Summary
Herpes simplex virus-1 (HSV-1) establishes lifelong latency in peripheral neurons where productive replication is suppressed. While periodic reactivation results in virus production, the molecular basis of neuronal latency remains incompletely understood. Using a primary neuronal culture system of HSV-1 latency and reactivation, we show that continuous signaling through the phosphatidylinositol 3-kinase (PI3-K) pathway triggered by nerve growth factor (NGF)-binding to the TrkA receptor tyrosine kinase (RTK) is instrumental in maintaining latent HSV-1. The PI3-K p110α catalytic subunit, but not the β or δ isoforms, is specifically required to activate 3-phosphoinositide-dependent protein kinase-1 (PDK1) and sustain latency. Disrupting this pathway leads to virus reactivation. EGF and GDNF, two other growth factors capable of activating PI3-K and PDK1 but that differ from NGF in their ability to persistently activate Akt, do not fully support HSV-1 latency. Thus the nature of RTK-signaling is a critical host parameter that regulates the HSV-1 latent-lytic switch.
Introduction
The ability of herpes simplex virus to establish and maintain a life-long latent infection in peripheral neurons is fundamental to its survival and function as a human pathogen. Classically, the latent state is defined as the absence of infectious virus production despite the presence of episomal viral genomes in neuronal nuclei. Expression of the more than 80 ORFs encoded by HSV-1 is highly restricted in latently infected neurons (Knipe and Cliffe, 2008). The exception is a latency-associated RNA transcript (LAT) that accumulates to high levels in the neuronal nucleus. Several functions have been proposed for LAT, including the ability to modulate the chromatin state of the viral episome, inhibit apoptosis, and produce microRNAs that suppress lytic gene expression (Bloom et al., 2010).
Periodically, the virus changes its relationship with the neuronal host and reactivation from latency ensues, resulting in the coordinate expression of lytic genes and production of infectious virus that spreads back to the epithelium. A variety of conditions can promote reactivation, including exposure to UV light, stress, fever, anxiety and nerve trauma (Cushing, 1905; Glaser and Kiecolt-Glaser, 2005; Warren et al., 1940; Wheeler, 1975). While herpes reactivation following surgery on the trigeminal ganglion was first reported over a century ago, the mechanisms underlying latency and reactivation remain largely unknown.
Experiments using animal model systems have been instrumental in understanding latency (Wagner and Bloom, 1997). In addition to defining viral genes required for reactivation, these systems have revealed important roles for components of both innate and acquired immunity in modulating viral reactivation (Knickelbein et al., 2008; Leib et al., 1989a; Leib et al., 1989b; Thompson et al., 2009). At its core, however, latency involves a precisely tuned interaction between the virus and host neuron. Consequently, the intricate details of this relationship are difficult to tease out in animal models due to the confounding influence of non-neuronal cells types and the actions of immune defenses. Instead, a detailed molecular understanding of HSV-1 latency in neurons requires a cell culture model that utilizes a homogenous neuronal population that faithfully recapitulates the hallmarks of latency and reactivation.
Sympathetic neurons can be cultured as a pure population of cells that depend upon trophic support from nerve growth factor (NGF) or glial-derived neurotrophic factor (GNDF) (Glebova and Ginty, 2005). Indeed, latency can be established in primary sympathetic neurons cultured in the presence of NGF (Wilcox and Johnson, 1988; Wilcox et al., 1990; Wilcox, 1987). This agrees with studies in latently-infected rabbits showing that NGF-withdrawal can induce HSV-1 reactivation in sensory and sympathetic neurons in vitro or after anti-NGF treatment in vivo (Hill et al., 1997). Importantly, NGF stimulates a range of physiological responses in neurons including but not limited to differentiation, survival, inflammation, regeneration, cell cycle arrest and cell death by interacting with multiple cell surface receptors and triggering at least five independent signaling pathways. Surprisingly, since publication of the initial reports describing NGF-dependent latency, the specific NGF-responsive receptors and signal transduction pathways required to maintain latency and prevent reactivation have not been deciphered.
Here we have developed a simple, real-time readout for reactivation in living neurons and employed small-molecule chemical inhibitors along with gene-silencing techniques to determine the signaling components that control HSV-1 latency. Significantly, we find that a continuous neuronal signaling program mediated by NGF through the TrkA receptor, PI3-kinase p110α isoform, PDK1 and Akt is required to suppress HSV productive (lytic) growth and maintain latency. Disrupting this signaling pathway, even transiently, using selective small molecule inhibitors or shRNA-mediated gene silencing resulted in efficient reactivation. Moreover, these studies reveal that the duration of growth factor signaling to Akt is a critical parameter regulating latency in neurons. Specific growth factors therefore have different abilities to support latency and suppress lytic HSV-1 replication.
Results
To define the cellular requirements to sustain HSV-1 latency in neurons, we modified a primary neuronal cell culture model for establishing HSV-1 latency in vitro (Wilcox and Johnson, 1988; Wilcox, 1987), such that reactivation can be monitored in real-time. Dissociated superior cervical ganglia (SCG) neurons from E21 rat embryos were cultured with 50 ng/ml NGF in the presence of 5-fluorouracil and aphidicolin to remove non-neuronal cells. SCG neurons isolated in this manner resulted in sufficiently pure populations of neurons to enable a study of virus-neuron interactions without interference from other cell types.
Once established, these neuronal cultures were subsequently infected with HSV-1 (Fig. 1A). An otherwise wild-type HSV-1 strain expressing GFP fused to the Us11 true-late (γ2) protein served as a reporter to follow the lytic phase of the viral life cycle and allowed reactivation to be detected in living neurons (Benboudjema et al., 2003). Replicate wells of virus-infected neurons were treated with acyclovir (ACV) for up to six days to suppress lytic HSV-1 replication. At this point, ACV can be removed and the infected cultures maintained for weeks without the production of infectious virus as detected by plaque assay (Fig. 1B). Likewise, there was no detectable expression of mRNA encoding ICP27 (Fig. 1C, lane 2-3), a critical immediate-early (IE) regulator essential for productive replication, indicating that the virus had entered a non-replicating state. This was reinforced by the accumulation of LAT transcripts, which were readily detected by RT-PCR in SCG neurons (Fig. 1C), and reproducibly found in 20% of the neuronal nuclei (3 independent experiments, n = 1500) by in situ hybridization after ACV removal (Fig. 1D). Finally, accumulation of GFP-Us11, a reporter gene expressed late in the productive growth cycle, was also not detected (Fig 1E, top panels). The absence of (i) detectable infectious virus production, (ii) detectable productive lytic cycle gene expression and (iii) the concurrent accumulation of nuclear LATs are accepted hallmarks of latency in neurons (Knipe & Cliffe, 2008; (Bloom et al., 2010).
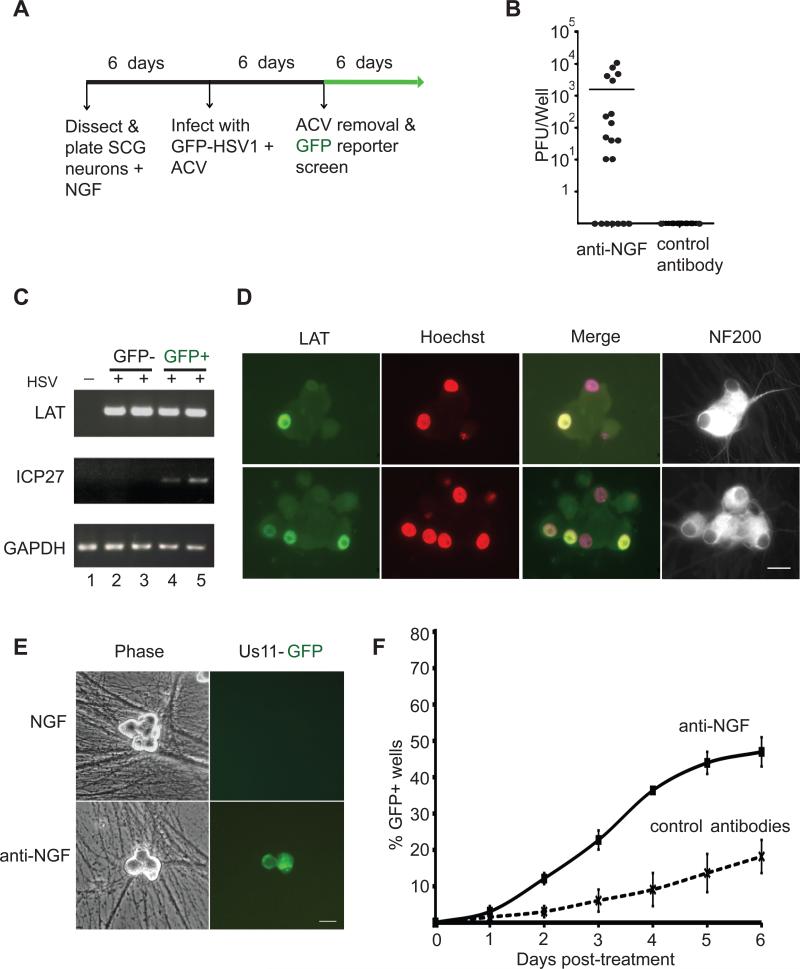
Fig 1. Establishment of a non-replicating HSV-1 infection in cultured SCG neurons.
(A) Protocol for establishment of HSV-1 infections in SCG cultures. Dissociated SCG were seeded in 96-well plates in media supplemented with 50 ng/ml NGF. After 6 d, cells were treated with 50 μM acyclovir (ACV), and 1 d later infected with HSV-1 EGFP-Us11 (MOI = 1 based upon titer on Vero cells). Infected cultures were maintained with ACV for an additional week to allow the virus to establish a non-replicating infection.
(B) Plaque assays were performed after 6 d of treatment with blocking antibodies against NGF or BDNF (control). Twenty individual wells were assayed for each condition. Bar show the average number of plaque forming units (PFU) per well.
(C) Detection of HSV-1 transcripts by RT-PCR. After establishing a non-replicating HSV-1 infection in the presence of NGF and ACV, the ACV was removed and GFP fluorescence monitored over a 7 d period. RNA was collected from two GFP-negative samples (lanes 2 and 3) representing latently-infected cultures and two GFP-positive samples (lanes 4 and 5) representing the small number of cultures that spontaneously reactivated in the absence of any treatment. Semi-quantitative RT-PCR was used to determine the expression of LAT, ICP27 and cellular GAPDH RNA transcripts. Lane 1, uninfected SCG culture.
(D) Accumulation of LAT in nuclei of latently-infected neurons. HSV-1-infections were established as described in (A). After ACV removal, neurons were fixed and probed with a combination of fluorescent in situ hybridization (FISH) and indirect-immunofluorescence. Viral LAT RNA transcripts were detected by RNA FISH [green] and nuclear DNA was visualized using Hoechst stain [red]. The merge of the RNA FISH and Hoechst signals highlights the latently-infected neurons [yellow]. Neurofilament protein NF200, a neuronal cytoplasmic marker, was detected by indirect immunofluorescence [white]. Bar = 20 μm.
(E) Detection of reactivating virus by fluorescent microscopy. Phase contrast and fluorescent images of representative clusters of latently-infected neurons. Reactivation was induced by NGF-deprivation, resulting in the expression of the HSV-1 EGFP-Us11 fusion protein as a true late (γ2) gene product. The image was captured on day 3 following NGF-depletion. Bar = 20 μm.
(F) Reactivation assay showing the percentage of wells containing GFP-positive cells detected by fluorescent microscopy of living neurons over a 6 d period after the removal of ACV and NGF. NGF was depleted using anti-NGF blocking antibodies and compared with anti-BDNF (control) antibodies.
Depletion of NGF using an anti-NGF antibody, resulted in productive viral replication (reactivation), evident from the production of infectious virus measured 6 days after adding anti-NGF (Fig. 1B), the selective accumulation of ICP27 mRNA in GFP-positive cultures (fig 1C), and late GFP-Us11 reporter expression which was readily detected after 1 - 2 days, and steadily increased up until day 6 (Fig. 1E, F). LATs were detected in all cultures even during productive viral growth (Fig 1C), consistent with studies showing that LAT expression is not limited to latently infected cells (Devi-Rao et al., 1991)(our unpublished data). Importantly, GFP-US11 reporter accumulation was routinely observed in approximately 10 to 20% of wells in each experiment, representing a baseline level of spontaneous reactivation. Taken together, these results indicate that NGF-depletion reproducibly activated expression of viral productive cycle genes in latently-infected neurons and thereby verified the reported requirement for NGF to suppress productive replication and maintain latency in cultured sensory neurons (Smith et al., 1994). Activation of productive cycle lytic genes in latently-infected neurons, culminating in the release of infectious virus, is the hallmark of HSV-1 reactivation from latency.
NGF-withdrawal results in apoptosis of SCG neurons (Deckwerth and Johnson, 1993) and it is conceivable that HSV-1 reactivation occurs through activation of a cell death pathway. To address this, a pan-caspase inhibitor, Z-VAD-fmk, was added to the cultures prior to NGF withdrawal. While the inhibitor effectively prevented cell death in response to NGF-depletion under these conditions (Fig. 2A), latently-infected SCGs reactivated to equivalent levels (Fig. 2B). In the absence of Z-VAD-fmk, GFP-positive cells induced by NGF-withdrawal displayed intact nuclei by Hoechst staining (data not shown). Thus, caspase-dependent apoptosis per se was not required for viral reactivation induced by NGF-deprivation.
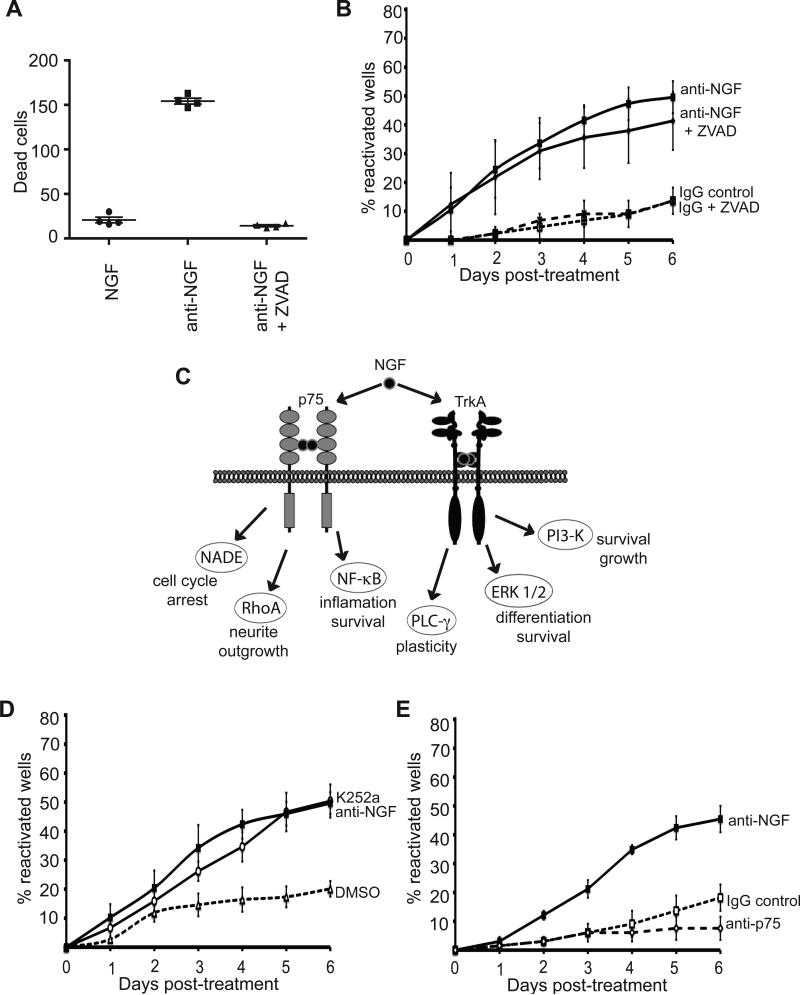
Figure 2. Inhibition of TrkA induces HSV-1 reactivation.
(A) Pan-caspase inhibitor Z-VAD-fmk does not prevent the reactivation after NGF withdrawal. GFP-HSV-1 infected SCG neuron cultures were treated with anti-NGF or control antibodies in the presence or absence of 20 μM Z-VAD-fmk. Plot shows the number of GFP+ wells, indicative of reactivated virus, scored over a 6 d period.
(B) Z-VAD-fmk was effective in blocking apoptosis. Cultured neurons were treated with anti-NGF antibody in the presence or absence of Z-VAD-fmk (20 μM). After 48 h, neurons were stained with Hoechst 33342 dye and scored for nuclear fragmentation indicative of apoptosis. Bar indicates mean (n = 200).
(C) Schematic showing signaling pathways activated by NGF. SCG neurons express two NGF receptors, p75 or TrkA and activate multiple pathways that give rise to different biological effects (Chao, 2003).
(D) Reactivation assay comparing treatments with the Trk inhibitor K252a (200 nM in DMSO), anti-NGF antibody (positive control) or DMSO alone. See also Figure S1.
(E) Reactivation assay comparing anti-p75 (9651) blocking antibody with anti-NGF and IgG control antibodies.
Latency requires NGF-TrkA signaling
Next we began to explore the mechanism(s) by which NGF suppressed lytic replication and maintained latency. NGF interacts with two receptors, the TrkA receptor tyrosine kinase (RTK) and the p75 neurotrophin receptor (Fig. 2C). The earlier in vitro studies were carried out prior to the identification of TrkA as an NGF receptor (Kaplan et al., 1991; Wilcox and Johnson, 1988; Wilcox et al., 1990) and before the multiple NGF signaling pathways were defined; consequently little information is available on the role of individual NGF receptors in controlling HSV-1 latency. A large body of work has established that NGF signaling through the Trk and p75 receptors is remarkably complex and capable of triggering at least five major signaling pathways that orchestrate diverse physiological responses (Chao 2003).
To address the receptor requirements for NGF-dependent latency, infected SCG cultures were treated with the pharmacological agent K252a (Fig. 2D), at a concentration (200 nM) that selectively blocks Trk receptors (Fig. S1), but not other RTKs (Berg et al., 1992). Addition of K252a resulted in reactivation levels and kinetics similar to those observed upon NGF-depletion using anti-NGF antibody. To test whether the p75 receptor participates in HSV-1 reactivation, cells were treated with the anti-p75 antibody (9651), which blocks NGF binding to the receptor and consequently ablates downstream signaling (Huber and Chao, 1995; Skoff and Adler, 2006). Reactivation was not detected in latently-infected SCGs treated with 9651 (Fig. 2E). Taken together, these results indicate that reactivation of latent HSV-1 upon NGF-depletion specifically involved TrkA, but not p75. Moreover, the results suggest that signals emanating from the NGF-bound TrkA receptor are required to suppress lytic replication and maintain latency.
Requirement for PI3-kinase to suppress reactivation and maintain latency
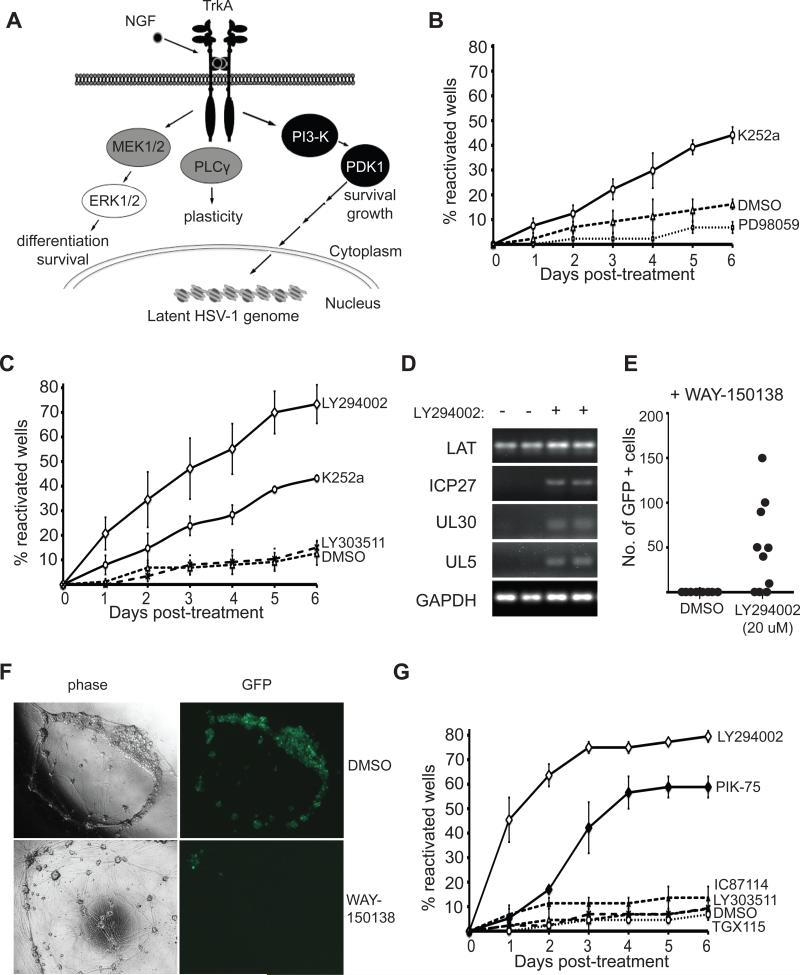
Binding of NGF to the TrkA receptor can activate the mitogen-activated protein (MAP) kinase pathway, phospholipase Cγ and phosphatidyl inositol-3-kinase (PI3-K) (Fig. 3A). To determine which of these pathways were required to maintain latency, we first treated sympathetic cultures with a panel of well-characterized chemical inhibitors that have been used previously to examine TrkA signaling in sympathetic neurons (MacInnis and Campenot, 2002; Ye et al., 2003). While PD98059 inhibited MAP kinase kinase (MEK), and consequently blocked ERK activation in these neuronal cultures (Fig. S2A), reactivation was not detected compared to cultures treated with the TrkA inhibitor K252a (Fig. 3B). Importantly, inhibition of lytic replication by PD98059 was not observed in acutely infected SCG neuron cultures, indicating that ERK activity was not required for the productive cycle of HSV-1 replication (Fig. S2BC). To determine whether PI3-K signaling contributes to the maintenance of latency in neurons, cultures were treated with the broad specificity PI3-K inhibitor LY294002. Remarkably, while inhibiting ERK activation did not induce reactivation, the PI3-K inhibitor LY294002 resulted in robust reactivation, with a greater fraction of wells showing reactivation than with the TrkA inhibitor K252a (Fig. 3C). The effect of LY294002 was specific because LY303511, a close structural analog of LY294002 that does not inhibit PI3-K, did not result in detectable HSV-1 reactivation (Fig. 3C). The ability of LY294002 to block PI3-K signaling was readily demonstrated by monitoring phosphorylation of a downstream target (Fig. S2D).
Figure 3. PI3-K is required in latency.
(A) Schematic of divergent TrkA-mediated signaling pathways. Molecules that were tested and found to be either required (black), or not required (gray), for maintenance of latency are highlighted. Shc, Src homologous and collagen-like adaptor protein; Grb2, growth factor receptor-bound protein 2; Gab1, Grb2-associated binder-1; PI3-K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide-dependent kinase 1; MEK, mitogen-activated protein kinase (MAPK)/ERK kinase; PLCγ, phospholipase Cγ; Trk, tropomyosin-related kinase receptor, NGF, nerve growth factor.
(B) Reactivation assay comparing the response of GFP-HSV-1 infected SCG neurons treated with MEK inhibitor PD98059 (50 μM) to Trk inhibitor K252a (200 nM) or vehicle control DMSO. See also Figure S2 A-C.
(C) Reactivation of HSV-1 after 6 d of treatment with the PI3-K inhibitor LY294002 (10 μM), LY303511 (an inactive analogue of LY294002; 10 μM), the Trk inhibitor K252a (200 nM) or DMSO. See also Figure S2 D.
(D) Detection of HSV-1 transcripts by RT-PCR. After establishing a non-replicating HSV-1 infection in the presence of NGF and ACV, the ACV was removed and the cultures were treated with LY294002 or DMSO. The RNA was collected 20 h later. Semi-quantitative RT-PCR was used to determine the expression of LAT, ICP27, UL30, UL5 and cellular GAPDH RNA transcripts.
(E) Number of GFP+ neurons after 48 h of treatment with LY294002 or vehicle DMSO in the presence of WAY-150138 (20 μg/ml). Ten wells were scored for each condition and data points indicate the number of individual GFP+ cells in a well containing 103 SCG neurons.
(F) WAY-150138 prevents HSV-1 spread in SCG neurons. Cultures were infected with HSV-1 Us11-GFP at a MOI of 0.1 in the presence of WAY-150138 or DMSO and the spread of virus visualized by GFP fluorescence after 72 h.
(G) Selective inhibition of the PI3-K catalytic subunit p110α leads to reactivation. Infected cultures were treated with the PI3-K inhibitor LY294002, LY303511, PIK75 (PI3-K p110α inhibitor, 0.116 μM), TGX115 (PI3-K p110βδ inhibitor, 2.6 μM), IC87114 (PI3-K p110δ inhibitor, 2.6 μM), or DMSO.
Lytic gene transcription occurs within hours of inhibiting the PI3-kinase pathway
Although Us11-GFP fluorescent protein provides a convenient real time marker for HSV-1 reactivation, it relies on the accumulation of sufficient protein quantities for detection by live fluorescent imaging. This likely contributes to the gradual increase in positive wells in the time courses. As an alternative, we prepared RNA from infected cultures (in duplicate) collected 20 h after exposure to LY294002 and performed RT-PCR to detect representative IE (ICP27), early (UL30 and UL5) lytic transcripts (Fig. 3D). As expected LAT RNA was readily detected before and after LY294002 treatment, whereas the lytic genes were only detected after addition of the inducer.
To evaluate the number of neurons undergoing independent reactivation events we pre-treated cultures with WAY-150138, a compound that specifically blocks viral spread by preventing encapsidation of the viral DNA genome (Newcomb and Brown, 2002; Pesola et al., 2005; van Zeijl et al., 2000). Infected sympathetic neuron cultures were treated with WAY-150138 and reactivation induced with LY294002. Small but significant numbers of GFP-positive neurons could be detected in 70% of wells indicating that a number of independent reactivation events occur per individual culture (Fig. 3E). Presumably some or all of these reactivation events give rise to infectious virus that spreads to neighboring cells. This provides a basis for scoring the number of GFP positive wells rather than individual cells. The effectiveness of the compound in preventing the spread of virus in cultured SCG neurons was addressed by performing a lytic infection at a MOI of 0.1 and by visualizing the infected neurons by fluorescence microscopy (Fig. 3F). After 72 h, the majority of neurons expressed GFP but in the presence of WAY-150138 only the cluster of neurons that were initially infected were GFP positive.
Subunit specific PI3-kinase signaling suppresses HSV-1 reactivation
The PI3-K holoenzyme comprises an 85-KDa regulatory subunit partnered with one of three catalytic subunits (p110 α, β, and δ), each of which is expressed in sympathetic neurons (Bartlett et al., 1999). LY294002 is a broad-spectrum inhibitor capable of antagonizing all PI3-K p110 isoforms, but small molecule inhibitors selective for each isoform have also been characterized (Feldman et al., 2005; Knight et al., 2006). Latently-infected cultures were treated with three of these inhibitors: TGX115, a selective inhibitor of p110β and p110δ, IC87114 selective for p110δ and PIK75, an inhibitor of p110α. Surprisingly, treatment with p110α-selective inhibitor PIK75 resulted in substantial reactivation that was nearly as efficient as LY294002 (Fig. 3D). In contrast, treatment with the p110β and p110δ inhibitors TGX115 and IC87114 did not result in reactivation (Fig. 3D). Thus the catalytic activity of the PI3-K p110α subunit is most critical for maintaining latent HSV-1 in cultured sympathetic neurons.
Depletion of PDK1 with shRNAs results in HSV-1 reactivation
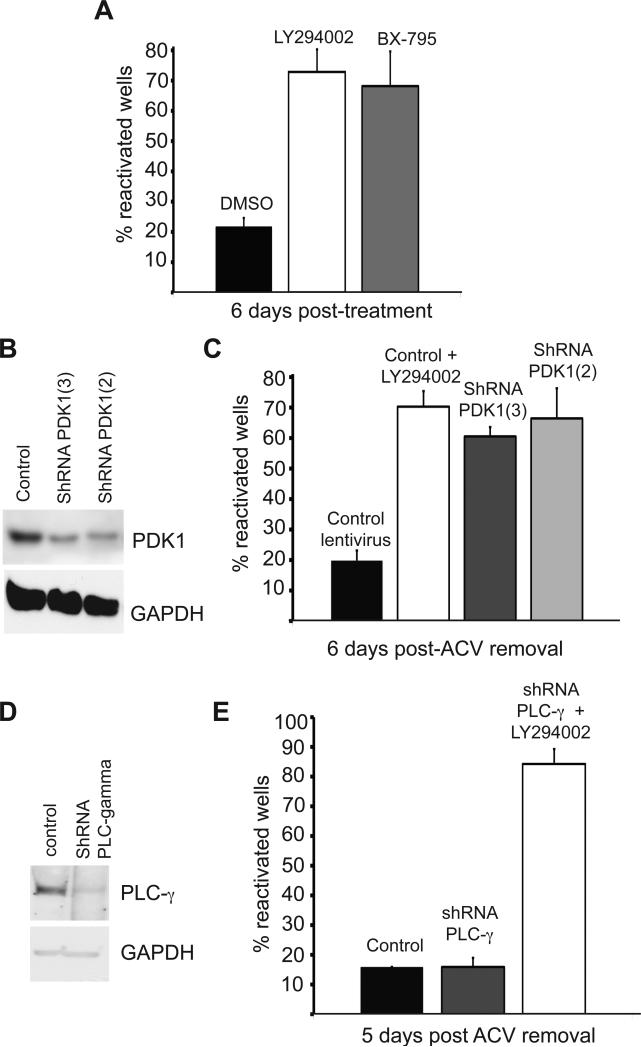
Activation of PI3-K stimulates phosphatidylinositol phosphorylation and leads to the recruitment of 3-phosphoinositide-dependent protein kinase-1 (PDK1) to the plasma membrane. We examined the involvement of PDK1 in maintaining latency, using BX-795, a pyrimidine-derivative that inhibits PDK1 by competing for the ATP-binding pocket of the catalytic site (Feldman et al., 2005). BX-795-treatment resulted in levels of reactivation similar to those induced by LY294002 (Fig. 4A). Again, inhibition could be readily demonstrated by monitoring phosphorylation of a downstream substrate (Fig. S3).
Figure 4. PDK-1 is required in latency.
(A) Inhibition of PDK1 induces reactivation. Reactivation in the presence of BX-795 (PDK1 inhibitor, 1 μM), LY294002 or vehicle control DMSO. See also Figure S3.
(B) Depletion of PDK1 was verified by immunoblot analysis of lysates prepared after 6 d of infection with each lentivirus. GAPDH served as a loading control.
(C) Depletion of PDK1 using shRNAs promotes reactivation. Following ACV removal, GFP-HSV-1 infected cultures were infected with two different lentiviruses expressing an mCherry fluorescent marker and shRNAs against rat PDK1. Cultures infected with a control lentivirus in the presence or absence of LY294002 acted as positive and negative controls respectively. Number of wells expressing GFP was scored after 6 d.
(D) Depletion of PLCγ was verified by immunoblot analysis of lysates prepared after 5 d of infection with each lentivirus. GAPDH served as a loading control.
(E) Depletion of PLCγ using shRNAs does not induce or prevent reactivation. Following ACV removal, cultures harboring latent GFP-HSV-1 were infected with a control lentivirus or with a lentivirus expressing an shRNA against rat PLCγ. Addition of LY294002 acted as a reactivation control. Number of wells expressing GFP was scored after 5 d.
Next the requirement for PDK1 was confirmed using RNA interference, an independent approach that does not rely upon chemical inhibitors. PDK1 was depleted using shRNAs expressed from a pLVTHM lentiviral vector (Fig. 4B) that had been modified to express mCherry thereby allowing lentiviral infection and HSV-1 reactivation to be monitored simultaneously in live cells. Infection with two different PDK1 shRNA lentiviruses successfully depleted endogenous PDK1 protein levels and significantly, resulted in reactivation at levels comparable to LY294002 (Fig. 4C). Parallel infections with a control lentivirus did not induce reactivation unless neurons were treated with LY294002, confirming that coinfection with a lentivirus does not have a detectable effect on HSV-1 latency or reactivation.
We also tested a lentivirus expressing shRNA to phospholipase Cγ (PLCγ), an independent arm of TrkA signaling (Fig. 3A). While PLCγ levels were reduced significantly by the shRNA (Fig. 4D), no increase in HSV-1 reactivation was detected (Fig. 4E). Cultures treated with PLCγ shRNAs were still capable of reactivation in response to LY294002 (Fig. 4E), demonstrating that PLCγ was not required for productive replication. Thus, loss of the PLCγ from NGF-TrkA signaling is not sufficient to reactivate latent HSV-1. This result also strengthens the observations made with the PDK1 shRNAs by showing that the methodology does not necessarily give rise to reactivation. Taken together, these findings show that specifically interrupting the PI3-K signaling pathway either by inhibiting PDK1 activity or by selectively depleting PDK1 protein using shRNA resulted in efficient reactivation. Moreover, these experiments clearly demonstrate that shRNAs can provide an effective tool to study HSV-1 latency.
Differential ability of growth factors to support HSV-1 latency
NGF is not alone in its ability to bind its receptor and trigger PI3-K-mediated signaling. Indeed, it is surprising that a relatively ubiquitous RTK-linked signal pathway component such as PI3-K would be involved in suppressing HSV-1 lytic replication and maintaining latency. This raises the intriguing possibility that other growth factors that act through the PI3-kinase pathway and are expressed in SCG neurons, such as EGF and GDNF, might also regulate HSV-1 latency.
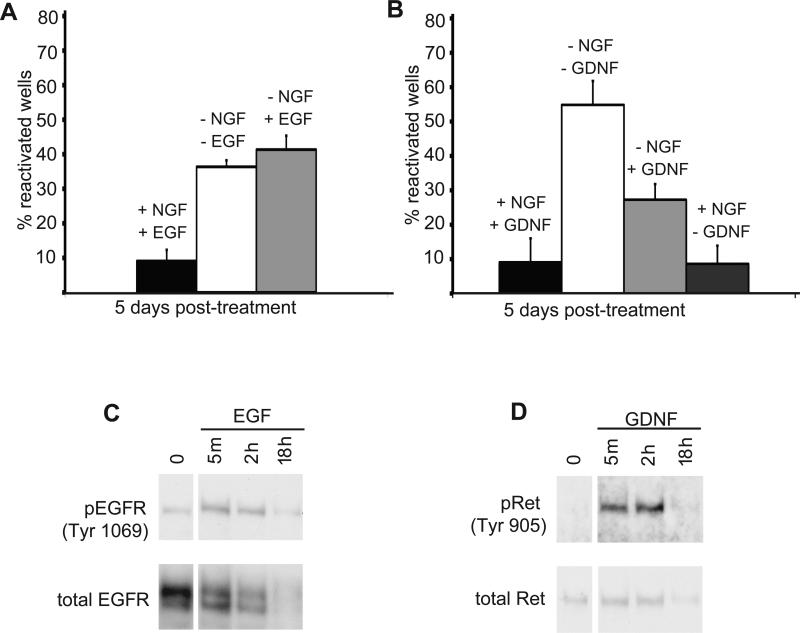
To address this, SCG neuron cultures were established and maintained in media containing either NGF and EGF, or NGF and GDNF (each at 50 ng/ml). Latent HSV-1 infections were then established in each culture and assayed for reactivation using blocking antibodies to individual growth factors. Removal of NGF resulted in reactivation regardless of the presence or absence of EGF (Fig. 5A). In contrast, inclusion of GDNF resulted in smaller numbers of GFP+ wells suggesting that GDNF has some capacity to maintain latency after NGF-depletion (Fig. 5B). Removal of both NGF and GDNF was required to achieve maximal reactivation in cultures established and maintained in the presence of both factors. The differential ability of EGF and GDNF to maintain HSV-1 latency was not due to lack of RTK activity, since both factors stimulated their respective receptors, EGFR and c-RET (Fig. 5C, D). Thus, despite their ability to bind ligand and stimulate RTK-signaling via a PI3K-dependent pathway, NGF, EGF, and GDNF differed in their ability to suppress lytic replication and maintain HSV-1 latency in neurons.
Figure 5. Differential effects of growth factors on HSV-1 reactivation.
(A) EGF does not substitute for NGF in maintaining latency. GFP-HSV-1 latently infected SCG neuron cultures were established in the presence of NGF and EGF (50 ng/ml each), washed and then cultured for 5 d in media containing NGF (filled bar), EGF and anti-NGF antibody (shaded bar) and anti-NGF antibody only (open bar).
(B) GDNF can partially substitute for NGF. As in A, except that neurons were cultured in media containing NGF and GDNF (50 ng/ml). Neurotrophins were removed by addition of either anti-NGF or anti-GDNF receptor antibodies.
(C) and (D) Cultured SCG neurons express functional receptors for EGF and GDNF. Neurons cultures were maintained for 6 h in media lacking growth factors and treated with either EGF or GDNF (50 ng/ml each) and lysates prepared after 5 min, 2 h or 18 h. Levels of activated receptor were detected by immunoblotting using antibodies to phospho-EGFR (C) and phospho-RET, the GDNF receptor (D). Down-regulation of the receptor (shown here at 18 h) after stimulation has been reported previously (Beguinot et al., 1984; Pierchala et al., 2006; Richardson et al., 2006; Stoscheck and Carpenter, 1984).
Duration of Akt activation is critical to maintain latency in neurons
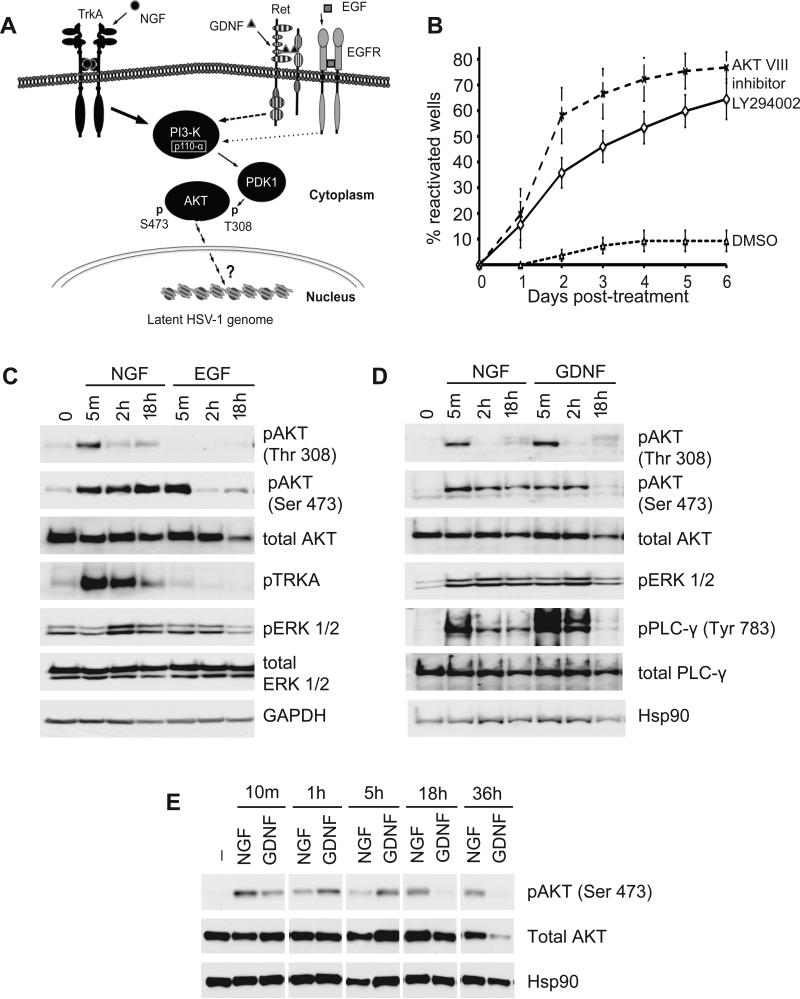
The serine/threonine kinase Akt represents a key component of the PI3-kinase pathway and regulates fundamental cellular processes such as apoptosis and protein synthesis. Because Akt is a prominent substrate for PDK1-mediated phosphorylation, we treated latently infected neurons with AKT inhibitor VIII, a cell permeable allosteric inhibitor of Akt (Calleja et al., 2009), in the presence of NGF (Fig. 6B). Treatment with the inhibitor resulted in robust activation with 60% of wells scoring positive for GFP in 2 days. The ability of this compound to prevent activation of Akt as measured by phosphorylation at serine-473 was confirmed by immunoblotting (Fig. S4). This result demonstrates that activation of Akt is necessary to maintain latent HSV-1 in sympathetic neuron cultures.
Figure 6. NGF, EGF, and GDNF activate equivalent downstream pathways in cultured sympathetic neurons but with different kinetics.
(A) Sustained RTK-mediated activation of Akt is required to maintain HSV-1 latency. GDNF and EGF signal through the Ret and EGFR tyrosine kinase receptor, respectively and like NGF-TrkA, they activate PI3-K-PDK1-Akt, albeit with different kinetics. The ability of different growth factors to maintain latency is directly proportional to the duration of PI3-K signaling. NGF induces prolonged PI3-K signaling to maintain latency. GDNF has an intermediate ability to sustain the PI3-K pathway and latency. Finally, EGF provides a transient signal and is unable to maintain latency. In black are elements of the pathway tested that maintain latency. PI3-K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide-dependent kinase 1; Trk, tropomyosin-related kinase receptor, NGF, nerve growth factor, Ret, Ret tyrosine kinase receptor; EGFR, epidermal growth factor receptor.
(B) Reactivation of latent HSV-1 after treatment with AKT inhibitor VIII (5 mM for 16 h), PI3-K inhibitor LY294002 (10 mM) or DMSO. See also figure S4.
(C) and (D) Kinetics of growth factor signaling in SCG neurons. Cultures (4×105 cells/lane) were maintained for 6 h in media lacking growth factors and then supplemented with NGF, EGF or GDNF (50 ng/ml each). Lysates were prepared after 5 min, 2 h or 18 h and analyzed by immunoblotting to detect phosphorylated forms of TrkA, AKT, ERK and PLCγ. NGF treatment was compared to EGF (A) or GDNF (B). GAPDH and Hsp90 serve as loading controls.
(E) Profile of Akt phosphorylation by NGF and GDNF. Cultures with supplemented with NGF or GDNF and harvested after 10 min, 1 h, 5 h, 18 h and 36h. Lysates were analyzed by immunoblotting with antibodies against Akt phospho-Ser-473 and total Akt. In the absence of trophic support neurons begin to die at around 36 h as indicated by decreased levels of Hsp90. Note that the NGF 5 h sample is slightly under-loaded.
The differential ability of NGF, EGF and GDNF to maintain latency cannot be explained by a simple lack of receptor expression or PI3-K activity and suggests that the duration of signaling might be more important. Therefore, the kinetics of growth factor signaling in sympathetic neurons was examined. We focused on two key phosphorylation sites on Akt: threonine-308 (T308), a major PDK1 substrate and serine-473 (S473), a target for phosphorylation by mTORC2, both of which are accepted indicators of Akt activation. Uninfected cultures of SCG neurons were treated with each growth factor and lysates were prepared after different time intervals and analyzed by immunoblotting. As shown in Fig. 6C and D, each growth factor produced a strikingly different profile. In the presence of NGF, Akt was rapidly phosphorylated on T308 and remained phosphorylated at S473 over the 18 h time period, whereas EGF gave only a short-lived increase in phosphorylation at S473 and no detectable phosphorylation at T308, even at the shortest time point. These responses indicated that NGF and EGF can both activate Akt, but do so with very different kinetics as measured by phosphorylation on T308 and S473.
Treatment with GDNF showed an intermediate profile, with a very similar profile to NGF at 2 h but differed at 18 h when the phospho-S473 signal had returned to background levels. To address this further, we performed a second time course analysis choosing additional time points at which to compare phosphorylation at S473 in the presence of NGF or GDNF (Fig. 6E). As before, both growth factors gave a similar profile at early times but differed significantly at 18 h and 36 h. The inability of GDNF to activate Akt (phospho-S473) for long periods is consistent with its reduced ability to support HSV-1 latency in neuron cultures. Taken together, these results argue that differential ability of individual growth factors to maintain latency and suppress HSV-1 reactivation is directly related to their differing abilities to provide sustained signaling through PI3-K and Akt.
Discussion
The remarkable ability of HSV-1 to stably colonize and periodically reactivate from peripheral neurons is well-accepted, but the cellular and molecular mechanisms responsible for maintaining life-long latency punctuated by episodic reactivation remain enigmatic. The underlying disparity in our understanding of latency compared to the productive replication cycle largely reflects the absence of a tractable experimental system to ask mechanistic questions about fundamental interactions between the virus and host neuron.
Here we describe a modified primary neuron cell culture system capable of supporting a stable, non-productive HSV-1 infection that exhibits key hallmarks of latency, including nuclear LAT accumulation and the absence of detectable lytic gene expression. Lytic reactivation in live neurons can be scored in real-time using a GFP-reporter virus and the cultures are amenable to chemical or biological manipulations, permitting mechanistic studies. Significantly, we have found that continuous signaling through the canonical PI3-Kinase (PI3-K) pathway triggered by NGF binding to the TrkA receptor was instrumental in maintaining HSV-1 latency in primary neurons. PI3-K p110α catalytic subunit activity, but not the alternative β or δ isoforms, was specifically required to suppress lytic replication and sustain latency. Surprisingly, not all growth factors capable of stimulating PI3-K signaling were equally effective at supporting HSV-1 latency, and the ability to activate Akt in a sustained manner appears to be a critical parameter.
The importance of continuous PI3-K signaling in maintaining latency highlights the role of the host neuron and cell-type specific signal pathways. While this does not diminish the contribution of the host innate and acquired immune responses to suppress reactivation in disease pathogenesis (Knickelbein et al., 2008), or the potential for LATs to suppress lytic IE gene expression (Garber et al., 1997), it directly demonstrates that fundamental features of latency can be reconstituted by infecting pure neuronal cultures with HSV-1 and illustrates that a pivotal neuron-specific signal transduction pathway is a critical regulator of the virus. Importantly, these findings suggest that neuronal targets of PI3-K/Akt signaling are the likely cellular effectors responsible for maintaining latency. Alterations to these cellular targets may transmit the initial reactivation signal(s) to the repressed viral genome. Prolonged signaling through the PI3-K/Akt axis could conceivably maintain critical aspects of the latent state, including nuclear LAT accumulation, viral microRNA production, cytoplasmic HCF-1 localization, and maintenance of the viral genome in repressive chromatin state (Umbach et al., 2008; Kristie et al, 1999; (Bloom et al., 2010). Alternatively, other cellular functions known to be regulated by PI3-K/Akt, such as cap-dependent translation, may emerge as important regulators. The cell-type dependent expression of receptors such as TrkA that display the appropriate PI3-K/Akt activation profile are likely to be a critical determinant that limits latency to peripheral neurons. Future studies using this neuronal culture system will determine which parameters are most relevant to latency.
Signaling through the PI3-K pathway is emerging as part of a general mechanism to control the replication of a number of important viruses (Buchkovich et al., 2008). For example, activation of the PI3-K pathway by the Epstein-Barr virus latent membrane protein 2A (LMP2A) promotes the survival of the host lymphocyte and prevents EBV reactivation (Morrison et al., 2003; Swart et al., 2000). Along similar lines, recent work with the Kaposi's sarcoma-associated herpesvirus has shown that inhibition of PI3-K signaling facilitates reactivation from latency (Peng et al., 2010). This study shows for that differences in the duration of PI3-K-mediated Akt activation by RTK-signaling directly correlate with the ability to maintain HSV-1 latency. Differential outcomes resulting from NGF compared to EGF signaling have also been reported in uninfected cultured cells including PC12 cells (Chao, 1992). Furthermore, related strategies relying on differential signal strength and duration dictate decisions in BDNF-induced neuronal branching and plasticity, lineage commitment in the immune system, differentiation and development (Calao et al., 2008; Hogquist, 2001; Ji et al., 2010; Murphy and Blenis, 2006; Schmierer and Hill, 2007).
While much has been learned from studying latency in small animal models, each model present challenges when it comes to identifying the molecular processes that regulate latency. There is compelling evidence, for example, that both the innate and acquired immune responses contribute to the control of latency at the whole animal level (Khanna et al., 2003). From an investigative standpoint, this imposes a substantial layer of complexity that can confound studies of the intimate virus-neuron relationship. Pharmacological inhibitors that target pathways within the neuron may alter the behavior of immune cells and regulatory factors that control the virus within a neuron may be essential for immune function or even the viability of the experimental animal. By utilizing a pure population of neurons that can be readily manipulated, it becomes easier to interpret studies of the discrete interactions between the virus and its host cell. By the same logic, defining the spectrum of virus-neuron interactions will ultimately highlight the unique contributions of the immune response.
While the mechanistic connections between natural reactivation cues such as exposure to UV radiation, physical trauma and psychological stress and the PI3-kinase signaling pathway have yet to be established, there are clear indications that ablation of NGF signaling can be a potent reactivation stimulus in vivo. Firstly, anti-NGF treatment in humans and latently-infected rabbits stimulates reactivation and is associated with aggravated herpetic keratitis, the leading cause of infectious blindness (Lambiase et al., 2008; Mauro et al., 2007). Secondly, surgical axotomy (retrogasserian rhizotomy) for the treatment of trigeminal neuralgia is a known inducer of HSV-1 reactivation in humans and can be reproduced in experimental animals (Carton and Kilbourne, 1952; Cushing, 1905; Walz et al., 1974). NGF is normally taken up at nerve terminals and transported in a retrograde manner to the cell body located in the ganglia (Ginty and Segal, 2002). Severing of nerve axons blocks transport of NGF-receptor complexes from the periphery to the cell body and is approximated in our system by addition of anti-NGF antibodies to the culture media.
In addition to using selective chemical inhibitors to target specific pathways, we have shown that host gene involvement can be queried by shRNA-mediated silencing. Future application of genome-wide shRNA screening techniques could potentially define neuronal genes required to maintain latency or transition to productive replication. Conversely, a battery of stimuli or small molecules can be tested for their ability to provoke reactivation in the presence of NGF. Other pathways capable of controlling reactivation independent of PI3K-signaling may thus be revealed. The extent to which other classical reactivation stimuli identified in humans and animals act on a neuron-autonomous level, or via influencing secondary systems can also be addressed. Basic questions in HSV biology such as the role of LAT RNAs and the temporal pattern of viral gene expression in reactivating neurons can also now be explored in detail.
Experimental Procedures
Cell culture and HSV-1 infection
Superior cervical ganglia (SCG) neurons from E21 rat embryos were dissociated in trypsin (0.1%) at 37°C for 30 min. Approximately 5000 neurons per well were plated in a 96-well plate coated with rat tail collagen (0.66 mg/ml, 08-115, Millipore). SCG neurons isolated in this manner provide a relatively pure population of neurons expressing the TrkA receptor (Glebova and Ginty, 2005) and contain few non-neuronal cells. The cells were maintained with neurobasal media, glucose (0.4%), B27 supplement, NGF (50 ng/ml) and glutamine (2 mM) and treated with 5-fluorouracil and aphidicolin to eliminate any dividing cells that contaminate the cultures. After 6 d, the cells were pretreated with acyclovir, (ACV, 50 mM) for 20 h, and subsequently infected with HSV-1 (Patton strain GFP-Us11; multiplicity of infection (MOI) = 1 based upon titer on Vero cells) for 2 h in the presence of ACV to block productive HSV-1 replication (Efstathiou and Preston, 2005). Neurons were maintained in ACV for at least 6 d. After ACV removal, infected neuronal cultures were exposed to different reactivation stimuli. In an experiment, 22 independently infected wells were analyzed per individual stimulus. Graphs summarize a minimum of 3 separate experiments and error bars indicate the standard error of the mean.
RT-PCR
RNA was isolated from approximately 30,000 latently-infected neurons and analyzed by standard methodologies. The primer sequences are posted in the supplementary section.
Combined Fluorescent In situ Hybridization and Indirect Immunofluorescence
Cells were cultured and infected with HSV-1 (GFP-Us11) as described above but plated onto 8-well chamber slides at a density of ~2×104 neurons/chamber. In situ Hybridizationwas performed by adding a mix containing four LAT probes (200 nM each) for 5 h at 42°C. LAT-specific oligonucleotides were designed against the ~2 kb intron region of HSV-1 strain 17 (Farrell et al., 1991), and were synthesized with a fluorescein tag on the 5’ end. All subsequent incubations for immunofluorescence (IF) were done at RT. Additional details can be found in the supplement.
shRNA Lentivirus Infection
Lentiviruses expressing shRNAs against rat PDK1 and rat PLCγ were generated using a pLVTHM vector that included an mCherry expression cassette. SCG cultures were infected with lentivirus (MOI = 5) for 12 h prior infection with HSV-1. The efficiency of lentiviral infection as judged by mCherry expression was approximately 90%. The shRNA sequences are posted in the supplementary section.
Plaque assay
Cell lysates were prepared from neuron cultures by freeze-thawing, and the amount of infectious virus determined by plaque assay using Vero cells and serial dilutions of each lysate.
Viability assay by Hoechst staining
One week after plating, SCG cultures where treated with anti-NGF blocking antibodies in the presence or absence of 20 μM ZVAD-fmk (627610, Calbiochem). After 48 h, cells were stained with Hoechst 33342 (Molecular Probes) and visualized by light microscopy. The number of apoptotic nuclei was determined by counting 200 cells.
Supplementary Material
Acknowledgements
We thanks Kevan Shokat for the specific PI3K inhibitors, Josh Bloom (Wyeth) for WAY-150138 and Eugene Johnson for advice. This work was supported by grants to MVC (NS21072, HD23315), ACW (GM61139, S10RR017970) and IM (AI073898, GM056927) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Primary sympathetic neuronal cultures support HSV infections resembling natural latency
- NGF and TrkA receptor tyrosine kinase-mediated PI3K-signaling maintains neuronal HSV latency
- NGF, EGF and GDNF activate PI3K but differ in the ability to support latency
- Duration of PI3K and Akt activation by receptor tyrosine kinases governs HSV-1 latency
Additional materials and methods can be found in the online supplemental section
References
- Bartlett SE, Reynolds AJ, Tan T, Heydon K, Hendry IA. Differential mRNA expression and subcellular locations of PI3-kinase isoforms in sympathetic and sensory neurons. J Neurosci Res. 1999;56:44–53. doi: 10.1002/(SICI)1097-4547(19990401)56:1<44::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Beguinot L, Lyall RM, Willingham MC, Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci U S A. 1984;81:2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benboudjema L, Mulvey M, Gao Y, Pimplikar SW, Mohr I. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J Virol. 2003;77:9192–9203. doi: 10.1128/JVI.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010:246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem Sci. 2008;33:339–349. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carton CA, Kilbourne ED. Activation of latent herpes simplex by trigeminal sensory-root section. N Engl J Med. 1952;246:172–176. doi: 10.1056/NEJM195201312460503. [DOI] [PubMed] [Google Scholar]
- Chao MV. Growth factor signaling: where is the specificity? Cell. 1992;68:995–997. doi: 10.1016/0092-8674(92)90068-n. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cushing H. The surgical aspects of major neuralgia of the trigeminal nerve. JAMA. 1905;XLIV:773–779. [Google Scholar]
- Deckwerth TL, Johnson EM., Jr. Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi-Rao GB, Goodart SA, Hecht LM, Rochford R, Rice MK, Wagner EK. Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol. 1991;65:2179–2190. doi: 10.1128/jvi.65.5.2179-2190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S, Preston CM. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 2005;111:108–119. doi: 10.1016/j.virusres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci U S A. 1991;88:790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- Garber DA, Schaffer PA, Knipe DM. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol. 1997;71:5885–5893. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Hill JM, Garza HH, Jr., Helmy MF, Cook SD, Osborne PA, Johnson EM, Jr., Thompson HW, Green LC, O'Callaghan RJ, Gebhardt BM. Nerve growth factor antibody stimulates reactivation of ocular herpes simplex virus type 1 in latently infected rabbits. J Neurovirol. 1997;3:206–211. doi: 10.3109/13550289709018295. [DOI] [PubMed] [Google Scholar]
- Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. A potential interaction of p75 and trkA NGF receptors revealed by affinity crosslinking and immunoprecipitation. J Neurosci Res. 1995;40:557–563. doi: 10.1002/jnr.490400415. [DOI] [PubMed] [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Coassin M, Costa N, Lauretti P, Micera A, Ghinelli E, Aloe L, Rama P, Bonini S. Topical treatment with nerve growth factor in an animal model of herpetic keratitis. Graefes Arch Clin Exp Ophthalmol. 2008;246:121–127. doi: 10.1007/s00417-007-0593-6. [DOI] [PubMed] [Google Scholar]
- Leib DA, Bogard CL, Kosz-Vnenchak M, Hicks KA, Coen DM, Knipe DM, Schaffer PA. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989a;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989b;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro C, Pietro L, Emilio CC. The use of nerve growth factor in herpetic keratitis: a case report. J Med Case Reports. 2007;1:124. doi: 10.1186/1752-1947-1-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JA, Klingelhutz AJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J Virol. 2003;77:12276–12284. doi: 10.1128/JVI.77.22.12276-12284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Newcomb WW, Brown JC. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. J Virol. 2002;76:10084–10088. doi: 10.1128/JVI.76.19.10084-10088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Wu TT, Tchieu JH, Feng J, Brown HJ, Li X, Qi J, Deng H, Vivanco I, Mellinghoff IK, et al. Inhibition of the phosphatidylinositol 3-kinase-Akt pathway enhances gamma-2 herpesvirus lytic replication and facilitates reactivation from latency. J Gen Virol. 2010;91:463–469. doi: 10.1099/vir.0.015073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesola JM, Zhu J, Knipe DM, Coen DM. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J Virol. 2005;79:14516–14525. doi: 10.1128/JVI.79.23.14516-14525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala BA, Milbrandt J, Johnson EM., Jr. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DS, Lai AZ, Mulligan LM. RET ligand-induced internalization and its consequences for downstream signaling. Oncogene. 2006;25:3206–3211. doi: 10.1038/sj.onc.1209349. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430–436. doi: 10.1016/j.expneurol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Smith RL, Escudero JM, Wilcox CL. Regulation of the herpes simplex virus latency-associated transcripts during establishment of latency in sensory neurons in vitro. Virology. 1994;202:49–60. doi: 10.1006/viro.1994.1321. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984;98:1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74:10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M, Fairhurst J, Jones TR, Vernon SK, Morin J, LaRocque J, Feld B, O'Hara B, Bloom JD, Johann SV. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J Virol. 2000;74:9054–9061. doi: 10.1128/jvi.74.19.9054-9061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EK, Bloom DC. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz MA, Price RW, Notkins AL. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974;184:1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Warren SL, Carpenter CM, Boak RA. Symptomatic Herpes, a Sequela of Artificially Induced Fever : Incidence and C Aspects; Recovery of a Virus from Herpetic Vesicles, and Comparison with a K Strain of Herpes Virus. J Exp Med. 1940;71:155–168. doi: 10.1084/jem.71.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CE., Jr. Pathogenesis of recurrent herpes simplex infections. J Invest Dermatol. 1975;65:341–346. doi: 10.1111/1523-1747.ep12607603. [DOI] [PubMed] [Google Scholar]
- Wilcox CL, Johnson EM., Jr. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CL, Smith RL, Freed CR, Johnson EM., Jr. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CLJ, E. M., Jr. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987;61:2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.