Abstract
The Na+ −K+ −2Cl− cotransporter NKCC1 is expressed in sensory neurons where it accumulates intracellular Cl− and facilitates primary afferent depolarization. Depolarization of primary afferent fibre terminals interferes with the gating of incoming sensory signals to the spinal cord. The cotransporter belongs to a family of ion transporters which are sensitive to changes in cell volume. Cell shrinkage, through mechanisms that are still unknown, leads to the phosphorylation and activation of NKCC1. Similarly, axotomy results in increased NKCC1 phosphorylation in dorsal root ganglion (DRG) neurons. This review summarizes the work on the kinases that directly mediate NKCC1 activation. These are the sterile-20-like kinases SPAK and OSR1. Upon their activation through phosphorylation by upstream kinases, SPAK and OSR1 bind to specific peptides located in the cytosolic N-terminal tail of NKCC1, phosphorylate, and stimulate cotransport activity. Expression of SPAK and OSR1 varies from tissue to tissue, but in DRG neurons and in spinal cord, SPAK and OSR1 expression levels are similar. In DRG neurons, both kinases participate in the modulation of NKCC1, as the knockdown of one kinase only results in a partial decrease of NKCC1 function, while the knockdown of both kinases is additive. The identity of the kinases (e.g. WNK kinases) that possibly act upstream of SPAK and OSR1 is also discussed.
Eric Delpire is Professor of Anesthesiology and Molecular Physiology & Biophysics at Vanderbilt University, Nashville, Tennessee. His expertise is in the molecular physiology of membrane transporters involved in cell ion and volume homeostasis. Intracellular Cl−, for instance, is tightly regulated in neurons and the modulation of its concentration greatly affects inhibitory synaptic transmission. Neuronal Cl− homeostasis is particularly relevant in the modulation of CNS excitability and the gating of sensory signals.

The sensation of touch is an important physiological process that informs an organism of its surrounding environment. The sensation of pain, if it is of a short duration, can also be beneficial as it protects an organism from harmful situations. Chronic pain, however, is typically not physiological, but the result of damage to the central or peripheral nervous system. The pathogenesis of neuropathic pain is intricate, due to the complexity of the pathways and processing of pain signals. Sensory signals are detected at the periphery (skin, organs, etc.), then translated into electrical signals that travel to the spinal cord through sensory nerve fibres. The terminals of these fibres connect to central neurons which relay the information through ascending pathways to diverse brain structures. Information is also sent back from the brain through descending pathways, thereby altering the processing of future incoming sensory signals. As a result, there are many locations where sensory signals can be modified and where abnormal processing can occur.
The GABAA receptor and its ligand γ-amino butyric acid (GABA) modulate the gating of sensory signals. This inhibitory neurotransmitter plays a critical role, both in central and peripheral nervous systems. GABA action depends upon Cl− gradients which are maintained by secondary active cation–chloride cotransport mechanisms, such as the Na+ −K+ −2Cl− cotransporter (NKCC1) and the K+–Cl− cotransporter KCC2. If the Cl− concentration is kept low, as in many adult central neurons (Fig. 1), GABA elicits membrane hyperpolarization. The physiological significance of KCC2 in lowering intracellular Cl− in central neurons is discussed in detail in a recent review (Blaesse et al. 2009). If, however, the Cl− concentration is maintained high, as in peripheral neurons, GABA elicits membrane depolarization. Because the effect of GABA occurs pre-synaptically, the end result of GABA release is signal inhibition leading to reduction in sensory perception. Also note that in the absence of NKCC1 or KCC2 function, e.g. when the Cl− gradient in neurons has collapsed due to intense activity, efflux of HCO3− ions can also produce neuronal depolarization (Blaesse et al. 2009).
Figure 1. Schematic diagram of adult CNS neuron with low intracellular Cl− and immature CNS neuron or adult sensory neuron with high intracellular Cl−.
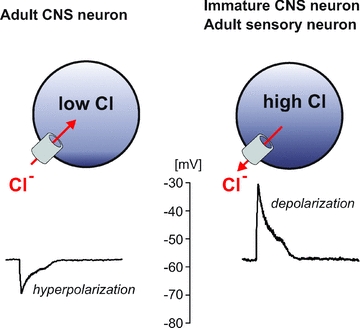
Opening of GABAA receptor in the membrane leads to Cl− entry and hyperpolarization in the first neuron, and Cl− exit and depolarization in the second neuron.
The cation–chloride cotransporters
The Na+ −K+ −2Cl− cotransporter (NKCC) and the K+–Cl− cotransporter (KCC) belong to the Slc12 family of solute carriers. These plasma membrane transporters promote the electroneutral movement of inorganic cations, tightly coupled to the movement of Cl−. These secondary active transport mechanisms use the energy of the Na+ and K+ gradients generated by the Na+/K+-ATPase. NKCC is driven mostly by the Na+ and Cl− gradients and transports inwardly, whereas KCC is generally driven by the K+ gradient and transports outwardly (however, under high neuronal activity, local K+ and Cl− gradients collapse and the direction of K+–Cl− cotransport reverses). Both NKCC and KCC are sensitive to cellular volume, with NKCC activated by cell shrinkage, and KCC activated by cell swelling. Whether or not these osmotic properties are at play during neuronal activity is still unknown. In brain, NKCC1 expression and activity are highest when neurons are born and migrate from the paraventricular zone to their final destination in the brain (Owens et al. 1996; Plotkin et al. 1997b). As neurons settle and mature, expression of NKCC1 decreases. Upon injury, this phenotype reverses and NKCC1 function is again increased (van den Pol et al. 1996), suggesting that NKCC1 could be involved in cellular repair mechanisms. One component of neuronal repair involves re-establishing connections by extending new processes which requires localized shape and volume changes. Therefore, the osmotic properties of NKCC1 might be important components of neuronal repair.
Primary afferent depolarization and presynaptic inhibition in spinal cord
The central nervous system is flooded with sensory information coming from peripheral organs, such as skin, muscle, visceral organs, etc. Presynaptic inhibition in the vertebrate spinal cord is a mechanism by which unnecessary sensory noise is filtered before it reaches neurons that project to the brain or back to the periphery. Inhibition of sensory signal transmission occurs in part at axo-axonic synapses through the depolarization of primary afferent terminals (referred to as primary afferent depolarization or PAD). Several hypotheses have been proposed to explain PAD. They include the local accumulation of external K+ ions, GABAA-mediated depolarization, and volume-transmitted autocrine and paracrine transmissions. These hypotheses have been extensively reviewed in Rudomin & Schmidt (1999). The prevalent hypothesis is of last-order GABAergic interneurons synapsing onto the terminals of Ia, Ib and group II primary afferent fibres and inhibiting the gating of sensory signals.
The circuitry of pain
Small size (Aδ and C) afferent fibres carry nociceptive information from the periphery to output neurons located in lamina I and II layers of the dorsal horn of the spinal cord (Fig. 2). Although axo-axonic synapses have been more difficult to identify at Aδ and C afferent fibre terminals, PAD of these fine afferents has been experimentally observed (for references, see Willis, 2006). Interneurons, located in layers III and IV, project towards the superficial output neurons and synapse onto the terminals of the afferent fibres. These interneurons can be stimulated by projection neurons (also located in deeper laminae) and stimulated by large myelinated Aβ fibres carrying tactile information. Upon stimulation, the interneurons release GABA onto the presynaptic terminal of nociceptive fibres, depolarizing the membrane, and inhibiting inputs coming from the periphery. Through this circuitry, one can explain why gentle stimulation of the skin around an injury would decrease the transmission of pain signals to spinothalamic tract pathways.
Figure 2. Schematic representation of the pain circuitry in the spinal cord.
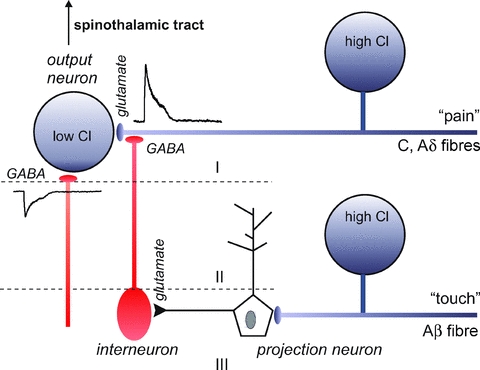
Unmyelinated (C) and thinly myelinated (Aδ) afferent fibres bring pain signals to output neurons in lamina I and II in the spinal cord. Interneurons activated by projection neurons in deeper layers release GABA at the terminals of the C and Aδ fibres. As the Cl− concentration is high in these afferent neurons, GABA produces depolarization of the terminal. This depolarization inhibits incoming pain signals coming from the periphery.
NKCC1 function permits primary afferent depolarization
The first observation that a Na+ −K+ −2Cl− cotransporter was involved in accumulating Cl− was made in amphibian sensory neurons in 1988 (Alvarez-Leefmans et al. 1988). Using microelectrodes, Alvarez-Leefmans demonstrated that the Cl− accumulation was dependent upon external Na+, K+ and Cl− concentrations. Furthermore, the accumulation was sensitive to the loop diuretic furosemide (frusemide). After cloning the mouse NKCC1 isoform of the Na+ −K+ −2Cl− cotransporter (Delpire et al. 1994), and developing an NKCC1-specific polyclonal antibody (Kaplan et al. 1996), we demonstrated that this cotransporter was highly expressed in DRG neurons (Plotkin et al. 1997a). Then, we demonstrated that absence of the cotransporter in DRG neurons from NKCC1 knockout mice resulted in a significant decrease in intracellular Cl− (Sung et al. 2000). Gramicidin-perforated patch recordings of wild-type DRG neurons at resting membrane potential demonstrated GABA-mediated depolarization. It is assumed that the Cl− concentration is high in the axon terminal, and therefore GABA elicits depolarization of the terminal. As mentioned above, this primary afferent depolarization has been demonstrated in both primary afferent fibres that innervate muscle stretch receptors and cutaneous mechanoreceptors, and unmyelinated and thinly myelinated C and Aδ fibres (for review, see Alvarez-Leefmans, 2009).
Pain, hyperalgesia and neurogenic inflammation
Tissue injury and inflammation can cause pain, hyperalgesia and allodynia both at the injured area and at sites beyond this area. Peripheral inflammation and injury that produces persistent nociceptor activation may lead to excessive primary afferent depolarization, reaching a threshold for the trigger of dorsal root reflexes (DRRs) (Sluka et al. 1995; Rees et al. 1996; Lin et al. 1999; Valencia-de Ita et al. 2006). Hence, the normally inhibitory PAD can become excitatory if the depolarization is large enough to trigger spikes in nociceptive afferents. Evidence has also shown that DRRs conducted centrifugally can produce neurogenic inflammation and hyperalgesia (reviewed in Willis, 2006). As introduced in a previous section, under ordinary physiological conditions, stimulation of peripheral mechanoreceptors connected to Aβ afferents produces PAD in nociceptive afferents via GABAergic interneurons, thereby reducing nociceptive transmission. Following tissue injury and inflammation, the PAD evoked by tactile stimuli may become significant enough to evoke DRRs in nociceptive afferents. The DRRs conducted centripetally may excite dorsal horn neurons that are normally stimulated by nociceptors and evoke mechanical allodynia (Cervero & Laird, 1996; Cervero et al. 2003; Price et al. 2005; Pitcher et al. 2007).
Several studies provide evidence of NKCC1 being involved in nociceptive processing and in the generation and maintenance of hyperalgesic states and neurogenic inflammation. NKCC1 knockout mice exhibit an increased pain threshold to noxious heat and reduced allodynia following intradermal capsaicin injection (Laird et al. 2004). Local peripheral or intrathecal administration of NKCC antagonists has a significant anti-nociceptive effect in the formalin model of tissue injury-induced pain (Granados-Soto et al. 2005). In addition, NKCC inhibitors stop the dermal manifestations of histamine in human skin (Willis et al. 2004) and block cough produced by irritants of the tracheal mucosa (Mazzone & McGovern, 2006). It has also been shown that noxious visceral stimulation with intracolonic capsaicin induces an increase in NKCC1 and its phosphorylation in the lumbosacral spinal cord of mice. This phosphorylation could cause the enhancement of GABA-mediated PAD that leads to DRRs in nociceptive afferents (Galan & Cervero, 2005). Spinal administration of bumetanide, an NKCC inhibitor, reduces DRR activity, mechanical allodynia and hyperalgesia produced by intradermal injection of capsaicin (Valencia-de Ita et al. 2006).
It is still uncertain how upregulation of NKCC1 transforms PAD into DRRs. The nociceptive barrage that occurs during tissue injury leads to increased GABA activity in the dorsal horn, producing a transient decrease in intra-terminal chloride concentration. This could cause phosphorylation of NKCC1, which would lead to a rebound increase in the chloride concentration. Note, however, that NKCC1 activity is itself highly sensitive to changes in the intracellular Cl− concentration, with increases in intracellular Cl− reducing cotransport activity (Lytle & McManus, 2002). If the intracellular chloride concentration is increased above basal levels, it may result in a depolarizing shift in the GABA electrochemical gradient to values equalling its spike threshold. This increase in chloride concentration could be due to a shifting in the NKCC1 set point, enhanced surface expression of NKCC1, or trafficking of another hypothetical chloride uptake mechanism.
Regulation of NKCC1 by kinases
We know from experiments performed in the early 1990s that stimulatory hormones and hyperosmolarity increase simultaneously NKCC1 phosphorylation and activity (Lytle & Forbush, 1992; O’Donnell et al. 1995; Lytle, 1997). It took another 10 years for the identity of the kinases to be revealed through a yeast 2-hybrid screen that identified protein–protein interaction between SPAK (sterile-20 (Ste20)-related proline-alanine-rich kinase), OSR1 (oxidative stress response) and the cytoplasmic N-terminal domain of the cation–chloride cotransporters (Piechotta et al. 2002).
SPAK and OSR1 belong to a family of mammalian kinases that share homology with the yeast Ste20/Sts1 kinases (Dan et al. 2001; Delpire, 2009). Overall, SPAK shares 66% amino acid identity with OSR1, from which it probably evolved as a result of gene duplication during late vertebrate evolution (Delpire & Gagnon, 2008). SPAK and OSR1 share 90% amino acid identity in their N′ terminal catalytic domain and 56% amino acid identity in their C-terminal regulatory domain. The regulatory domain can be divided into three regions, based on amino acid conservation. The region proximal to the catalytic domain shares 70% amino acid identity. This region, called PF1 in Lee et al. (2009), is followed by 39–67 residues sharing less than 10% amino acid identity, and a very highly conserved (80%) distal region, called PF2 (Lee et al. 2009) or CCT (Villa et al. 2007; see Fig. 3).
Figure 3. Schematic representation of SPAK and OSR1 kinases with short N-terminal domain, followed by catalytic domain, and large C-terminal regulatory domain.

Numbers represent the percentage amino acid identity between mouse SPAK and OSR1 sequences within the delimited region. Red lines indicate the position of a putative caspase cleavage site. PAPA = Proline Alanine rich region.
While the first functional experiments performed in Xenopus laevis oocytes failed to demonstrate SPAK activation of NKCC1 (Piechotta et al. 2003), expression of a catalytically inactive kinase exhibited dominant-negative attributes by completely abrogating cotransporter function (Dowd & Forbush, 2003; Gagnon et al. 2006b). Conversely, co-expression of the catalytically inactive SPAK with KCC2 stimulates cotransport function (Gagnon et al. 2006b).
The first molecular details of SPAK activation of NKCC1 have been worked out in several studies. First, it was determined that the last 90 residues of SPAK are required for binding to RFx[V/I] sequences located in many proteins, including the N-terminal tail of NKCC1 and the regulatory domain of WNK kinases (Piechotta et al. 2002, 2003; Delpire & Gagnon, 2007). Indeed, the crystal structure of the CCT domain of OSR1 confirmed the presence of a hydrophobic pocket which accommodates RFx[V/I] peptides (Villa et al. 2007). Second, studies demonstrate that WNK4 and WNK1 phosphorylate SPAK at two specific serine/threonine residues (Vitari et al. 2005; Moriguchi et al. 2006). The first residue is a conserved threonine (mouse Thr243) which is located in the activation loop of the kinase (Gagnon et al. 2006a); the second residue is a regulatory C-terminal serine (mouse Ser383) which is conserved among SPAK and OSR1 from all species. Although the precise mechanism of activation is still unknown, phosphorylation of both residues is necessary for SPAK activation (Gagnon & Delpire, 2010b). One possible model would be that the PF1 domain interacts with the kinase domain thus creating an inactive conformation. Upon phosphorylation of Ser383, this interaction is broken. Further conformational changes occur due to the phosphorylation of the activation loop Thr243, altogether resulting in the creation of an active kinase. Once activated SPAK phosphorylates specific threonine residues located in the cytoplasmic N-terminal tail of NKCC1 (mouse Thr199, Thr201, Thr206). Third, the crystal structure of the OSR1 kinase domain revealed that the enzyme forms a domain-swapped dimer (Lee et al. 2009). Domain swapping is a mechanism by which two monomers form a dimer by exchanging identical structural elements or domains (Liu & Eisenberg, 2002). The domain exchanged in mouse OSR1 comprises the P + 1 loop, helix αEF, and five extra residues (Phe186–Gly203). The physiological significance of this dimer is still unknown. Two possibilities for dimer formation are (i) the kinase in its dimer conformation is inactive, or (ii) the dimer conformation is active. The crystal structure of OSR1 provides several clues that the dimer is in an inactive conformation. However, this inactive conformation in the crystal might not predict the function/activity of the dimer in vivo. In the first scenario, WNK phosphorylation could trigger the dimer to come apart and release activated monomers. These monomers can then interact and phosphorylate the cotransporter. The second scenario is that WNK phosphorylation could prime monomers to form dimers. The checkpoint kinase 2 (chk2) provides a precedent for this second possibility. Indeed, when chk2 is phosphorylated at Thr68 by the ATM kinase, it places the kinase in a state competent for autophosphorylation of two activation loop threonine residues (Oliver et al. 2006). The authors suggested that the main effect of Thr68 phosphorylation was to promote homo-dimerization. Within the dimer, one molecule could phosphorylate the T-loop of the other molecule, and vice versa. Because WNK4 not only phosphorylates Ser383 in the regulatory domain of SPAK, but also phosphorylates Thr243 in the activation loop, it is difficult to imagine how trans-autophosphorylation would participate in SPAK/OSR1 activation. This could only work if the trans-autophosphorylation would involve residues other than Thr243. We previously demonstrated that Thr231, Thr236 and Thr247 also incorporated 32P from 32P-ATP (Gagnon et al. 2006a). Among these three residues, however, only the mutation of Thr247 into an alanine was shown to affect SPAK function (Gagnon et al. 2006a). Interestingly, this residue is part of the domain that is swapped or exchanged between the two monomers. Whether phosphorylation of Thr247 stabilizes an active dimer or triggers the dissociation of the dimer is still unknown. Work will be needed in this area to pinpoint the true relevance of the dimer.
How is NKCC1 regulated in dorsal root ganglion neurons?
A recent study that used axotomy as a model of peripheral nerve injury demonstrated a depolarizing shift in the GABAA reversal potential (Pieraut et al. 2007). This shift, which was indicative of an increased intracellular Cl− concentration, was probably due to increased NKCC1 activity. In fact, the shift was sensitive to bumetanide, an inhibitor of the Na+ −K+ −2Cl− cotransporter, and was absent in NKCC1 knockout mice. Furthermore, the use of a phosphopeptide antibody revealed that the shift was associated with increased phosphorylation of the cotransporter.
Studies performed in Xenopus laevis oocytes show that SPAK and OSR1 are each able to increase NKCC1 activity when co-injected with WNK4. Does this observation mean that cells co-expressing the two kinases use them interchangeably or that SPAK and OSR1 function is identical and redundant? We started addressing these questions by utilizing an established cell line (50B11) derived from rat dorsal root ganglion neurons (Chen et al. 2007). These cells are able to differentiate into cells that extend long neurites, express neuronal markers and generate action potentials. Furthermore these cells exhibit many of the properties of nociceptive neurons (Chen et al. 2007). Even undifferentiated 50B11 cells express the Na+ −K+ −2Cl− cotransporter, SPAK, OSR1, and all WNK isoforms (Geng et al. 2009). Because quantitative Western blot analysis demonstrated that these cells express SPAK and OSR1 at similar levels, they were deemed suitable for examining cotransporter activity upon genetic silencing of each kinase. These experiments demonstrated that short-hairpin-mediated silencing of either kinase resulted in a significant reduction in bumetanide-sensitive K+ uptake, whereas the silencing of both kinases had a greater effect (Geng et al. 2009). Rescue experiments demonstrated that SPAK overexpression could overcome OSR1 silencing, restoring cotransporter activity, and vice versa (Fig. 4).
Figure 4. NKCC1 activity, as measured through bumetanide-sensitive K+ uptake, in wild-type and SPAK- or OSR1-knockdown 50B11 cells.
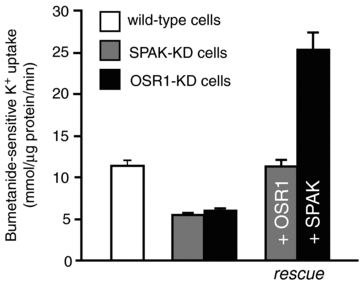
Note the reduction in activity in both knockdown cell lines. OSR1 overexpression was able to restore NKCC1 activity in SPAK-knockdown cells. Similarly, SPAK over-expression was able to activate NKCC1 in OSR1-knockdown cells. Redrawn from Geng et al. (2009).
Our laboratory has generated both SPAK and OSR1 knockout mice (Geng et al. 2009, 2010), and disruption of the OSR1 gene produced animals that died in utero from an unknown developmental defect. In contrast, disruption of the SPAK gene resulted in viable mice with no overt deleterious phenotype. SPAK knockout mice, however, exhibit a pain perception phenotype as demonstrated by an increased latency to respond to heat-evoked noxious stimuli, as well as a significant locomotor phenotype (Geng et al. 2010). To address the role of SPAK in regulating NKCC1 in native sensory neurons, we isolated DRG cells from SPAK knockout mice and wild-type mice and assessed bumetanide-sensitive Na+ −K+ −2Cl− cotransport activity using a fluorescent thallium-sensitive dye. Activity of NKCC1 was reduced by one-half in DRG neurons isolated from SPAK knockout mice, indicating that some Na+ −K+ −2Cl− cotransporters were functional due to the activity of OSR1 (Geng et al. 2009). Altogether, the data obtained in 50B11 knockdown cells and in the SPAK knockout mice indicate that both SPAK and OSR1 stimulate Na+ −K+ −2Cl− cotransport activity in dorsal root ganglion neurons. It should be pointed out that because the two kinases seem to interchangeably phosphorylate and activate the cotransporter, this does not necessarily mean that they have redundant function. Indeed, they could very well be part of distinct signalling cascades that converge to the cotransporter. For this possibility to be true, there must be different activating proteins upstream of SPAK and OSR1.
What do we know about upstream signalling molecules?
Figure 5 summarizes the regulatory elements of NKCC1. There is growing evidence that SPAK and OSR1 are the primary kinases phosphorylating and activating NKCC1. This, however, does not exclude the participation of other protein kinases in the modulation of NKCC1 function. Two mass spectrometry-Edman sequencing studies provide conflicting data that could be interpreted as evidence for multiple kinases activating NKCC1. Indeed, Darman & Forbush (2002) demonstrated that Thr206, Thr211 and Thr224 (mouse NKCC1 sequence) were phosphorylated upon forskolin activation. However, Vitari and coworkers (Vitari et al. 2006) identified Thr197, Thr201 and Thr206, but not Thr211 or Thr224, as targets of SPAK phosphorylation. Therefore, if the absence of Thr211 and Thr224 phosphorylation by SPAK is correct, this would indicate that a kinase other than SPAK or OSR1 mediates the forskolin-induced activation of NKCC1.
Figure 5. Illustration of different regulatory elements of NKCC1.
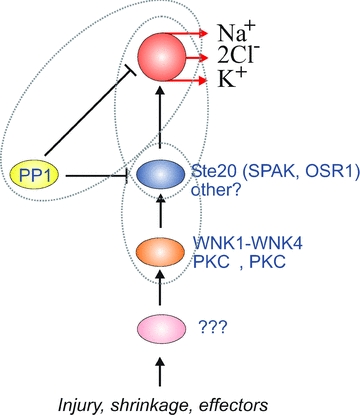
SPAK and OSR1 represent kinases that are directly responsible for phosphorylation and activation of NKCC1. Both NKCC1 and these direct kinases are inactivated through dephosphorylation by PP1. WNK kinases and several PKC isotypes are located upstream of SPAK and OSR1. These kinases are also probably the target of other unidentified effectors. Venn diagrams represent necessary protein–protein interaction between regulatory elements.
NKCC1 inactivation is mainly mediated by protein phosphatase 1 (PP1; Darman et al. 2001). Recent work from this laboratory has shown that not only NKCC1 is dephosphorylated and inactivated by PP1, but SPAK is also dephosphorylated by the phosphatase. Kinase inhibition by PP1 is made possible by the scaffolding of these two proteins on the cytoplasmic N-terminal tail of the cotransporter (Gagnon & Delpire, 2010a).
As previously noted, WNK kinases regulate cation–chloride cotransporter function by acting upstream of SPAK and OSR1. The WNK kinases in question are WNK4 (Piechotta et al. 2003; Vitari et al. 2005; Gagnon et al. 2006b), WNK1 (Vitari et al. 2005; Anselmo et al. 2006; Moriguchi et al. 2006) and WNK3 (Rinehart et al. 2005; Ponce-Coria et al. 2008). In addition, there is additional evidence that WNKs affect the trafficking of the cotransporters to the plasma membrane through mechanisms independent of the Ste20 kinases (Gamba et al. 2009). As each WNK isoform also generates multiple splice variants, there is great diversity in the proteins that can act upstream of SPAK and OSR1. This diversity could be part of distinct signalling pathways that lead to NKCC1 activation. Furthermore, WNK kinases might not be the only kinases that are acting upstream of SPAK/OSR1, as Li and coworkers demonstrated that PKCθ phosphorylated and activated SPAK in T lymphocytes (Li et al. 2004). More recently, Smith et al. demonstrated that PKCδ acted upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells (Smith et al. 2008).
The participation of PKCδ in hypertonic stimulation of NKCC1 is interesting. Indeed experiments performed in X. laevis oocytes with catalytically inactive SPAK and WNK4 revealed that SPAK had dominant-negative effects and significantly reduced the activation of NKCC1 under hypertonicity, whereas inactive WNK4 had no effect on hypertonic stimulation of the cotransporter. As PKCδ represents a parallel pathway to the activation of SPAK, these data could demonstrate independence of pathways that lead to the activation of the cotransporter.
Summary
SPAK and OSR1, which belong to the germinal center kinase VI branch of mammalian sterile-20 kinases, modulate NKCC1 activity in DRG neurons through binding and phosphorylation. Cotransporter activity in turn results in increased intracellular Cl−, which facilitates primary afferent depolarization, presynaptic inhibition, and diminished transmission of nociceptive signals. Cotransporter activity is also increased via phosphorylation after nerve injury, indicating a possible role for the cotransporter in neuronal repair. While molecular details of SPAK/OSR1 activation are continually being worked out, the signalling cascades that lead to their activation are still unknown. Several kinases that activate SPAK and OSR1 have been identified in DRG neurons, but their specific roles have not yet been defined.
Acknowledgments
This work was supported by NIH grant GM074771 to E.D. T.M.A. is a Vanderbilt Department of Anesthesiology B.H. Robbins Scholar.
References
- Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporter and Channels in the Nervous System: From Molecules to Diseases. London: Academic Press; 2009. pp. 439–470. [Google Scholar]
- Alvarez-Leefmans FJ, Gamiño SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. Neuroreport. 1996;7:526–528. [PubMed] [Google Scholar]
- Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–351. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Chen W, Mi R, Haughey N, Oz M, Höke A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J Peripher Nerv Syst. 2007;12:121–130. doi: 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- Darman RB, Flemmer A, Forbush B. Modulation of ion transport by direct targeting of protein phosphatase type 1 to the Na-K-Cl cotransporter. J Biol Chem. 2001;276:34359–34362. doi: 10.1074/jbc.C100368200. [DOI] [PubMed] [Google Scholar]
- Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem. 2002;277:37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- Delpire E. The mammalian family of sterile20p-like protein kinases. Pflugers Arch. 2009;458:953–967. doi: 10.1007/s00424-009-0674-y. [DOI] [PubMed] [Google Scholar]
- Delpire E, Gagnon KB. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics. 2007;28:223–231. doi: 10.1152/physiolgenomics.00173.2006. [DOI] [PubMed] [Google Scholar]
- Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na-K-2Cl cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem. 1994;269:25677–25683. [PubMed] [Google Scholar]
- Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function. The N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) and PP1. J Biol Chem. 2010a;285:14115–14121. doi: 10.1074/jbc.M110.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl−, cotransport activity. Am J Physiol Cell Physiol. 2010b doi: 10.1152/ajpcell.00074.2010. DOI 10.1152/ajpcell.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006a;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KB, England R, Delpire E. Volume sensitivity of cation-chloride cotransporters is modulated by the interaction of two kinases: SPAK and WNK4. Am J Physiol Cell Physiol. 2006b;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- Galan A, Cervero F. Painful stimuli induce in vivo phosphorylation and membrane mobilization of mouse spinal cord NKCC1 co-transporter. Neuroscience. 2005;133:245–252. doi: 10.1016/j.neuroscience.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Gamba G, Garbarini NJ, Delpire E. Regulation of cation-chloride cotransporters. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporter and Channels in the Nervous System: From Molecules to Diseases. London: Academic Press; 2009. pp. 357–381. [Google Scholar]
- Geng Y, Byun N, Delpire E. Behavioural analysis of Ste20 kinase SPAK knockout mice. Behav Brain Res. 2010;208:377–382. doi: 10.1016/j.bbr.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Hoke A, Delpire E. The Ste20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem. 2009;284:14020–14028. doi: 10.1074/jbc.M900142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. Peripheral and central antinociceptive action of Na+ −K+ −2Cl− cotransporter blockers on formalin-induced nociception in rats. Pain. 2005;114:231–238. doi: 10.1016/j.pain.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium and the glomerular afferent arteriole. J Clin Invest. 1996;98:723–730. doi: 10.1172/JCI118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci Lett. 2004;361:200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Cobb MH, Goldsmith EJ. Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci. 2009;18:304–313. doi: 10.1002/pro.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu J, Vita R, Sun B, Tabata H, Altman A. SPAK kinase is a substrate and target of PKCθ in T-cell receptor-induced AP-1 activation pathway. EMBO J. 2004;23:1112–1122. doi: 10.1038/sj.emboj.7600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Liu Y, Eisenberg D. 3D domain swapping: as domains continue to swap. Protein Sci. 2002;11:1285–1299. doi: 10.1110/ps.0201402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. Activation of the avian erythrocyte Na-K-Cl cotransport protein by cell shrinkage, cAMP, fluoride, and calyculin-A involves phosphorylation at common sites. J Biol Chem. 1997;272:15069–15077. doi: 10.1074/jbc.272.24.15069. [DOI] [PubMed] [Google Scholar]
- Lytle C, Forbush B., III The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992;267:25438–25443. [PubMed] [Google Scholar]
- Lytle C, McManus T. Coordinate modulation of Na-K-2Cl cotransport and K-Cl cotransport by cell volume and chloride. Am J Physiol Cell Physiol. 2002;283:C1422–C1431. doi: 10.1152/ajpcell.00130.2002. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE. Na+-K+-2Cl− cotransporters and Cl− channels regulate citric acid cough in guinea pigs. J Appl Physiol. 2006;101:635–643. doi: 10.1152/japplphysiol.00106.2006. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Urushiyama S, Hisamoto N, Iemura SI, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2006;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- O’Donnell ME, Martinez A, Sun D. Endothelial Na-K-Cl cotransport regulation by tonicity and hormones: phosphorylation of cotransport protein. Am J Physiol Cell Physiol. 1995;269:C1513–C1523. doi: 10.1152/ajpcell.1995.269.6.C1513. [DOI] [PubMed] [Google Scholar]
- Oliver AW, Paul A, Boxall KJ, Barrie SE, Aherne GW, Garrett MD, Mittnacht S, Pearl LH. Trans-activation of the DNA-damage signalling protein kinase Chk2 by T-loop exchange. EMBO J. 2006;25:3179–3190. doi: 10.1038/sj.emboj.7601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechotta K, Garbarini NJ, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: Evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278:52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- Piechotta K, Lu J, Delpire E. Cation-chloride cotransporters interact with the stress-related kinases SPAK and OSR1. J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- Pieraut S, Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, Mersel M, Delpire E, Valmier J, Scamps F. NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J Neurosci. 2007;27:6751–6759. doi: 10.1523/JNEUROSCI.1337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher MH, Price TJ, Entrena JM, Cervero F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol Pain. 2007;3:17. doi: 10.1186/1744-8069-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol. 1997a;272:C173–C183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997b;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloridesensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Sluka KA, Lu Y, Westlund KN, Willis WD. Dorsal root reflexes in articular afferents occur bilaterally in a chronic model of arthritis in rats. J Neurophysiol. 1996;76:4190–4193. doi: 10.1152/jn.1996.76.6.4190. [DOI] [PubMed] [Google Scholar]
- Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Westlund KN, Willis WD. Fibre types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol. 1995;74:981–989. doi: 10.1152/jn.1995.74.3.981. [DOI] [PubMed] [Google Scholar]
- Smith L, Smallwood N, Altman A, Liedtke CM. PKCδ acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem. 2008;283:22147–22156. doi: 10.1074/jbc.M801752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K-W, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-de Ita S, Lawand NB, Lin Q, Castañeda-Hernandez G, Willis WD. Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95:3553–3561. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. J Neurosci. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DMF. Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep. 2007;8:839–845. doi: 10.1038/sj.embor.7401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis EF, Clough GF, Church MK. Investigation into the mechanisms by which nedocromil sodium, frusemide and bumetanide inhibit the histamine-induced itch and flare response in human skin in vivo. Clin Exp Allergy. 2004;34:450–455. doi: 10.1111/j.1365-2222.2004.01898.x. [DOI] [PubMed] [Google Scholar]
- Willis WD. John Eccles’ studies of spinal cord presynaptic inhibition. Prog Neurobiol. 2006;78:189–214. doi: 10.1016/j.pneurobio.2006.02.007. [DOI] [PubMed] [Google Scholar]


