presented in pseudorandom order, intermingled with 60 fixation trials in which the 500 msec MSIT stimulus was replaced with a fixation cross (see Supplement: Figure S1). Trials were presented over five runs (3 minutes each). Subjects were trained on the task in a MR simulator and encouraged to either speed up (accuracy > 95%) or slow down (accuracy < 75%) to maintain individual error rates at approximately 10– 20% during the experiment.
fMRI Acquisition
Task
A 3.0 T GE Signa scanner was used to acquire an axial T1-image for alignment; a reverse spiral sequence (23) for T2* weighted images (GRE, TR = 2000ms, TE = 30ms, FA = 90, FOV = 20cm, 40 slices, 3.0mm/slice, 64×64 matrix); and, a high resolution T1 scan (3D SPGR, 1.5 mm slices, 0 skip) for anatomic normalization. Subject head movement was minimized through instructions to the participant and packing with foam padding.
Connectivity
fcMRI data was acquired using the same T2*sequence as above, over 8 minutes, for a total of 240 volumes, while cardiac and respiratory cycles were recorded. Subjects were instructed to keep eyes open and fixate on a white crosshair on a black background while “allowing the mind to wander”. A 9-year old female patient withdrew from the scanner before the completion of the resting state scan, leaving 17 age- and gender-matched pairs of OCD (14.1 +/− 2.6; 11F) and healthy youth (13.9 +/− 2.6; 11 F) for inclusion in the connectivity analyses.
Pre-processing
Functional data were sinc-interpolated, slice-time corrected (24), realigned to the first image acquired (“mcflirt”, 25), and thresholded to exclude extra-parenchymal voxels. Functional volumes were warped into common stereotactic space using the MNI152 template in SPM5 (26). Excessive movement (> 1 mm or degree on average, > 2 mm or degrees for any TR) led to the exclusion of several runs (1 run: 4 OCD, 2 Controls; 2 runs: 3 OCD, 1 Control). For fcMRI data, image reconstruction proceeded as above, followed by band pass filtering (0.01–0.1Hz) and the regression of physiological confounds and motion parameters from the time series.
Data Analysis
Behavioral
Accuracy and response times (RT) were entered as dependent measures in a two-way ANOVA using group (OCD vs. healthy) as the between-subjects factor and condition (incongruent vs. congruent) as the within-subjects factor. Average movement parameters for included runs were compared between groups using 2-sample t-tests (p < .05, 2- tailed).
Task
Functional data was analyzed using a standard random effects analysis within the framework of the modified General Linear Model (27) in SPM5. Correct incongruent, correct congruent, and commission error trials were modeled against fixation trials as implicit baseline. Omission trials were modeled as a covariate of no interest. Activation maps were derived for linear contrasts of interest, Incongruent – Congruent (correct trials only) and Error against implicit baseline (i.e., Fixation)1, including subjects with at least 5 commission errors across conditions (12). Contrasts were entered into “second order” random effects analyses to produce activation maps (28) that were examined within and between groups. To test a priori hypotheses concerning vMFC and pMFC function, we used search volumes defined by super-group (OCD plus healthy youth) activations (pMFC: −12, 24, 30; k = 2448 and vMFC: 0, 60, −9; k = 52). A whole brain search was also performed. Alpha thresholds were p < 0.05, correcting for false discovery rate (FDR, 29).
Connectivity
Task-related areas of group difference in the pMFC and vMFC were used as seed regions in psychophysiological interaction (PPI) analyses testing for task-dependent functional connectivity (30). A PPI variable was created for each subject by multiplying a “psychological” variable (representing the sequential ordering of incongruent and congruent stimuli)2 by mean seed region time series (i.e., physiological variable), and then regressing the PPI variable on times series for each voxel in the brain (30). The resulting voxel-specific r-coefficients were converted to Z-scores images and entered into random effects analyses testing for group differences at pFDR < 0.05.
For the resting state analyses, mean time-series from the pMFC and vMFC seeds were regressed on fcMRI data for the whole brain for each subject, followed by group analyses conducted in the same manner as above. To test a priori hypotheses regarding vMFC connectivity within the DMN, a PCC search volume (−6, −60, 12; k = 2164) was based on supergroup connectivity for the vMFC seed. Connectivity of the pMFC seed to the LFC was also hypothesized but, since we did not have specific predictions regarding laterality or LFC subregion, no search volume was used.
Correlations with Behavioral Measures
Contrast estimates for interference and errors, and Z-score measures of connectivity were extracted from areas of group difference and tested for associations with OCD symptom severity and performance. In analyses that collapsed across subjects, the relation between brain response and behavioral measures was tested while controlling for age, since age may impact both variables (31).
Results
Behavioral
Pediatric OCD and healthy youth were less accurate on incongruent than congruent trials (Table 1), with a significant effect of condition [F(1, 34) = 84.0, p < .001], but no effect of group (p = 0.74) and no interaction (p = 0.22). Response latencies were slower for incongruent than congruent trials, again with a significant effect of condition [F(1,34) = 495.0, p < .001], but no effect of group (p = 0.23) and no interaction (p = 0.93). There were no between-group differences for any motion parameter.
Table 1.
Interference: Activations and deactivations for pediatric OCD and healthy control subjects.
| OCD | Healthy Control | |
|---|---|---|
| Accuracy | ||
| Incongruent | 0.89 +/− 0.07% | 0.90 +/− 0.06% |
| Congruent | 0.99 +/− 0.02% | 0.98 +/− 0.05% |
| Response Times | ||
| Incongruent | 1098.8 +/− 270.7 | 1013.6 +/− 209.5 |
| Congruent | 799.5 +/− 219.1 | 712.0 +/− 154.4 |
Task
Interference
During interference-processing, pediatric OCD and healthy youth activated task control regions [e.g., pMFC, the inferior frontal gyri and anterior insula or “anterior operculum”, parietal cortex (13); Table 2, Figure 1A and B]. OCD patients activated a large MFC area, from the pMFC into the vMFC. In contrast, healthy controls deactivated (congruent > incongruent) the vMFC.
Table 2.
Activations and deactivations for OCD and healthy youth during interference
| Region (Brodmann Area) | OCD | Healthy | ||||
|---|---|---|---|---|---|---|
| Cluster | Coordinates | Z-Scorea | Cluster | Coordinates | Z-Scorea | |
| Activations | ||||||
| pMFC (32/6/8/9) | 1341 | −12, 21, 20 | 4.91 | 601 | −6, 12, 48 | 4.52 |
| 3, 36, 21 | 4.15 | −3, 33, 30 | 3.92 | |||
| 6, 30, 27 | 4.02 | 3, 30, 45 | 3.81 | |||
| rACC (32) | 3, 48, 9 | 3.44 | ||||
| vMFC (10) | 9, 45, −6 | 2.93 | ||||
| Bilateral Anterior Opercula (47/13/45) | 195 | −39, 24, −12 | 3.35 | 119 | −48, 6, 30 | 3.71 |
| −33, 18, 6 | 4.30 | |||||
| 90 | 51, 15, −3 | 3.20 | 108 | 45, 27, −9 | 3.43 | |
| 33, 21, 9 | 3.11 | 39, 18, −6 | 4.02 | |||
| Right Middle - Superior Frontal Gyri (9/10) | 212 | 27, 51, 27 | 3.78 | 222 | 36, 36, 27 | 4.11 |
| 24, 54, 18 | 3.66 | 42, 45, 27 | 3.55 | |||
| 30, 60, 15 | 3.26 | |||||
| Bilateral Parietal (40/7) | 1162 | 48, −57, 54 | 5.36 | 211 | 48, −39, 48 | 4.15 |
| −36, −60, 51 | 4.91 | 36, −63, 51 | 3.71 | |||
| 447 | −36, −51, 42 | 4.04 | ||||
| −57, −45, 36 | 3.78 | |||||
| Bilateral Occipital (17/18/19/23) | 1381 | 21, −90, 6 | 4.63 | 82 | 36, −96, −3 | 3.65 |
| −9, −96, 0 | 4.53 | 54 | −6, −96, −6 | 4.27 | ||
| Deactivations (Cong > Incong) | ||||||
| vMFC (BA 11) | 42 | 6, 57, −15 | 4.42b | |||
| Midline PCC/Precuneus (23/31) | 285 | 12, −60, 24 | 4.16c | |||
| −9, −57, 18 | 4.12 | |||||
pFDR < 0.05, whole brain search.
pFDR < 0.05, vMFC search volume.
Significant at cluster level (pcorr < 0.0001), but subthreshold for peak (pFDR = .14) in whole brain search.
Figure 1.
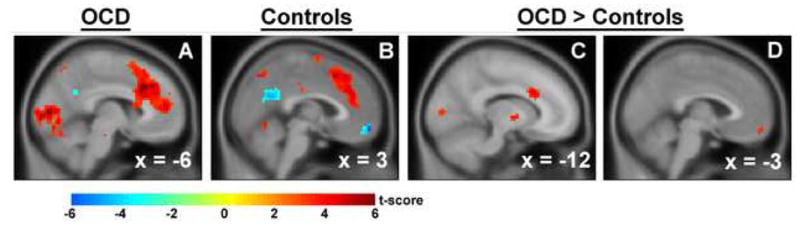
During interference, patients with pediatric OCD (A) and healthy subjects (B) activated the pMFC. Deactivation of the vMFC was also observed in healthy youth (B). Patients exhibited greater interference-related activation of the dACC (C) and the vMFC (D) than healthy youth.
Greater activations were exhibited by OCD than healthy youth in pMFC, specifically left dorsal ACC (dACC, −12, 21, 27; Z = 4.07; k = 43, Figure 1C), and vMFC (−3, 57, −15; Z = 3.29; k = 12, Figure 1D) search volumes. The vMFC group difference was driven by greater activity for congruent than incongruent trials in healthy youth, i.e., relative deactivation during interference, than in pediatric OCD. No significant group differences survived correction for multiple comparisons across the whole brain.
Errors
Fifteen OCD (10 F, 14.5 +/− 2.3 years) and 12 healthy youth (7 F, 14.9 +/− 2.0 years) exhibited sufficient error rates for inclusion in the contrast of error and fixation trials. As with interference, task control regions were activated in both groups (Table 3, Figure 2A and B). Similar to results from interference contrasts, healthy controls deactivated the vMFC, while patients did not.
Table 3.
Activations and deactivations for OCD and healthy youth during error-processing
| OCD | Healthy | |||||
|---|---|---|---|---|---|---|
| Region (Brodmann Area) | Cluster | Coordinates | Z-Score a | Cluster | Coordinates | Z-Score a |
| Activations | ||||||
| pMFC (32/6/8/9) | 381 | 0, 30, 30 | 4.17 | 880 | −6, 33, 33 | 4.87 |
| −9, 30, 36 | 3.82 | 3, 30, 42 | 4.84 | |||
| −9, 30, 48 | 3.78 | 3, 15, 45 | 4.30 | |||
| Bilateral Anterior Opercula (47/13/45) | 107 | 48, 12, −6 | 3.42 | 234 | 45, 21, −6 | 4.69 |
| 36, 12, 6 | 3.33 | 60, 15, 3 | 3.29 | |||
| 39, 24, −18 | 3.14 | 30, 30, −6 | 3.21 | |||
| 246 | −42, 12, 15 | 3.86 | 142 | 30, 36, 0 | 4.44 | |
| −30, 30, −6 | 3.63 | −33, 24, 0 | 3.58 | |||
| Right Lateral OFC (10) | 74 | 39, 48, −3 | 4.30 | 90 | 42, 54, 12 | 4.05 |
| Right Middle Frontal Gyrus (10) | 45, 48, 0 | 3.31 | ||||
| Right Middle Frontal Gyrus (9/8/6) | 177 | 39, 6, 54 | 4.58 | |||
| 45, 9, 48 | 3.79 | |||||
| 48, 24, 42 | 3.78 | |||||
| Left Middle Frontal Gyrus (9/8/6) | 298 | −48, 0, 42 | 4.26 | |||
| −54, 6, 27 | 4.20 | |||||
| −45, 30, 39 | 3.68 | |||||
| Right Superior Frontal Gyrus (10/9) | 93 | 30, 48, 33 | 3.55 | |||
| 21, 54, 30 | 3.51 | |||||
| Bilateral Parietal (40/7) | 240 | 42, −66, 51 | 3.90 | 258 | 42, −57, 48 | 4.21 |
| 54, −57, 39 | 3.80 | 36, −66, 54 | 4.15 | |||
| 80 | −57, −36, 45 | 3.31 | 557 | 48, −39, 54 | 4.36 | |
| −54, −39, 36 | 3.00 | −42, −45, 54 | 4.27 | |||
| Right Occipitocerebellum (18/17/30/19) | 1160 | 9, −81, −21 | 5.05 | 71 | 9, −81, −18 | 3.64 |
| 15, −69, −3 | 4.63 | 6, −69, −12 | 2.91 | |||
| Left Occipital (18) | 174 | −36, −81, −18 | 4.71 | |||
| Right Temporal Lobe (21) | 101 | 60, −33, −3 | 3.68 | |||
| 51, −30, −6 | 3.29 | |||||
| Left Thalamus/Caudate | 189 | −6, −12, 15 | 3.62 | |||
| −3, −21, 18 | 3.49 | |||||
| 6, −21, 24 | 3.45 | |||||
| Deactivations: Fix > Error | ||||||
| vMFC (11) | 20 | 3, 57, −15 | 3.66b | |||
| PCC/Precuneus (31) | 126 | 12, −57, 24 | 3.47c | |||
| −9, −57, 12 | 3.42 | |||||
| −6, −66, 24 | 3.32 | |||||
pFDR < 0.05, whole brain search.
pFDR < 0.05, vMFC search volume.
Significant at cluster level (pcorr = 0.004), but subthreshold for peak (pFDR = .52) in whole brain search.
Figure 2.
During error-processing, patients with pediatric OCD (A) and healthy subjects (B) activated the pMFC. Deactivation of the vMFC was also observed in healthy youth (B). Patients exhibited greater error-related activation of the vMFC (C) than healthy youth. Parameter estimates for error, incongruent and congruent trials relative to implicit baseline (i.e., fixation) were extracted from the vMFC for patients (shown in red) and controls (shown in blue); estimates were positive for patients (i.e., activation), but negative for healthy youth (i.e., deactivation) across both error and incongruent trials (D).
Greater activation was exhibited by OCD patients than healthy controls in the vMFC (0, 57, −12; Z = 3.57; k = 45, Figure 2C) search volume, driven by greater deactivation in controls than patients. A similar pattern was noted in the rostral ACC (rACC, 15, 39, −12; k = 29; Z = 3.40), but did not did not reach statistical significance. No significant group differences were observed in the pMFC search volume or in the whole brain search.
To further explore group differences in the vMFC, estimates for error, incongruent and congruent trials relative to implicit baseline (i.e., fixation trials) were extracted for patients and controls, revealing that the vMFC was deactivated by healthy youth, but activated by patients for both error and incongruent trials (Figure 2D).
Connectivity
pMFC
A pMFC seed region was defined based on the group difference (OCD > Controls) observed in the dACC during task. The PPI analysis showed greater functional coupling of this region within the vMFC search volume (−3, 63, −15; Z = 3.32, k = 19) during incongruent relative to congruent trials, driven by PPI beta estimates that were positive for OCD patients, but negative for controls (Figure 3). No group differences survived correction in the whole brain search.
Figure 3.
Psychophysiological interaction analyses revealed greater connectivity of the dACC to the vMFC during incongruent relative to congruent trials in OCD patients compared to controls (A). PPI beta estimates from the vMFC were positive for OCD patients, but negative for controls (B).
At resting state, the dACC showed significant connectivity with task control regions in both groups (Figure 4A and B), but patients exhibited a trend towards less connectivity with the right anterior operculum than controls (39, 36, 0; Z = 4.71; k = 141, pFDR = 0.09 for peak) that was significant for cluster, with whole brain correction (pcorr = 0.01, Figure 4C). No other significant group differences were observed in the whole brain search.
Figure 4.
Resting state connectivity of the dACC seed for patients (A) and controls (B). Compared to controls, patients exhibited reduced connectivity to the right anterior operculum (C); inset shows OCD patients in open circles (medicated) and red circles (unmedicated) on the left, and control subjects in gray circles on the right.
vMFC
The vMFC group difference during task was examined as a seed for PPI and resting state connectivity analyses. There were no significant findings for the PPI analysis. For the resting state analysis, the vMFC showed significant connectivity with the PCC in patients and healthy youth (Figure 5A and B), consistent with previously described “default mode” connections between these regions (15). Compared to controls, patients exhibited less vMFC connectivity within the PCC search volume (6, −42, 39, Z = 3.93, k = 88, Figure 5C) during resting state. No significant group differences were observed in the whole brain search.
Figure 5.
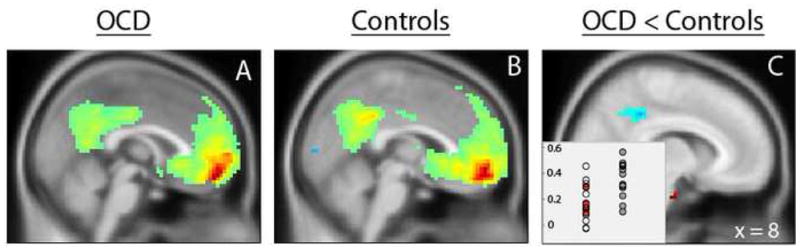
Resting state connectivity of the vMFC seed for patients (A) and controls (B). Connectivity of the vMFC to the PCC was reduced for patients compared to controls (C); inset shows OCD patients in open circles (medicated) and red circles (unmedicated) on the left, and control subjects in gray circles on the right.
Task and Connectivity Correlations with Behavioral Measures
Interference-related activation of the dACC correlated with higher error rates on incongruent trials across the combined group of subjects (r = 0.4, p = 0.03), after covarying for age, but there was no difference by group. There were no other associations of either task-related activations or measures of connectivity with performance, obsessive compulsive or depressive symptom severity. Scatter plots suggested that medication status did not differentiate patients or drive between-group results (Supplement: Figure S2; Figures 4 and 5, insets).
Discussion
Posterior (dACC, interference) and ventral (vMFC, interference and errors) areas of MFC hyperactivity during performance monitoring were used as seeds for connectivity analyses during task and resting state in patients with pediatric OCD compared to healthy youth. In patients, the dACC showed increased connectivity with the vMFC during task, and reduced connectivity with the right anterior operculum during resting state. Patients also exhibited a failure of task-related deactivations to interference and errors in the vMFC which, during the resting state, showed reduced connectivity to the PCC. Taken together, our findings point towards a novel approach for considering pMFC and vMFC involvement in the pathophysiology of OCD.
In the absence of differences on behavioral measures of performance, pMFC hyperactivation to interference in pediatric OCD, and in prior studies of adult patients (5–7), suggests reduced efficiency. Across our combined group of subjects, greater dACC activation was associated with higher error rates, consistent with the notion that increasing levels of pMFC engagement are required to maintain performance in OCD. Alternatively, the general drive towards pre-potent responding may be stronger in OCD, producing greater interference load. In either case, OCD patients might be able to maintain performance when faced with simple cognitive interference, but succumb to conflict from security drives (4) known to evoke pre-potent response tendencies even among the healthy individuals (32, 33).
A richer understanding of interference-related hyperactivation of the dACC in pediatric OCD derives from its occurrence in the context of abnormal engagement of the vMFC, and increased dACC-vMFC interactions. The dACC is typically activated during interference-processing (10, 34), while the vMFC typically deactivates during cognitively demanding tasks (35–37), as seen in our healthy subjects. In contrast, activation of the VMFC normally occurs during appraisal of emotional salience, including self-referential judgments (36), evaluation of internal emotional states (38), response to reward (39) and punishment (11), suppression of emotional conflict (40), and motivation for particular outcomes (41). Others have hypothesized that vMFC deactivation during cognitive tasks may reduce attention to internally-focused affective processes to facilitate response to external demands (15, 37), and recent work supports this notion by relating better task performance to greater vMFC deactivation (42–44). Although speculative, the atypical engagement of the vMFC in OCD may reflect increased emotional salience of cognitive stimuli during interference processing which, in turn, could require increased pMFC activity to maintain ongoing performance.
Alternatively, functional abnormalities during performance monitoring in pediatric OCD could stem from fundamental alterations in the dACC-based task control network, given the reduced resting state connectivity of the dACC to the right anterior operculum in patients. The dACC and operculum co-activate across a variety of tasks (13), remain connected during the resting state (13, 45), and may drive reciprocal interactions between the frontal-parietal network for executive control and the vMFC-PCC network for default mode function (46). In OCD, reduced connectivity of the dACC with the right anterior operculum could compromise dACC-based interference-processing function in association with the emergence of inappropriate vMFC activation during task.
Differential activity (OCD activating, healthy youth deactivating) contributed to the vMFC finding for the interference contrast, but also to significant group differences in the same location for the contrast of errors against implicit baseline, suggesting that a failure to deactivate ventral medial cortex may characterize both performance monitoring functions in pediatric OCD. A similar pattern of differential activation contributed to a subthreshold cluster in the rACC that bears comment given its location in the same region as in previous studies of adult OCD (6, 8) and its emergence, at lower thresholding, as a sub-peak within the broader vMFC region of group difference in our pediatric sample. We have previously theorized that error-related hyperactivation of the rACC in adults with OCD may reflect exaggerated affective response to errors (8, 47), however, a novel (and potentially complementary) interpretation derives from considering that rACC “hyperactivity” may represent a failure of the more broadly defined vMFC to deactivate during task. Alternatively, vMFC may be functionally dissociable from rACC (38), as supported by the contrast between our finding of vMFC deactivation in response to both interference and errors in healthy youth, and prior work showing less rACC deactivation as distinguishing errors from interference-processing (42). In addition, failure to confirm the vMFC group difference for errors compared to correct trials (see Supplement) prevents conclusions about “error-processing,” per se. Indeed, patients’ hyperactivation of the vMFC for errors compared with fixation may have been driven by interference, since the majority of errors occurred on incongruent trials.
Nonetheless, failure to deactivate the vMFC on incongruent and error, but not congruent trials suggests a critical alteration of the default mode network, originally defined by vMFC-PCC deactivations across a range of cognitively demanding tasks (37, 48). The possibility of DMN alteration in OCD gains additional support from the failure of task-related PCC deactivations during performance monitoring in our pediatric patients and in a prior study of adult OCD (6), as well as its reduced connectivity to the vMFC in our sample. Resting state correlations between the vMFC and PCC have been posited to mediate self-related, episodic memories during “stimulus-independent thought” (15, 36, 37, 49) – processes which must be suppressed to facilitate performance when external, cognitive demands are high (14, 42). The DMN has not been well-studied in OCD, but our results suggest that the alteration of its reciprocal interactions with dACC-based task control networks could relate to the intrusive, distressing thoughts that interrupt resting state consciousness in OCD and are difficult for patients to suppress.
Small sample size across a wide age range prevented us from testing the effects of age on fMRI or connectivity measures, yet our findings raise the possibility that altered development of canonical networks for task control and default mode function may contribute to pediatric OCD. In healthy youth, segregation between and integration within these networks increase gradually with age (50–52). In pediatric OCD, atypical engagement of the vMFC and increased dACC-vMFC interactions during task suggest a failure to segregate task control from default mode regions, while reduced resting state connectivity of the dACC to right operculum and vMFC to PCC suggest a failure to integrate within these respective networks.
Our experiment was not designed to address the specificity of altered MFC performance monitoring function or connectivity for OCD, however, the extant literature provides some relevant insight. At resting state, reciprocal interactions between task control and default mode networks are decreased in attention deficit hyperactivity disorder (53), but increased in schizophrenia (54), suggesting that relations between these networks may be differentially impacted across different mental disorders (55). Similar to our findings in patients with OCD, patients with autism fail to deactivate the vMFC during interference-processing (56), and show reduced connectivity of the DMN at resting state (56, 57), possibly contributing to the preoccupations that intrude on resting state consciousness in both disorders. In contrast, patients with MDD exhibit increased vMFC-PCC connectivity, potentially distinguishing the relationship between depression and the DMN (58). During performance monitoring, vMFC hyperactivity has been demonstrated in OCD (8), ASD (59), and MDD (60), while hypoactivation of the vMFC (61, 62) and the pMFC (63–65) occurs in attention deficit hyperactivity disorder (ADHD), suggesting that alternate patterns of MFC-based task control activity may differentiate internalizing and externalizing disorders. Contradictory reports (62) may stem from differences in the fMRI task used (61) and the impact of accuracy on MFC activation (31). Nevertheless, combining fcMRI with carefully designed fMRI studies may help to elucidate the role of task control and default mode network interactions in OCD compared to other psychiatric illness.
Limitations
Our failure to find an association between OCD symptom severity and task-related MFC hyperactivations represents a departure from some (5), (8), but not all (6, 7), previous studies of performance monitoring in OCD. Small sample size, low current symptom severity in some patients, and SSRI medication use may have limited power to detect a relationship between CYBOCs scores and functional brain alterations. The same factors may also have contributed to our failure to demonstrate pMFC hyperactivity to errors in pediatric OCD, contrasting with some (5, 6), but not all (8) prior work in adult patients. However, scatter plots of contrast estimates for key findings revealed little difference by patients’ medication status, and recent work suggests MFC-based performance monitoring is not altered by SSRI use (66) and remains hyperactive in pediatric OCD even after symptom remission (67). From the perspective of paradigm design, induction of more congruent errors would have enabled contrasts separating errors versus correct trials across incongruent and congruent conditions to more clearly distinguish the effect of interference from errors, while longer trial duration might have allowed for the temporal resolution of response preparation from evaluation (42). Finally, studies using larger samples, longitudinal designs and including at risk youth are needed to establish whether dysfunction of task control and default mode networks precedes OCD, occurs at its onset, or develops as a consequence of the disorder.
Conclusion
We observed pMFC hyperactivity to interference, atypical engagement of the vMFC to interference and errors, and altered connectivity between the dACC and vMFC during task, and the dACC-operculum and vMFC-PCC during rest in pediatric OCD. These findings suggest that altered function and connectivity in canonical networks for task control and default mode may represent early markers of OCD. Theoretically, such alterations could disrupt reciprocal interactions between these networks, potentiating the intrusion of vMFC-based affectively laden, self-referential thoughts while disrupting pMFC-based performance-monitoring function.
Supplementary Material
Acknowledgments
Drs. Fitzgerald and Taylor received funding from Dana Foundation (KDF), NARSAD (KDF), the Todd Ouida Clinical Scholars Award (KDF) and the NIMH (KDF: K23-MH082176, SFT: R01- MH071821)
Footnotes
Presented at the American Academy of Child and Adolescent Psychiatry, October 30, 2009 and the American College of Neuropsychopharmacology, Hollywood, FL, December 6, 2009.
An analysis of error compared to correct trials is included in the Supplement to facilitate comparison with some prior work (8)
The PPI analysis focused on interference (Incongruent vs. Congruent) rather than errors (Errors vs. Fix, or Errors vs. Correct), because the imbalanced number of error relative to fixation (or correct) trials precluded the construction of a mean-centered psychological variable, confounding the PPI (30).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baxter LR, Jr, Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, et al. Brain Mediation of Obsessive-Compulsive Disorder Symptoms: Evidence From Functional Brain Imaging Studies in the Human and Nonhuman Primate. Semin Clin Neuropsychiatry. 1996;1:32–47. doi: 10.1053/SCNP00100032. [DOI] [PubMed] [Google Scholar]
- 2.Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28:334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz JM. A role for volition and attention in the generation of new brain circuitry: toward a neurobiology of mental force. Journal of Conciousness Studies. 1999;6:115–142. [Google Scholar]
- 4.Szechtman H, Woody E. Obsessive-compulsive disorder as a disturbance of security motivation. Psychol Rev. 2004;111:111–127. doi: 10.1037/0033-295X.111.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- 6.Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 9.Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 10.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 13.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 15.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 18.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs M. Children’s Depression Inventory (CDI) manual. New York: 1992. [Google Scholar]
- 20.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 21.Geller DA, Biederman J, Faraone S, Agranat A, Cradock K, Hagermoser L, et al. Developmental aspects of obsessive compulsive disorder: findings in children, adolescents, and adults. J Nerv Ment Dis. 2001;189:471–477. doi: 10.1097/00005053-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- 23.Stenger VA, Boada FE, Noll DC. Multishot 3D slice-select tailored RF pulses for MRI. Magn Reson Med. 2002;48:157–165. doi: 10.1002/mrm.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre GK, Zarahn E, D’Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 26.Kang E, Lee DS, Kang H, Lee JS, Oh SH, Lee MC, et al. Age-associated changes of cerebral glucose metabolic activity in both male and female deaf children: parametric analysis using objective volume of interest and voxel-based mapping. Neuroimage. 2004;22:1543–1553. doi: 10.1016/j.neuroimage.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. Neuroimage. 1997;6:305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- 28.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 29.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 30.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald KD, Perkins SC, Angstadt M, Johnson T, Stern ER, Welsh RC, et al. The development of performance-monitoring function in the posterior medial frontal cortex. Neuroimage. 2010;49:3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salkovskis PM, Harrison J. Abnormal and normal obsessions--a replication. Behav Res Ther. 1984;22:549–552. doi: 10.1016/0005-7967(84)90057-3. [DOI] [PubMed] [Google Scholar]
- 33.Apter A, Fallon TJ, Jr, King RA, Ratzoni G, Zohar AH, Binder M, et al. Obsessive-compulsive characteristics: from symptoms to syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35:907–912. doi: 10.1097/00004583-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- 35.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 39.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 42.Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- 44.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 45.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- 48.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 49.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 55.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 58.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, et al. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 61.Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 62.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zang YF, Jin Z, Weng XC, Zhang L, Zeng YW, Yang L, et al. Functional MRI in attention-deficit hyperactivity disorder: evidence for hypofrontality. Brain Dev. 2005;27:544–550. doi: 10.1016/j.braindev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 65.Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 66.de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





