Abstract
Previous research has demonstrated that administration of μ-opioid receptor agonists into the nucleus accumbens increases high-fat diet consumption in sated rats and has shown a role of basolateral amygdala (BLA) activity in mediating this response. The present experiments were conducted to examine the role of BLA opioid transmission in mediating high-fat feeding driven by either intra-accumbens opioid activation or 24-hr home cage food deprivation. Injection of the μ-opioid agonist, D-Ala2-NMe-Phe4-Glyol5-enkephalin (DAMGO) into the nucleus accumbens (0.25 μg/0.5μl/side) increased consumption of a high-fat diet, and this effect was attenuated by pretreatment with the opioid antagonist, naltrexone (5μg/0.25μl/side) administered into the BLA. In contrast, intra-BLA naltrexone administration had no influence on the increase in high-fat intake following 24-hr food deprivation. These findings suggest that BLA opioid transmission is an important mediator of palatability-driven feeding as modeled by intra-accumbens opioid activation, while BLA opioid transmission has no significant influence on the increase in high-fat feeding driven by short-term negative-energy balance.
1. Introduction
Feeding behavior is mediated by an assortment of factors, including homeostatic mechanisms (Saper et al., 2002), learned associations (Petrovich et al., 2007), and the rewarding nature of the food (Berridge, 2009; Berthoud, 2004). The process by which the rewarding, or hedonic, characteristic of food drives feeding (Kelley et al., 2005) is vital to understanding the current obesity trend. A well-characterized animal model of this process involves opioid activation of the nucleus accumbens (Kelley et al., 2005). Intra-accumbens administration of μ-opioid receptor agonist D-Ala2-NMe-Phe4-Glyol5-enkephalin (DAMGO) produces exaggerated consumption, specifically of palatable foods such as a high-fat diet, and enhances operant responding for food reward (Bakshi & Kelley, 1993; Zhang & Kelley, 2002). Additionally, intra-accumbens μ-opioid receptor activation increases “liking” reactions displayed by rats in taste reactivity responses to intra-oral sucrose administration (Pecina and Berridge, 2000). These studies and others suggest that activation of the nucleus accumbens opioid system increases consumption by altering the hedonic impact (or palatable nature) of food (Kelley et al., 2005).
The potentiation of high-fat intake following intra-accumbens opioid activation is dependent on a distributed network of feeding-related regions located throughout cortical, limbic, and brainstem areas (Will et al., 2003). The basolateral amygdala (BLA), shown to be important for regulating emotion and motivation, is an integral part of this distributed opioid-driven feeding network (Will et al., 2004). The BLA is well positioned to communicate with brain regions that influence processes that drive feeding behavior, including emotional and sensory inputs from prefrontal and gustatory cortex (Shi et al., 1998) and brainstem parabrachial nuclei (Fulwiler & Saper, 1984; Saper & Loewy, 1980), and critical outputs to the hypothalamus, ventral striatum, and motor output regions necessary to express feeding behavior (Kelley et al., 1982; Swanson, 1982; Alheid, 2003).
It has been demonstrated that activation of the BLA is required to observe the increased consumption of a high-fat diet following intra-accumbens opioid activation (Will et al., 2004), as temporary inactivation of the BLA with the GABAA agonist muscimol prevented the robust increase in high-fat intake following intra-accumbens DAMGO, yet having no influence on baseline intake (Will et al., 2004). In contrast, intra-BLA muscimol had no influence on high-fat consumption following 24-hr food deprivation, suggesting a specific role for BLA activity in mediating feeding driven by striatal opioid activation (Will et al., 2009). Together, these data point to an important BLA signaling pathway that specifically drives opioid-induced high-fat feeding beyond satiety, as opposed to driving feeding towards satiety through a short-term energy-deficit.
The present experiments aimed to examine the specific neurochemical nature of this BLA signaling that selectively contributes to the opioid-driven, but not energy-deficit driven feeding. BLA opioids are a likely candidate, considering that BLA opioids have been shown to influence reward-related behaviors associated with both drug (Marinelli et al., 2010) and natural rewards (Wassum et al, 2009; Petrovich et al., 2003). Therefore, the involvement of BLA opioid transmission was examined using the general nonselective opioid antagonist naltrexone. Rats were given ad libitum access to a high-fat diet following bilateral administration of naltrexone or vehicle into the BLA immediately prior to administration of DAMGO or vehicle into the nucleus accumbens. A second group of rats were given ad libitum access to a palatable diet immediately after bilateral administration of naltrexone or vehicle into the BLA following either 24-hr home cage food deprivation or no deprivation. Automated feeding chambers simultaneously assessed general locomotor activity, number and duration of food hopper entries, and food consumption.
2. Methods
2.1. Subjects
18 adult male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing 300–400 g, were housed in groups of two in Plexiglas cages in a climate-controlled colony room at a temperature of 22 °C. The rats were maintained on a 12-hr light- dark cycle, and all experiments were conducted during the light phase (0700 –1900) between the hours of 1200 and 1500. Unless otherwise noted, rats had free access to laboratory chow and drinking water before and throughout the experiment. Groups contained 8–10 rats. All experimental procedures were in accord with protocols approved by the University of Missouri Institutional Animal Care and Use Committee.
2.2. Surgery
Rats were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 9 mg/kg, respectively; Sigma, St. Louis, MO), and 2 sets of guide stainless steel cannulas (23 gauge, 10 mm) were sterotaxically targeted bilaterally above the border of the nucleus accumbens core and lateral shell and above the BLA (Experiment 1). For Experiment 2, each rat was only implanted with bilateral cannulas targeted above the BLA. Therefore, each rat was implanted with four cannulas in Experiment 1 and two cannulas in Experiment 2. Guide cannulas were secured to the skull with stainless steel screws and light curable resin (Dental Supply of New England, Boston) using standard flat-skull techniques. After surgery, wire stylets were placed in the guide cannulas to prevent occlusion. Coordinates for the aimed sites are as follows: nucleus accumbens: AP, +1.4;ML, ±2.0; DV,−7.8 and BLA: AP,−2.8; ML, ±4.7; DV: −8.6. The coordinates for both regions were chosen to allow valid cross-study comparisons to an earlier study (Will et al., 2009). While the targeted region for the accumbens is near the border of the core and lateral shell, the injection volume used (0.5μl) likely allows diffusion beyond the core and lateral shell. Therefore, the region being studied is referred to more generally as the `nucleus accumbens' throughout the paper.
2.3. Apparatus
Behavioral assessment of feeding took place in a room separate from the colony room in eight Plexiglas (30.5 cm × 24.1 cm × 21.0 cm) custom-built feeding chambers (Med Associates, St. Albans, VT). Rats had access to water ad libitum and approximately 35 g of palatable diet. Feeding chambers were equipped with four infrared locomotor activity beams located 6 cm apart across the length of the chamber and 4.3 cm above the floor. An automated weigh scale for the food hopper monitored the consumption of food. An additional infrared beam spanning the entrance of the food hopper determined the number and duration of each head entry into the hopper area. The feeding hopper and water bottle were located on the same side (opposite corners) of one chamber wall, and a removable waste tray was located beneath the bar floor. The measurements included locomotor activity (number of horizontal beam breaks), duration of hopper entry (duration of beam break at the entrance of the hopper), hopper entries (number of beam breaks at the entrance to the hopper), and amount consumed (grams of diet consumed). Testing periods consisted of behavioral monitoring in the feeding chambers by a computer running Med-PC software (Med Associates Version IV, St. Albans, VT).
2.4. Drug Microinjection
D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO; Research Biochemicals, Natick, MA) and naltrexone (Sigma, St. Louis, MO) were both dissolved in sterile 0.9% saline. The vehicle control was always sterile 0.9% saline. Infusions were delivered with a microdrive pump (Harvard Apparatus, South Natick, MA), connected by means of polyethylene tubing (PE-10), while rats were gently handheld. Thirty-three-gauge 12.5-mm injectors were used, extending 2.5 mm beyond the end of the guide cannulas. The rate of injection was 0.32 μl/min for the nucleus accumbens and 0.16 μl/min for the BLA, with the total duration of infusion being 93 s, resulting in 0.5-μl and 0.25-μl volumes, respectively. One additional minute was allowed for diffusion.
2.5. Behavioral Assessment of Feeding
All behavioral testing began 1-week post-surgery and occurred in the Med-Associates monitors described. Rats were placed in these monitors for 2 hr daily until stable food consumption across 3 days was obtained, usually occurring within 6 days. Animals were given 2 days of sham injections over the last 2 days of the baseline period to ensure acclimation to the treatment procedure. On the first day of this acclimation procedure, a 10-mm injector was inserted and left in place for 2 min, with no volume injected. The following day, a 12.5-mm injector was inserted, and saline was administered for 93 s. In Exp. 1, naltrexone (5μg/0.25 μl/side bilaterally) or saline was infused into BLA, followed immediately by DAMGO (0.25μg/0.5 μl/side bilaterally) or saline into the nucleus accumbens, thus resulting in four possible treatment combinations. In Experiment 2, naltrexone (5μg/0.25 μl/side bilaterally) or saline was administered into the BLA after either a 24-hr period of home cage chow deprivation or ad libitum access. The test session began immediately after the last injection. There were at least 2 days between treatment sessions that were scheduled in a counter-balanced order.
2.6. Specialized Diet
The specialized sweetened high-fat diet was obtained from Teklad, Inc., Madison, WI. The diet contained 278.3 g/kg vitamin free casein, 4.2 g/kg DL-methionine, 100.0 g/kg sucrose, 441.2 g/kg shortening, 77.7 g/kg safflower oil, 26.3 g/kg cellulose, 53.3 g/kg mineral mix, 15.2 g/kg vitamin mix and 3.8 g/kg choline chloride. All components are expressed as weight (g). Based on energy, the diet is 6.2 kcal/g.
2.7. Histology
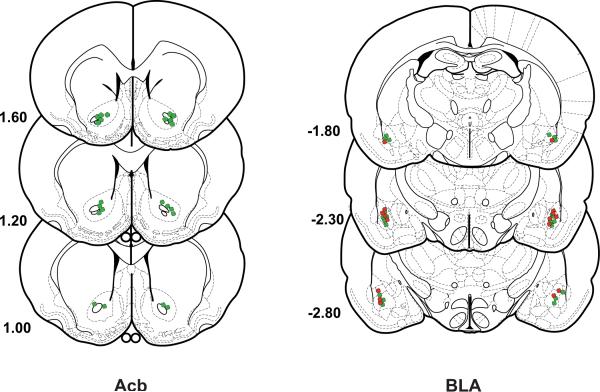
After behavioral testing was completed, subjects were overdosed with sodium pentobarbital and perfused transcardially with heparinized saline (200 ml), followed immediately by 500 ml of a 10% buffered formalin solution. The brains were then removed and placed in 10% formalin-20% sucrose for 1 week. Frozen serial sections (40 μm) were collected through the entire extent of the injection site, mounted on gelatinized slides, and counter-stained with cresyl violet. Cannula placement profiles were then analyzed for accuracy and data from rats with misplaced cannula were not included in the analyses. The placement of all BLA cannula and nucleus accumbens cannula are represented in histological schematic drawings (Fig. 3).
Figure 3.
Histological analysis and placement for DAMGO injections into the nucleus accumbens and naltrexone injections into the BLA for Experiment 1 (green) and Experiment 2 (red). Drawings and coordinates are based on the atlas of Paxinos and Watson (1998).
2.8. Statistical Analysis
For Experiment 1 (n=10), all feeding measures (food consumption, locomotor activity, hopper entries, duration of hopper entry) for the total 2hr session and across the various treatment conditions were analyzed using a two-factor within-subjects analysis of variance (ANOVA: nucleus accumbens treatment × BLA treatment), with the levels for each factor being either vehicle or drug. For Experiment 2 (n=8), these same measures were also analyzed using a two-factor within-subjects analysis of variance (ANOVA: Food deprivation state × BLA treatment), with the levels for each factor being either non-deprived or deprived and vehicle or naltrexone. Preplanned contrasts of means were conducted across treatments, between drug and vehicle and between each brain region (Exp. 1) or drug or vehicle and deprivation state (Exp. 2).
3. Results
3.1. Experiment 1 – Influence of intra-BLA naltrexone on the increased high-fat feeding behavior following intra-accumbens DAMGO
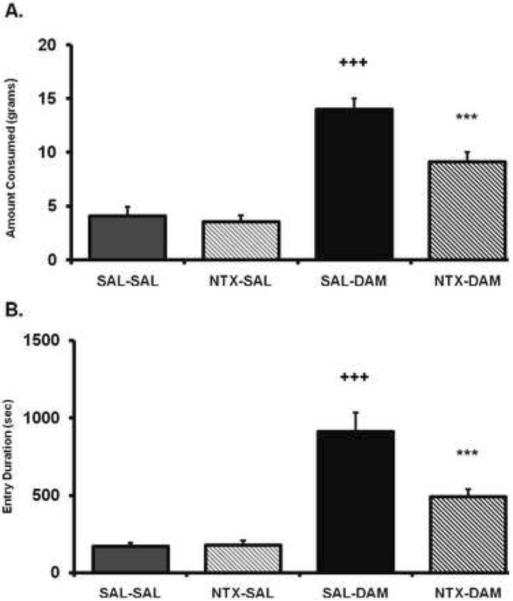
An ANOVA conducted on the food consumption data for Exp. 1 revealed a significant main effect of intra-accumbens DAMGO treatment (F(1,9)=164.7, p < .0001), intra-BLA naltrexone treatment (F(1,9)=41.58, p < .0001), and a significant nucleus accumbens × BLA treatment interaction (F(1,9)=16.78, p < .005). As displayed in Fig. 1, post-hoc comparisons revealed that naltrexone administration into the BLA had no effect on baseline consumption by itself (p = .48). Intra-BLA naltrexone treatment significantly reduced the increased food consumption driven by DAMGO administration into the accumbens (p < .0001), yet remained significantly elevated compared to baseline levels (p < .0001). An ANOVA conducted on the total duration of all hopper entries across the entire feeding session revealed a significant main effect of intra-accumbens DAMGO treatment (F(1,9)=77.67, p < .0001), intra-BLA naltrexone treatment (F(1,9)=16.75, p < .005), and a significant nucleus accumbens × BLA treatment interaction (F(1,9)=17.24, p < .005). Post-hoc comparisons revealed that naltrexone had no effect compared to saline treatment (p = .89). Naltrexone treatment significantly reduced the increased DAMGO-driven hopper entry duration driven by food-deprivation (p < .0005), yet remained significantly elevated compared to baseline levels (p < .005).
Figure 1.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper)after intra-accumbens DAMGO administration (DAM; 0.25 μg/0.5μl) or saline (SAL) immediately after either naltrexone (NTX; 5 μg/0.25μl per side) or saline (SAL) administration into the BLA. The x axis labels refer to treatment for the two regions (i.e., nucleus accumbens treatment-BLA treatment). Values represent group means (+/− S.E.M.). Plus sign represents SAL-SAL versus SAL-DAM. Asterisk represents SAL-DAM versus NTX-DAM Level of significance in all noted comparison, p < .0005.
An ANOVA conducted on the number of hopper entries across the entire feeding session revealed a significant main effect of intra-accumbens DAMGO treatment (F(1,9)=34.23, p < .001), but there was no effect of intra-BLA naltrexone treatment (F(1,9)=1.74, ns). Post-hoc comparisons revealed that intra-accumbens DAMGO increased the total number of hopper entries compared to saline treatment (p < .005) and while intra-BLA naltrexone reduced the total number of hopper entries elicited by DAMGO, this did not quite reach significance (p = .08) (see Table 1). The trend for naltrexone to decrease hopper entries was not related to general activity, as an ANOVA conducted on locomotor activity revealed no effect of these drug treatments across the feeding session for intra-accumbens DAMGO treatment (F(1,9)=2.28, ns), intra-BLA naltrexone treatment (F(1,9)= 0.23, ns), or nucleus accumbens × BLA treatment interaction (F(1,9)=1.1, ns) (Table 2).
Table 1.
Food Hopper Entries
| Pre-treatment Post-treatment |
Saline Saline |
NTX Saline |
Saline DAMGO |
NTX DAMGO |
|---|---|---|---|---|
| Exp. 1 | 70.3 ± 12.2 | 67.5 ± 17.9 | 210.5 ± 37.5* | 145.1 ± 17.8 |
| Pre-treatment Post-treatment |
Non-Restricted Saline |
Restricted Saline |
Non-Restricted NTX |
Restricted NTX |
|---|---|---|---|---|
| Exp. 2 | 118.1 ± 20.6 | 137.9 ± 25.1 | 92.5 ± 15.0 | 93.5 ± 11.6 |
Note. Values represent group means plus or minus the standard error of the mean for a 2-hr measure of food hopper entries (no. of beam breaks at entry of hopper). For Experiment 1, a superscript asterisk (* p < .005) represents DAMGO/SAL vs. SAL/SAL (each pairing represents the order of administration). For Experiment 2, there were no significant differences.
Table 2.
Activity
| Pre-treatment Post-treatment |
Saline Saline |
NTX Saline |
Saline DAMGO |
NTX DAMGO |
|---|---|---|---|---|
| Exp. 1 | 1556 ± 194.9 | 1356 ± 241.8 | 2028 ± 484 | 2524 ± 594.6 |
| Pre-treatment Post-treatment |
Non-Restricted Saline |
Restricted Saline |
Non-Restricted NTX |
Restricted NTX |
|---|---|---|---|---|
| Exp. 2 | 869 ± 118.9 | 910 ± 124.2 | 789 ± 121.7 | 785 ± 66.7 |
Note. Values represent group means (+/− S.E.M.) for a 2-hr measure of general activity (beam breaks). In both experiments, there were no differences in activity.
3.2. Experiment 2 – Influence of intra-BLA naltrexone on high-fat feeding following 24-hour food deprivation
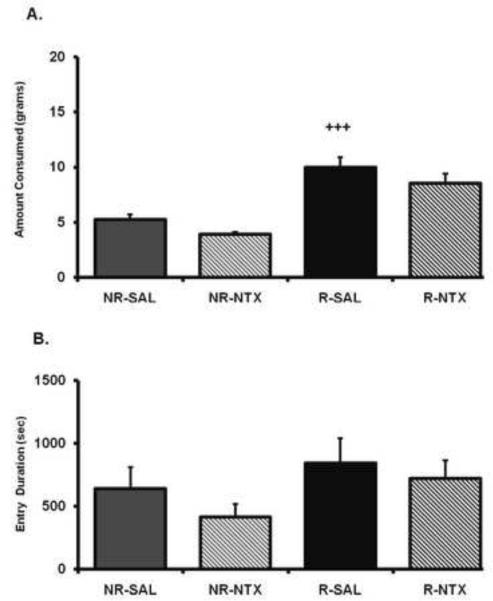
24-hr food deprivation led to a significant increase in palatable diet consumption, as an ANOVA conducted across the entire session revealed a significant main effect of deprivation state (F(1,7)=58.47, p < .001), a non-significant trend for an effect of BLA treatment (F(1,7)=5.123, p=.06), but no interaction (F(1,7,=.005, p=.94). In contrast to the DAMGO-driven food consumption observed in Experiment 1, post hoc contrasts revealed that intra-BLA naltrexone did not significantly reduce the increased food consumption driven by food restriction (p=.092) (Fig. 2). This treatment also still produced a marked consumption increase compared to non-deprived control treatment (p < .005). There was a trend for naltrexone to reduce intake by itself in the non-deprived condition, but this was not significant (p = .11). An ANOVA conducted on the combined duration of all hopper entries across entire feeding session revealed no main effect of deprivation state (F(1,7)=4.84, ns) or intra-BLA naltrexone treatment (F(1,7)=2.89, ns). As shown in Table 1, there was no main effect of deprivation state (F(1,7)=0.89, ns) or intra-BLA naltrexone treatment (F(1,7)=4.95, ns) on the number of food hopper entries. Finally, an ANOVA conducted on the activity levels revealed no effect of deprivation state (F(1,7)=0.04, ns) or intra-BLA naltrexone treatment (F(1,7)=1.50, ns) (Table 2).
Figure 2.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper) after 24-hr food deprivation (R) or no deprivation (NR) after either naltrexone (NTX; 5 μg/0.25μl per side) or saline (SAL) administration into the BLA. The x axis labels refer to deprivation state and intra-BLA treatment (i.e., deprivation state-BLA treatment). Values represent group means (+/− S.E.M.). Plus sign represents NR/SAL versus R/SAL. Level of significance noted, p < .001.
4. Discussion
The present experiments investigated the potential role of BLA opioid transmission in mediating feeding of a high-fat diet driven by either intra-accumbens opioid activation or 24-hr food deprivation. Consistent with previous research (Zhang & Kelley, 2002, Will et al., 2003, Will et al., 2004, Kelley et al., 2005; Will et al., 2009), intra-accumbens DAMGO administration significantly increased consumption of the high-fat diet. Prior administration of intra-BLA naltrexone significantly reduced the intra-accumbens DAMGO driven consumption of the high-fat diet. In regard to other measured feeding behaviors, intra-BLA naltrexone also significantly reduced the DAMGO-driven food hopper entries and hopper entry durations. These approach behaviors were not associated with changes in general locomotor activity levels as intraaccumbens DAMGO administration led to similar locomotor activity levels, regardless of the BLA treatment.
In the second experiment, this same intra-BLA naltrexone treatment was without influence on the increased high-fat intake produced by 24-hr home cage food deprivation. There was a trend for intra-BLA naltrexone treatment to reduce intake, yet this was observed for both non-deprived and deprived conditions to a similar degree. It is known that general inactivation of the adjacent central nucleus of the amygdala robustly reduces baseline feeding (Will et al., 2009), so the possibility of diffusion to this site may explain this trend. Together, these findings suggest that BLA opioid transmission is a key signaling event that specifically mediates opioid-driven feeding of a high-fat intake driven by ventral striatal opioid activation. In contrast, BLA opioid transmission does not appear to be involved in the increased feeding of a high-fat diet following short-term food deprivation.
Previous evidence suggesting a role for BLA neurons in mediating opiate reward processes include its role in altering sensory properties (Fontanini et al., 2009), learned reward associations (Schoenbaum et al., 1998; Tye and Janak, 2007) and taste reactivity and consumption behaviors (Berridge, 1996; Will et al., 2009). Moreover, BLA opioids in particular have been shown to influence reward-related behaviors associated with both drug (Marinelli et al., 2010) and natural rewards (Wassum et al, 2009; Petrovich et al., 2003). The anatomical connectivity of the BLA includes the major output pathway of the BLA to the accumbens (Kelley et al., 1982) and the ventral tegmental area, therefore having potential to alter the mesolimbic dopaminergic pathway known to regulate opiate reward (Koob, 1992). The BLA also projects to the lateral hypothalamus both directly and indirectly through the central nucleus of the amygdala (Petrovich et al., 2001) and receives inputs from gustatory cortex, brainstem and thalamic nuclei (Shi et al., 1998; Fulwiler & Saper, 1984; Saper & Loewy, 1980). However, while the connectivity is well known, there is little evidence as to what influence blocking opioid transmission in the BLA would have on these pathways.
The influence of intra-BLA naltrexone on DAMGO-induced consumption produced the same effect as that found previously using intra-BLA muscimol administration (Will et al., 2009). However, while both drugs reduced the increased consumption, the magnitude of the effect produced by naltrexone in the present study was less. Whether this was due to the distinctive nature of the receptors targeted by each drug or a dose-response function is unknown, but a more extensive dose response study would address this issue. It is likely that muscimol, commonly used pharmacological tool to “inactivate” a region of interest (Herry et al., 2008), led to more generalized inhibition of the BLA, including output pathways that were unaltered by intra-BLA naltrexone administration.
Future studies examining the role of opioid transmission in the central nucleus of the amygdala would be of interest, as prior research has shown activation of the central nucleus is required for intra-accumbens DAMGO-driven feeding of a high fat diet (Will et al., 2009). While general inactivation of the central nucleus of the amygdala with muscimol blocks increased chow intake following a 24-hr food deprivation (Baldo et al., 2005), the involvement of the central nucleus of the amygdala on high-fat feeding following food-deprivation has not been explored. Administration of DAMGO and naltrexone into the central nucleus of the amygdala have been shown to increase or decrease chow feeding, respectively, in a study demonstrating a bi-directional μ-opioid–opioid connection between the central amygdala and the accumbens (Kim et al., 2004). It would be of interest to explore this defined network to potentially distinguish what the present results show to be dissociable systems underlying palatable feeding driven by opioids versus food deprivation.
In summary, the present experiments provide the novel finding that opioid transmission in the BLA is involved in mediating intra-accumbens opioid-driven feeding behavior associated with a high-fat diet. In contrast, feeding behavior driven by a short-term energy-deficit is not dependent on BLA opioid transmission, suggesting a specific role of BLA opioids in mediating palatability-driven feeding. This is especially intriguing when considering that one of the major underlying causes of the current obesity epidemic is overconsumption of palatable tasty food in a non-deprived state. Therefore, furthering our understanding of the feeding networks that drive consumption of highly palatable (i.e. densely caloric) diets based on their rewarding nature, rather than energy need, is of considerable importance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF. Extended amygdala and basal forebrain. Annals of the New York Academy of Sciences. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Sensitization and conditioning of feeding following multiple morphine microinjections into the nucleus accumbens. Brain Research. 1993;648:342–346. doi: 10.1016/0006-8993(94)91139-8. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Alsene KM, Negron A, Kelley AE. Hyperphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: Dependence on intact neural output from the central amygdaloid region. Behavioral Neuroscience. 2005;119:1195–1206. doi: 10.1037/0735-7044.119.5.1195. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology and Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiology and Behavior. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite, 43. 2004:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. Journal of Neuroscience. 2009;29(8):2486–2495. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982 Mar;7(3):615–30. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiology and Behavior. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kim EM, Quinn JG, Levine AS, O'Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Research. 2004;1029(1):135–139. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Lê AD. Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behav Brain Res. 2010;211(1):58–63. doi: 10.1016/j.bbr.2010.03.008. 2010 Jul 29. Epub 2010 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minano FJ, Meneres Sancho MS, Sancibrian M, Salinas P, Myers RD. GABAA receptors in the amygdala: Role in feeding in fasted and satiated rats. Brain Research. 1992;586:104–110. doi: 10.1016/0006-8993(92)91377-q. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiology and Behavior. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998 Oct 5;399(4):440–68. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Brooks BM, Neill DB. GABAergic inactivation of basolateral amygdala alters behavioral processes other than primary reward of ventral tegmental self-stimulation. Behavioural Brain Research. 2007;181:110–117. doi: 10.1016/j.bbr.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. Journal of Neuroscience. 2007;27(15):3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. PNASUSA. 2009;106(30):12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. Journal of Neuroscience. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pritchett CE, Parker KE, Sawani, Ma H, Lai AY. Behavioral Characterization of Amygdala Involvement in Mediating Intra-Accumbens Opioid-Driven Feeding Behavior. Behavioral Neuroscience. 2009;123:781–793. doi: 10.1037/a0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]