Muscle insulin resistance plays a major pathophysiological role in type 2 diabetes and is associated with major public health problems, including obesity and coronary artery disease. Given the dire consequences associated with sedentary living (in terms of individual disease risk and the economic burden on health care systems), the promotion of an active lifestyle is an international priority. Public health guidelines generally recommend that adults perform at least 150 min week−1 of ‘moderate-intensity’ aerobic physical activity (typically defined as 40–60% of maximal aerobic power (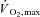 )) or a minimum of 60 min week−1 of ‘vigorous-intensity’ exercise (>60%
)) or a minimum of 60 min week−1 of ‘vigorous-intensity’ exercise (>60%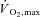 ) to promote health. These recommendations are based on robust evidence that suggests endurance training reduces the risk for chronic disease through the same general mechanisms that lead to improved athletic performance; for example, exercise-induced increases in muscle oxidative and glucose transport capacities have been linked to improved insulin sensitivity and glycaemic control. Unfortunately, most people fail to meet even the minimum physical activity guidelines, citing ‘lack of time’ as the major barrier to regular exercise participation. Innovations in exercise prescription that show benefits despite a minimal time commitment therefore represent a valuable strategy to encourage physical activity participation and reduce the risk of chronic diseases.
) to promote health. These recommendations are based on robust evidence that suggests endurance training reduces the risk for chronic disease through the same general mechanisms that lead to improved athletic performance; for example, exercise-induced increases in muscle oxidative and glucose transport capacities have been linked to improved insulin sensitivity and glycaemic control. Unfortunately, most people fail to meet even the minimum physical activity guidelines, citing ‘lack of time’ as the major barrier to regular exercise participation. Innovations in exercise prescription that show benefits despite a minimal time commitment therefore represent a valuable strategy to encourage physical activity participation and reduce the risk of chronic diseases.
A growing body of evidence suggests that high-intensity interval training (HIT) induces numerous physiological adaptations that are similar to traditional endurance training despite a lower total exercise volume and training time commitment (Gibala & McGee, 2008). Low-volume HIT is characterized by brief repeated ‘bursts’ of vigorous exercise interspersed with periods of rest or low-intensity exercise for recovery. A common model employed in many HIT studies is the Wingate test, which consists of a 30 s ‘all-out’ cycling effort against a standardized resistance. In a typical training session, subjects complete four to six Wingate tests interspersed with 4 min of rest, for a total of only 2 to 3 min of maximal exercise spread over a ∼15–30 min period. As little as six sessions of this low-volume HIT protocol over 2 weeks is a potent stimulus to increase muscle oxidative and glucose transport capacities (Gibala & McGee, 2008), but little is known about the effect of this type of training on common health status markers linked to disease risk.
In a recent issue of The Journal of Physiology, Richards et al. (2010) report that a Wingate-based HIT protocol consisting of only 16 min of all-out cycling over 14 days improved insulin sensitivity in previously sedentary or recreationally active young adults. Babraj and colleagues (2009) previously provided indirect evidence of improved insulin sensitivity based on oral glucose tolerance tests (OGTT) performed before and several days after an identical HIT protocol. However, the data from Richards et al. (2010) are more compelling, since insulin sensitivity was determined using the hyperinsulinaemic euglycaemic clamp technique, which is widely accepted as the reference standard for direct measurement in humans. Short-term HIT would not be expected to influence body composition and Richards et al. (2010) reported no change in several circulatory markers linked to insulin action, providing support for their conclusion that skeletal muscle adaptations probably contributed to the improved insulin sensitivity. As recognized by the authors, an important question pertinent to studies of this sort is whether the change in insulin sensitivity is due to training per se, or to the preceding exercise bout. While acute exercise effects are detectable for up to 48 h, the rigorous study design by Richards et al. (2010) suggests that the improved insulin sensitivity after HIT was a training-induced effect. Post-training measurements were made 72 h after the final training session, and no change in insulin sensitivity was observed in a control group that performed a single bout of Wingate-based exercise. However, the results are in contrast to a recent study (Whyte et al. 2010) that reported improved insulin sensitivity based on OGTT results 24 but not 72 h following a similar HIT training protocol in overweight and obese men. It has been suggested that the insulin-sensitizing effects of this type of exercise may be attenuated in obese and/or insulin-resistant adults and additional work is needed to clarify the effectiveness of HIT in different populations.
Wingate tests require a specialized cycle ergometer and the ‘all-out’ maximal effort necessitates an extremely high level of subject motivation. Therefore, it may not be safe or practical to implement this form of training in the general population. A recent study (Little et al. 2010) evaluated whether a more practical model of low-volume HIT could elicit metabolic and performance adaptations similar to Wingate-based HIT studies. The modified protocol involved eight to twelve 1 min intervals at an intensity that corresponded to ∼100%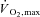 with 75 s of rest in between. While still a demanding form of exercise, the absolute work intensity corresponded to less than half of that achieved during an all-out Wingate test. The protocol was also time efficient in that only ∼10 min of exercise was performed over a 15–25 min period during each training session. Similar to Wingate-based HIT, six sessions of this modified HIT protocol over 2 weeks was a sufficient stimulus to increase skeletal muscle oxidative capacity and GLUT4 protein content. Unpublished work from our laboratory shows this HIT model is well tolerated and reduces hyperglycaemia in people with type 2 diabetes. Additional studies are needed to resolve whether low-volume HIT is a realistic, time-efficient exercise alternative to reduce the risk of metabolic disease in various populations.
with 75 s of rest in between. While still a demanding form of exercise, the absolute work intensity corresponded to less than half of that achieved during an all-out Wingate test. The protocol was also time efficient in that only ∼10 min of exercise was performed over a 15–25 min period during each training session. Similar to Wingate-based HIT, six sessions of this modified HIT protocol over 2 weeks was a sufficient stimulus to increase skeletal muscle oxidative capacity and GLUT4 protein content. Unpublished work from our laboratory shows this HIT model is well tolerated and reduces hyperglycaemia in people with type 2 diabetes. Additional studies are needed to resolve whether low-volume HIT is a realistic, time-efficient exercise alternative to reduce the risk of metabolic disease in various populations.
References
- Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL. Exerc Sport Sci Rev. 2008;36:58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. J Physiol. 2010;588:2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Metabolism. 2010 doi: 10.1016/j.metabol.2010.01.002. (in press) [DOI] [PubMed] [Google Scholar]


