Abstract
Costello syndrome (CS) is a rare multiple congenital anomaly disorder which is caused by germline mutations in the v-Ha-ras Harvey rat sarcoma viral oncogene homologue (HRAS) proto-oncogene. Experimental data suggest perturbing effects of the mutated protein on the functional and structural organization of networks of cerebral cortex and on the activity-dependent strengthening of synaptic transmission known as long term potentiation (LTP). In five patients with molecularly proven diagnosis of CS and in a group of 13 age-matched control subjects we investigated activity-dependent synaptic plasticity. To this end, we used a paired associative stimulation (PAS) protocol, in which left ulnar nerve stimuli were followed by transcranial magnetic stimulation (TMS) pulses to right cortical hand area, and recorded motor evoked potentials (MEPs) by single pulse TMS from left first dorsal interosseus (FDI) muscle before and after PAS. In 4 out of 5 CS patients and in a subgroup of nine control subjects we also evaluated the time course and the topographical specificity of PAS after-effects. In these two subgroups, MEPs were measured before, immediately after and 30 min after PAS in the left FDI and left abductor pollicis brevis (APB). While the PAS protocol led to a 65% increase of the FDI MEP amplitude in controls, the LTP-like phenomenon was significantly more pronounced in CS patients, with motor responses increased by 230%. In addition, CS patients showed a similar MEP increase in both muscles while control subjects showed a slight increase in APB and only immediately after PAS. We hypothesize that the extremely enhanced PAS after-effects could be due to the influence of HRAS activity on the susceptibility of synapses to undergo LTP.
Introduction
Costello syndrome (CS; [MIM] 218040) is the eponymous name for a rare multiple congenital anomaly disorder characterized by prenatal overgrowth, postnatal feeding difficulties and severe failure to thrive, distinctive ‘coarse’ facial features, mental retardation, cardiac defects (including hypertrophic cardiomyopathy and tachyarrhythmia), musculoskeletal and skin abnormalities, and predisposition to certain malignancies (Costello, 1977). CS is characterized by a relatively homogeneous clinical phenotype which is caused by a restricted spectrum of germline heterozygous missense mutations in the HRAS protooncogene ([MIM] 190020). To date only about 250 individuals with CS have been reported worldwide (Gripp & Lin, 2009).
Recent evidence in transgenic synRas mice expressing the constitutively active H-RasVal12 protein demonstrated that this GTPase exerts significant morphoregulatory effects on the dendritic phenotype as well as on the structural and functional synaptic connectivity (Arendt et al. 2004; Alpar et al. 2008), suggesting a relevant role of this protein for functional and structural organization of networks of cerebral cortex. The role of Ras GTPases in functional organization of cortical networks is also suggested by other studies reporting that Ras proteins are strictly connected to synaptic long-term potentiation (LTP) (English & Sweatt, 1997), a phenomenon of activity-dependent strengthening of synaptic transmission (Bi & Poo, 1998) considered to be involved also in learning and memory (Malenka & Bear, 2004). The recent introduction of techniques capable of stimulating the brain transcranially has made it possible to develop protocols of brain stimulation inducing LTP-like effects (Hallett, 2007). Repetitive transcranial magnetic stimulation (rTMS) techniques can induce changes in brain excitability that outlast the stimulation period (Cooke & Bliss, 2006). A rTMS approach for producing LTP-like changes in human brain is the so-called paired associative stimulation (PAS) (Stefan et al. 2000). This method is based on the Hebbian concept of spike-timing-dependent plasticity: two inputs, the first arising from electrical peripheral nerve stimulation and the second delivered over the motor cortex using TMS, are paired to activate brain networks approximately synchronously. If a sufficient number of pairs of stimuli is delivered, then the excitability of the sensorimotor cortex increases. PAS is a cortical phenomenon (Di Lazzaro et al. 2009) and pharmacological studies have shown that its effects are influenced by drugs that act on the NMDA receptor pathways, supporting the hypothesis that it involves LTP-like changes in cortical synapses (Stefan et al. 2002). Interestingly, by documenting that polymorphisms of the gene encoding brain-derived neurotrophic factor, which is involved in brain synaptic plasticity, influences PAS-induced LTP-like effects, Cheeran et al. (2008) provided evidence that PAS can be influenced by the genetic background of an individual.
Based on experimental evidence indicating that Ras proteins are involved in synaptic plasticity mechanisms, we hypothesized that LTP-like phenomena are altered in CS. The aim of this study was to evaluate LTP-like activity using PAS in a cohort of subjects with molecularly proven CS, and compare the results with those obtained in a control age-matched group.
Methods
Ethical approval
The study was performed according to the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty of the Catholic University of Rome. Informed consent was obtained based on a protocol approved by the institutional review board obtaining the consensus both from patients and their parents after a detailed explanation of the procedures and the aims of the study. Also the healthy subjects were informed about the aims of the study and gave written consent. rTMS was performed according to the recently published guidelines for the use of repetitive transcranial magnetic stimulation in subjects under 18 years (Rossi et al. 2009).
Patients
The study included five patients (mean age: 18.6 ± 5 years) with molecularly confirmed diagnosis. All the patients were clinically assessed. Diagnosis was obtained in relation to clinical characteristics (Fig. 1), and was confirmed by mutational screening of the HRAS gene. Relevant clinical, neuropsychological and molecular data are reported in Table 1. None of the patients was taking drugs acting on CNS or was affected by epilepsy.
Figure 1.
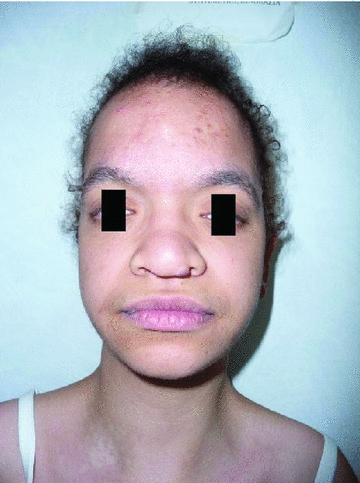
One of the studied patients presenting typical facial features of Costello syndrome
Table 1.
Dysmorphic, neurological, osteoarticular, cardiac and ectodermal features, and neoplasias in the subjects with Costello syndrome, heterozygous for the c.34G>A missense change (Gly12Ser) in HRAS, included in the study
| Features | |||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Sex | F | M | F | F | F |
| Age (years) | 15 | 16 | 24 | 24 | 14 |
| Growth* | |||||
| OFC (s.d.) | 0 | +2 | 0 | −2 | +1 |
| Height (s.d.) | −4,5 | −4,5 | −4,5 | −6 | −6 |
| Weight (s.d.) | −3,5 | −3,5 | −3,5 | −4 | −3 |
| Facial | |||||
| ‘Coarse’ face | + | + | + | + | + |
| Epicanthus | + | + | + | + | + |
| Strabismus | + | + | + | + | − |
| Depressed and wide nasal bridge | + | + | + | + | + |
| Bulbous tip of nose | + | − | + | + | + |
| Fleshy and cocked auricular lobes | + | + | + | + | + |
| Low set/posteriorly angulated ears | + | + | + | + | + |
| Full cheeks | + | + | + | + | + |
| Macrostomy/thick lips | + | + | + | + | + |
| Hoarse and deep voice | + | + | + | + | + |
| Mental development (IQ) | 52a | 41a | 52a | 48a | 51a |
| Dystonia (GDS/UDRS) | 8/6 | 7/5 | 12/9 | − | 6/4 |
| Epilepsy | − | − | − | − | − |
| MRI anomalies | − | − | Ch | − | Ch |
| Long Term Memory | |||||
| Verbal learning (IR/DR) | 9°/9° | 16°/25° | <1/2° | 2°/5° | 1°/2° |
| Visual learning (IR/DR) | 1°/<1° | <1°/1° | <1°/<1° | <1°/9° | 16°/2° |
| Spatial learning (IR/DR) | 2°/9° | 5°/5° | 9°/1° | 25°/37° | 16°/5° |
| Mental age | 6.8 | 7.8 | 12.2 | 11.0 | 7.0 |
| Heart | |||||
| Hypertrophic cardiomiopathy | + | + | + | + | + |
| Valvular anomalies | − | − | MVP | PVS | − |
| Arrhythmias | PAC | PVC | − | rBBB | − |
| Osteoarticular | |||||
| Short neck | + | + | + | + | + |
| Ulnar deviation of fingers | + | + | + | + | + |
| Interphalangeal laxity of fingers | + | + | + | + | + |
| Limited extension of joints | H, A | E, H, A | E, H, A | E, A | E |
| Spine | Hl | K | Hl, S | S | S |
| Skin and adnexa | |||||
| Deep palmar and plantar creases | + | + | + | + | + |
| Dark pigmentation | + | + | + | + | + |
| Thin dystrophic nails | + | + | + | + | + |
| Redundant skin | + | + | + | + | + |
| Hyperkeratosis | − | + | + | + | + |
| Curly hair | + | + | + | + | + |
| Neoplasias | |||||
| Papillomata | + | + | + | + | − |
| Other neoplasias | − | + | + | + | − |
Growth parameters referred to time of examination; A, Achilles tendon; Ch: Chiari 1 malformation; E, elbow; H, hip; Hl, hyperlordosis; Hy: hydrocephalus; IQ, intelligence quotient; MVP: mitral valve prolapse; PVS: pulmonary valve stenosis; PAC: premature atrial contraction; PVC: premature ventricular contraction; rBBB: right bundle brunch block; K, kyphoscoliosis; MRI: magnetic resonance imaging; S, scoliosis. IR, immediate recall. DR, delayed recall.
Wechsler intelligence scale. GDS, Global Dystonia Scale. UDRS, Unified Dystonia Rating Scale. OFC, Occipitoprontal circumference. S.D. standard deviations from standard median valves based on centers for disease control and prevention (CDC) Sex-specific OFC, height and weight-for-age growth charts.
A detailed neuropsychological assessment to evaluate their cognitive function was available for recruited patients. Cognitive development was assessed using the Wechsler intelligence Scale for Children-Revised (WISC-R) and the Wechsler Adult Intelligence Scale (WAIS), depending on the age of the subject and if appropriate for the level of functioning. Mental development was classified according to the classification reported in the Diagnostic and Statistical Manual of Mental Disorders, 1994 (DSM-IV). Memory function was investigated using the subtest of explicit/declarative long term memory of PROMEA, a battery of tests specifically designed for assessing memory and learning for which age specific normative data, collected in an Italian cohort, are available. The battery includes verbal, visual and spatial long term memory tasks. Verbal tasks included word-list learning with a list of 15 words of similar frequency of use that were not semantically related. The visual-object learning task included 15 figures of common objects (e.g. a tree, a knife, a flower). Fifteen figures of common objects were also presented in the study phase of the visual-spatial learning task. The test is subdivided into a study phase, in which the patient is asked to observe the objects presented and a test phase in which the patient is asked to recognize them. Both study and test phases were presented, in total, for three consecutive times (trials); each time the subject was asked to immediately recognise as many items as possible and the number of elements correctly recognised in each trial was scored. After the third trial with both study and test phase, the test phase only was repeated again after 15 min to assess long term memory (Vicari, 2007). Scores were calculated according to mental age. All patients had cognitive impairment with IQ ranging from 41 to 52. On the tests assessing long term memory, all patients showed low scores, even when the scores were calculated according to their mental age (Table 1).
As previously reported (Axelrad et al. 2004) our patients were social and cooperative despite their cognitive difficulties and they were all able to understand the simple task required for this electrophysiological study: to keep fully relaxed.
We evaluated the presence of dystonic symptoms in Costello patients by using the Unified Dystonia Rating Scale (UDRS) Revised score and the Global Dystonia Scale (GDS) (Table 1). For all the patients, brain and cervical spinal cord magnetic resonance imaging (MRI) were available.
Control electrophysiological data were obtained in a group of 13 age-matched subjects (mean age: 22.4 ± 5.2 years; P = 0.18) without any neurological and medical abnormality to compare the results obtained in CS patients with a healthy age-matched population. All the studied subjects were right-handed.
Transcranial magnetic stimulation
Focal TMS of the right hand M1 was performed with a high power Magstim 200 (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor responses in the contralateral FDI. Intensities were expressed as a percentage of the maximum output of the stimulator.
Resting motor threshold (RMT) was defined according to the recommendations of the IFCN Committee (Chen et al. 2008) as the minimum stimulus intensity that produced a liminal MEP (>50 μV in 50% of 10 trials) with the tested muscle at rest. The active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a liminal MEP (about 200 μV in 50% of 10 trials) during isometric contraction of the tested muscle. Throughout the entire study, both for patients and for healthy subjects, we used an orientation of the stimulating coil over the motor strip with the handle pointing backwards, with the induced current flowing in a posterior–anterior (PA) direction.
MEPs were band pass filtered (bandwidth 3 Hz to 3 kHz) (Digitimer D360 amplifiers) and each single trial was recorded on computer for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) and associated software. Recordings were made from the relaxed first dorsal interosseous muscle (FDI) of the left hand for both groups. The responses to 20 stimuli obtained at rest at an intensity of 120% RMT were averaged for the main experiment.
In a subgroup of healthy controls (10 subjects) and in two CS patients (patients 1 and 2), we also measured MEP amplitude at 140% RMT intensities (20 sweeps were averaged for each trial). We calculated the percentage increase in amplitudes observed at 140% RMT in respect of 120% RMT both in patients and in controls in order to evaluate whether the increase in amplitudes were comparable.
Short interval intracortical inhibition (SICI) and facilitation (ICF) were studied using the technique of Kujirai et al. (1993). Using a Bistim module, two magnetic stimuli were given through the same stimulating coil over the motor cortex and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. The conditioning stimulus was set at an intensity of 5% (of stimulator output) below active motor threshold. The second, test, shock intensity was adjusted to evoke a muscle response in relaxed FDI with a peak-to-peak amplitude of approximately 0.5 mV. The timing of the conditioning shock was altered in relation to the test shock: two inhibitory interstimulus intervals (ISIs) of 2 and 3 ms and one facilitatory ISI of 15 ms were investigated. Five stimuli were delivered at each ISI. The subject was given audio-visual feedback at high gain to assist in maintaining complete relaxation. The amplitude of the conditioned MEPs was expressed as a percentage of the amplitude of the test MEPs. The amplitude of the conditioned responses at the two inhibitory ISIs were averaged to give a grand mean value of SICI, and that of the conditioned responses at the facilitatory ISI was averaged to have a grand mean value of ICF.
The SP was elicited whilst subjects held a tonic voluntary contraction of approximately 50% of MVC. Five stimuli at 200% AMT were given. Ten trials were given at the left-FDI optimal scalp site using a stimulus intensity of 200% AMT. The duration of the SP was assessed in individual trials and was defined as the period from the onset of EMG suppression until the resumption of sustained post-stimulus EMG activity (McDonnell et al. 2006).
Paired associative stimulation
We used a high power Magstim 200 (Magstim Co.) connected to a figure-of-eight coil, with external loop diameters of 9 cm held over the right motor cortex at the optimum scalp position to elicit MEPs in the contralateral FDI. The induced monophasic current in the brain flowed in a posterior-to-anterior direction. The intervention consisted of single electrical stimuli delivered to the left ulnar nerve at the wrist at 300% of the sensory perceptual threshold (SPT), followed by TMS at an intensity sufficient to produce an unconditioned response amplitude of approximately 1 mV in the resting FDI. Ninety pairs were delivered at 0.05 Hz over 30 min at an interstimulus interval (ISI) of 25 ms. An ISI of 25 ms was used because this interval had been shown in previous experiments to be effective in increasing cortical excitability (Stefan et al. 2000).
We assessed for each subject and patient both sensory perceptual threshold (SPT) and ulnar pheripheral motor threshold (pMT). SPT was detected by increasing the stimulator intensity by steps of 0.1 mV until the sensation at the wrist was perceived. PMT was evaluated by increasing the stimulator intensity by steps of 0.1 mV, while recording from the left FDI, until a liminal compound motor action potential (CMAP) appeared.
To ensure that there was a similar timing for the arrival of the sensory afferent stimulus in motor cortex between groups, before baseline TMS assessment we recorded the N20 component of somatosensory evoked potentials (SEPs) after ulnar nerve stimulation in three CS patients (patients 1–3) and in all control subjects. To record SEPs, the active electrode was attached 3 cm posterior to C4 (10–20 system), and the reference electrode was at the right earlobe. Five hundred responses were averaged to identify the latency of the N20 peak.
Experimental design
Main experiment
We evaluated motor thresholds and MEPs recorded from left FDI in five Costello patients and 13 control subjects in baseline conditions and immediately after the end of PAS.
The EMG signal was monitored on a computer screen and via loudspeaker to provide feedback on the state of muscle relaxation. Trials contaminated with voluntary muscle activities were rejected. Moreover, we performed a quantitative off-line analysis of the pre-stimulus EMG activity for the 20 ms preceding cortical stimulus, by rectifying each recorded frame and calculating root means squares of the grand mean for each subject and for each patient.
Measures of baseline intracortical inhibition and facilitation
In a subgroup of Costello patients (patients 1, 2 and 3; mean age 18.3 ± 4.9 years, P = 0.24; Table 1) and in all control subjects we also evaluated baseline short interval intracortical inhibition (SICI) and facilitation (ICF) and cortical contralateral silent period (SP).
Time course of PAS-induced effects on left lFDI MEP and motor thresholds and somatotopy
In four CS patients (patients 1, 2, 3 and 5; mean age 17.2 ± 4.6 years) and in nine control subjects (mean age: 22.2 ± 4.9 years, P = 0.11), we probed the time course and topographical specificity of after-effects induced by PAS, performed as described above (i.e. ulnar electrical stimulation and TMS over left FDI motor cortex hot spot), by recording MEPs from left FDI and left abductor pollicis brevis muscle (APB) at three time points (before, immediately after and 30 min after PAS application). The sequence of recordings from FDI and APB was counterbalanced throughout this experimental condition. The optimal stimulation site over right motor cortex for left APB was identified and marked separately from that of left FDI. RMT, AMT and mean amplitude of MEPs evoked using an intensity of 120% RMT were assessed for both target muscles. The effects of paired associative stimulation on motor thresholds and resting amplitudes of left FDI MEP were compared to those of left APB for each time point.
PAS100 effects on left lFDI MEP and motor thresholds
In order to further exclude that variables such as attention and emotional factors that may arise through the fact that CS subjects were mentally retarded could interfere with the main results, in two patients (patients 2 and 3; aged 16 and 24 years) and in five control subjects (mean age: 24.8 ± 2.9, P = 0.18) we performed a PAS control experiment by modifying the timing of the TMS pulse related to ulnar nerve electrical stimulation. We used an ISI of 100 ms: the PAS100 protocol is not expected to produce any change in MEP amplitude (Stefan et al. 2000). Again, we measured RMT, AMT and mean MEP amplitude evoked using an intensity of 120% RMT at three time points (before, immediately after and 30 min after PAS).
Statistics
Main experiment
Since we aimed to disclose any difference in baseline motor cortex excitability and PAS-induced after-effects between the two studied groups, baseline MEP amplitude, RMT and AMT with PAS after-effects were compared in Costello patients and in the group of age-matched controls. Since MEP amplitude values tended to be log-normally distributed, a logarithmic transformation in order to best fit the ANOVA assumptions (gaussianity and homoschedasticity) was performed.
To compare motor thresholds between the two groups (Costello patients vs. healthy controls), we used a repeated measures analysis of variance (ANOVA) with PARAMETER (AMT and RMT), and TIME (baseline and post PAS) as within-subject factors and GROUP (Costello and Controls) as between-subject factors.
We compared MEP amplitudes in baseline conditions and after the application of PAS using a separated repeated measures ANOVA with TIME (baseline and post PAS) as within-subject factors and GROUP (Costello and Controls) as between-subject factor. When significant main effects or interactions were found, post hoc tests with correction for multiple comparisons were performed. The level of significance was set at P < 0.05.
We calculated the N20 latency normal limits in control subjects (mean ± 2.5 s.d.).
The comparisons of SRTs, pMTs and pre-stimulus EMG activity in baseline conditions between CS patients and healthy controls were performed by means of separated unpaired t tests with the level of significance set at P < 0.05.
In order to study the stimulus–response relationship in CS patients, we measured how far away the ratio of 140% RMT MEP amplitude and 120% RMT MEP amplitude of CS patients was from the mean ratio of the control subjects group by using a z-score.
Measures of baseline intracortical inhibition and facilitation
The mean amount of SICI and ICF and SP length were calculated in the CS patients group and the healthy control group. We calculated the normal limits of SICI, ICF and SP in control subjects (mean ± 2 s.d.).
Time course of PAS-induced effects on left FDI and motor thresholds and somatotopy
To explore the difference in motor thresholds between control subjects and patients, a four-way factorial ANOVA was performed with TIME (t0, t1, t2), TOPO (homo, hetero) and PARAMETER (RMT, AMT) as within-subject factors and GROUP (Costello and Controls) as between-subjects factor.
To assess the difference in MEP amplitude (after logarithmic transformation) between control subjects and patients, a three-way factorial ANOVA with TIME (t0, t1, t2) and TOPO (homo, hetero) as within-subject factors and GROUP (CS patients and controls) as between-subjects factor was performed. When significant main effects or interactions were found, post hoc tests with correction for multiple comparisons were performed. The level of significance was set at P < 0.05.
PAS100 effects on left FDI MEP and motor thresholds
We compared the effects of PAS100 between the two studied subgroups by performing two separated repeated measures ANOVAs, one for motor thresholds (PARAMETER (RMT, AMT) and TIME (T0, T1, T2) as within-subjects factors and GROUP (Costello and Controls) as between-subjects factor) and one for MEP amplitude (TIME(T0, T1, T2) as within-subject factor and GROUP (Costello and Controls) as between-subjects factor). When significant main effects or interactions were found, post hoc tests with correction for multiple comparisons were performed. The level of significance was set at P < 0.05.
Before entering ANOVA, all the data were tested for sphericity by using Mauchly's sphericity test.
Results
Main experiment
No significant difference was found in baseline cortical excitability parameters between patients and controls. Mauchly's test showed that sphericity was never violated in the rmANOVAs.
ANOVA for motor thresholds showed no significant TIME × GROUP interactions (F1,32 = 1.5; P = 0.23) (baseline motor thresholds, CS patients vs. Controls; RMT: 49.6 ± 5.0 vs. 47 ± 11.4, P = 0.63; AMT: 37.2 ± 5.3 vs. 35.4 ± 8, P = 0.65).
In the subgroup of healthy controls (10 subjects) and in two CS patients in whom we calculated the percentage increase in amplitudes observed at 140% RMT in respect of 120% RMT, the increase in amplitudes was not significantly different (z-score = −0.05; P = 0.96). The mean increase in amplitude in control subjects was 78 ± 55% and it was 52% in patient 1 and 100% in patient 2.
The ulnar nerve stimulation intensity used for PAS (300% SPT) was comparable in CS patients and in controls; it was 0.88 ± 0.13 times pMT in CS and 0.9 ± 0.1 times pMT in control subjects (P = 0.87, unpaired t test). N20 latency in CS was within normal limits: mean N20 latency was 18.6 ± 0.9 ms in healthy group (upper limit 20.8 ms; lower limit 16.3 ms), N20 latency was 17 ms for patient 1, 19 ms for patient 2, 20.5 ms for patient 3. Pre-stimulus background EMG activity (CS patients: 23,2 ± 5.5 μV; Controls: 18,2 ± 8,1 μV; P = 0.23) was comparable in the two groups.
None of the patients and controls experienced any adverse event during and after the application of PAS.
ANOVA for MEPs (ln μV) revealed a significant TIME × GROUP interaction (F1,16 = 9.5; P = 0.007). A post hoc unpaired t test showed no statistical difference for baseline MEPs between CS patients and controls (MEP amplitude (ln μV) (Costello vs. Healthy subjects): 6.3 ± 0.7 vs. 6.2 ± 0.5; P = 0.5). Remarkably, mean MEP amplitude was increased by about 230% in CS patients immediately after PAS, and by approximately 65% in healthy subjects (CS patients, baseline vs. post-PAS; controls, baseline vs. post-PAS: 695 ± 450 vs. 2300 ± 1500 μV; 570 ± 280 vs. 950 ± 430 μV) (Fig. 2). Moreover, after PAS application there was a significant difference in MEP amplitude both between groups (ln μV, CS patients vs. healthy subjects: 7.5 ± 0.8 vs. 6.7 ± 0.5; P = 0.02) and within each group (ln μV, CS patients, baseline vs. post-PAS: 6.3 ± 0.7 vs. 7.5 ± 0.7; P = 0.003; healthy subjects, baseline vs. post-PAS: 6.2 ± 0.5 vs. 6.7 ± 0.5; P = 0.0004). Figure 3 shows the individual mean MEPs in baseline conditions and after PAS application for each CS patient and for one representative control individual.
Figure 2. Mean peak-to-peak amplitude (μV) of motor evoked potentials recorded at rest in baseline conditions and after PAS in control subjects and in patients with Costello syndrome (CS).
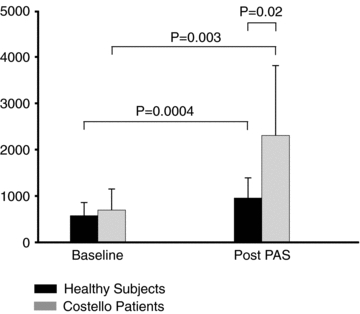
Each error bar is standard deviation. PAS led to an increase in MEP size in patients and controls. However, the facilitatory effect was significantly stronger in patients (65% increase in control subjects vs. 230% increase in CS patients).
Figure 3. Effects of paired associative stimulation (PAS) on the size of motor evoked potentials recorded at rest in one representative healthy control (upper trace) and in the five patients with Costello syndrome (lower traces).
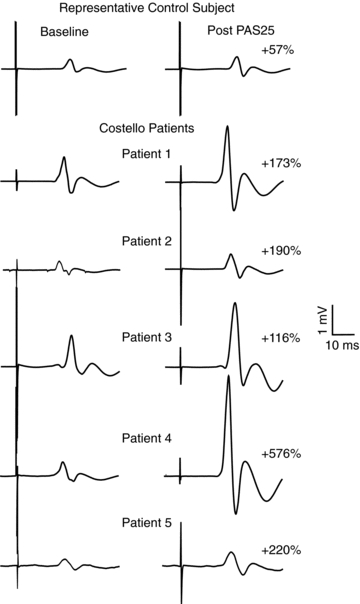
Each trace is the average of 20 sweeps. The percentage facilitation is reported for the control subject and patients. The facilitatory effects of PAS are extremely enhanced in patients.
Measures of baseline intracortical inhibition and facilitation
Mean SICI (Costello vs. Controls) was 33.2 ± 16.5 vs. 35.8 ± 18.1%, mean ICF was 130 ± 40 vs. 139 ± 49%, and mean SP was 131.4 ± 36.3 vs. 137.6 ± 27.1 ms. In CS, SICI was 52.2% for patient 1, 23.7% for patient 2 and 23.6% for patient 3 (lower limit 0%; upper limit 72%); ICF was 110% for patient 1, 104% for patient 2 and 176% for patient 3 (upper limit 237%); SP was 153 ms for patient 1, 89.4 ms for patient 2 and 151.8 ms for patient 3 (upper limit 192 ms).
Time course of PAS-induced effects on FDI MEP and motor thresholds and somatotopy
ANOVA for motor thresholds showed no significant TOPO × TIME × PARAMETER × GROUP interaction (F2,88 = 0.30; P = 0.74) so that, for motor thresholds, the two studied muscles had the same time course in both groups.
For MEP amplitude repeated measures ANOVA showed a significant main effect for TIME (F2,44 = 51.6; P < 0.0001) since an increase in mean MEP amplitudes after PAS was observed in both groups. Moreover there was a TIME × GROUP interaction (F2,44 = 13.5; P < 0.0001): PAS determined a larger increase in MEP amplitude in CS patients than control subjects. A statistical significance for TIME × TOPO interactions (F2,44 = 3.8; P = 0.02), but no significant TIME × GROUP × TOPO interactions (F1,72 = 0.97; P = 0.38) was found. In particular, it seemed that the facilitatory effect size of PAS is more pronounced in left FDI muscle than left APB muscle.
Since TIME × GROUP interaction was significant, we performed a post hoc t test between MEP amplitudes at the same time point and in the same muscle between CS patients and controls: this analysis revealed a statistical difference in the amplitudes measured at T1 and T2 in both muscles (FDI, T1 and T2: P = 0.04 and P = 0.03; APB, T1 and T2: P = 0.04 and P = 0.002) (Fig. 4).
Figure 4. Time course of the effects of ulnar paired associative stimulation (PAS) on MEPs in a subgroup of healthy controls (9 subjects) and in three patients with Costello syndrome (CS) for FDI muscle (homotopic effect) and APB muscle (heterotopic effect).
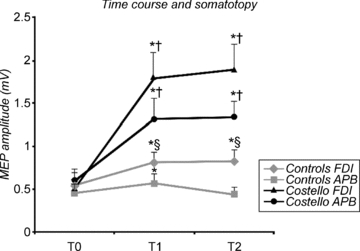
The figure shows that PAS induced an increase of MEP amplitude in both muscle for CS patients, but only in FDI for healthy subjects; values are means ± s.e.m.; T0 = baseline, T1 = immediately after the end of PAS, T2 = 30 min after the end of PAS. *P < 0.05 vs. baseline in the same group, and for the same muscle; §P < 0.05 vs. APB muscle in each time point and in each group; †P < 0.05 vs. the same muscle of controls group.
To explore within-group effects, we computed a separated two-factorial ANOVA for each group, with TIME (T0, T1, T2) and TOPO (homo, hetero) as within-subject factors. ANOVA for the control group revealed a main effect for TIME (F2,36 = 9.9; P = 0.004) and a significant TIME × TOPO interaction (F2,36 = 3.7; P = 0.01) that was due to a different pattern of PAS-induced effect on each studied muscle. An unpaired t test showed a significant difference between mean FDI MEP amplitude and mean APB MEP amplitude after PAS at T2 (P = 0.007) with a more pronounced increase of amplitude in FDI muscle. For FDI muscle a paired t test showed a significant difference both for T1 vs. T0 (P = 0.0007) and for T2 vs. T0 (P = 0.0005). For APB muscle a paired t test showed only a significant difference for T1 vs. T0 (P = 0.03) (see Fig. 4).
In the CS group, ANOVA showed a main effect for TIME (F2,12 = 54.6; P < 0.0001), but no TIME × TOPO interaction (F2,12 = 0.9; P = 0.44) that disclosed a similar effect of ulnar PAS in both muscles. An unpaired t test showed no significant difference between mean lFDI MEP amplitude and mean APB MEP amplitude after PAS (both at T1 and T2; P = 0.5 for both comparisons). In this group, a paired t test showed a significant difference both for T1 vs. T0 and for T2 vs. T0, and both for FDI and APB muscle, confirming the same time course after PAS in both muscles (FDI, T1 vs. T0, T2 vs. T0: P = 0.0008, P = 0.0009; APB, T1 vs. T0, T2 vs. T0: P = 0.01, P = 0.01) (see Fig. 4).
PAS100 effects on left FDI MEP and motor thresholds
ANOVA for motor thresholds showed no significant TIME × PARAMETER × GROUP interaction (F2,20 = 0.45; P = 0.64) so that motor thresholds had the same time course in both groups after PAS100 application. When ANOVA for mean MEP amplitude was performed, again, we noticed no significant TIME × GROUP interaction (F1,10 = 0.043; P = 0.96): PAS100 did not produce any change in MEP amplitude in both subgroups (CS patients (T0, T1, T2) and controls (T0, T1, T2): 636 ± 116 vs. 600 ± 20 vs. 630 ± 28 μV and 637 ± 391 vs. 527 ± 243 vs. 571 ± 217 μV).
Discussion
PAS represents a cortical phenomenon (Di Lazzaro et al. 2009) and is considered a marker of LTP-like plasticity in humans (Stefan et al. 2000; Wolters et al. 2003) because the after-effects are rapidly evolving, persistent, NMDA dependent and topographically specific (Stefan et al. 2000, 2002; Ridding & Taylor, 2001; Wolters et al. 2003). Accordingly to previous papers (Stefan et al. 2000; Quartarone et al. 2003, 2008), PAS induced a significant and long-lasting MEP increase with a different size effect in the studied hand muscles in healthy subjects. However, the main finding of the present study was the demonstration of very pronounced PAS-induced after-effects in CS patients: the increase of MEP amplitude after PAS was four times larger compared to that observed in age-matched healthy subjects. Furthermore, by studying the effects of interventional ulnar PAS on two different muscles we also disclosed a trend towards loss of PAS topographical specificity in CS patients, as these subjects, but not control individuals, showed a similar PAS-induced MEP increase that outlasted the stimulation period both in APB and FDI muscles. Changes in baseline cortical excitability could not be responsible for enhanced PAS effects in our patients because motor thresholds and amplitude of MEPs were within normal limits. Moreover, the input–output properties of cortical excitability, as evaluated by measuring the percentage increase of the size of motor evoked potentials elicited at two different stimulus intensities, were similar in patients and in controls. The level of excitability of GABA-related cortical circuits may also affect cortical plasticity (Ziemann et al. 1998; McDonnell et al. 2006; Teo et al. 2009). GABA related cortical activity as evaluated by measuring SICI and silent periods were within normal limits in CS patients; however, it should be considered that we studied SICI and silent period only in a subgroup of Costello patients and only using a conditioning stimulus intensity producing a strong SICI, and thus a slight change in SICI might be overseen in our study. Intracortical facilitation also was within normal limits in CS patients but the study of ICF has the same limitations of SICI study. It should also be considered that N20 latency of CS patients was within normal limits, so we could consider that the timing of synchronicity of events during PAS was comparable between the two groups. Moreover, we also demonstrated that CS patients were able to maintain muscular relaxation as well as controls and that the stimulation intensities used during the repetitive stimulation were comparable between the two groups. Furthermore the application of a PAS protocol with an ISI of 100 ms didn't show any difference between the studied subgroups and, accordingly to the data by Stefan and coworkers (2000), it did not induce any significant enhancement of cortical excitability in both healthy subjects and CS patients. Thus, by considering the above points, it seems unlikely that our results were influenced by CS patients’ bad performance in hand muscle relaxation or in keeping a constant level of attention. If that was the case, we probably would have found a MEP increase also after PAS100 application. Functional and structural CNS abnormalities, such as EEG abnormalities or Chiari I malformation, have been previously reported in CS (Der Kaloustian et al. 1991; Zampino et al. 2007). Only two of our patients exhibited a mild and asymptomatic Chiari I malformation and none had electrophysiological or clinical signs of epilepsy. Thus, the altered LTP activity in our cases cannot be explained by other concomitant or underlying neurological abnormalities.
What is the origin of enhanced LTP-like activity in Costello patients?
CS is caused by heterozygous germline, activating mutations of HRAS (Aoki et al. 2005), and belongs, together with neurofibromatosis type 1 (NF1), Noonan syndrome, Leopard syndrome and cardiofaciocutaneous syndrome, to a class of genetic syndromes linked to perturbation of function through the Ras pathway (Aoki et al. 2008). Approximately 90% of CS cases share an amino acid substitution affecting the glycine residue at codon 12, which is located in the guanine nucleotide binding site (Aoki et al. 2005). These mutations result in impaired GTP hydrolysis, and enhance HRAS function and upregulate signal flow through this GTPase. Ras genes are abundantly expressed in the adult CNS and several lines of evidence consistently indicate that Ras signalling plays a key role in synaptic plasticity (Mazzucchelli & Brambilla, 2000; Sweatt, 2004).
Previous experimental data based on a H-Ras knock-out mouse model supported a possible negative modulatory role for Ras signalling in NMDA receptor function (Manabe et al. 2000; Thornton et al. 2003). Accordingly, LTP experiments conducted in neurofibromatosis type 1 (NF1)-animals suggested that carrying a mutated neurofibromin with gain of function mutation in the Ras–MAPK pathway leads to a decrease rather than increase of LTP (Costa et al. 2002; Guilding et al. 2007).
In a recent review, Stornetta & Zhu (2010) report that both hypo- and hyperactivation of Ras signalling produced by several genetic defects impair the capacity of synaptic plasticity, underscoring the importance of a ‘happy-medium’ dynamic regulation of the signalling. Thus, the increased PAS after-effects observed in our patients appear in contrast with the strong evidence provided by the studies reviewed in the paper by Stornetta & Zhu of a correlation between altered Ras signalling and impairment of synaptic plasticity. Moreover, it appears difficult to reconcile the enhanced LTP-like activity with cognitive impairment in CS patients. Several hypotheses might explain these discrepancies. First, the Ras related mechanism for altering synaptic strength during plasticity is based on trafficking of AMPA-sensitive receptors (AMPA-Rs) at postsynaptic sites. Because different types of AMPA-Rs mediate transmission at intracortical and thalamocortical synapses (Kielland et al. 2009; Zhu, 2009), CS related alteration in Ras signalling might have a differential effect at thalamocortical and intracortical synapses. Because the LTP-like activity produced by PAS involves afferent stimulation and thus thalamocortical synapses (Oliviero et al. 2005), it can be hypothesized that high Ras related signalling drives AMPA-R into thalamocortical synapses resulting in enhancement of LTP-like activity. Another possible explanation is related to the role of Ras signalling in synaptic depotentiation (Stornetta & Zhu, 2010). If in CS patients there is a selective involvement of this Ras related modulation of synaptic function, PAS could result in an uncontrolled enhancement. However, at present there is no evidence in favour of this mechanism.
Though synaptic plasticity impairment seems to be present in all forms of genetically determined Ras signalling abnormality (Stornetta & Zhu, 2010), a slight enhancement of LTP has been reported in a single experimental study (Kushner et al. 2005). Kushner et al. (2005), by studying transgenic mice expressing a constitutively active form of H-Ras (i.e. G12V), documented a moderate LTP increase associated with the expression of hyperactive GTPase. Note that mice studied by Kushner revealed strong activation of downstream targets and enhanced learning and memory (Kushner et al. 2005) which is in contrast with the cognitive impairment and behavioural deficits observed in CS patients. Therefore, although the study by Kushner et al. (2005) seems to suggest an influence by ‘gain of function’ mutations of HRAS or, generally, of the Ras-MAPK pathway in enhancing LTP phenomena that is in agreement with our findings in CS, there is a clear discrepancy between the enhanced learning and memory of the mice studied by Kushner et al. (2005) and the impaired memory and learning of CS patients. It should be noted that the animal model studied by Kushner et al. (2005) might not be fully comparable to CS. Only recently, a new mouse model of CS has been described (Schuhmacher et al. 2008). These mice phenocopied some of the abnormalities observed in patients with CS, including facial dysmorphia and cardiomyopathies. These mice also exhibit behavioural phenotypes (Viosca et al. 2009) that resembled the hyperemotivity, hypersensibility and cognitive impairments observed in children with CS (Axelrad et al. 2007). The discrepancies between our findings and the results of experimental studies might also be related to the characteristics of the LTP protocol used. The type of protocol used for inducing LTP has been shown to influence the response in an animal model of altered Ras function. In NF1 mice brain slices, the effects of two different LTP inducing protocols were compared: theta burst stimulation (TBS) and high frequency stimulation (HFS) (Costa et al. 2002). The results of the study showed that while TBS-induced LTP was reduced, the LTP effects produced by HFS were normal. Thus, NF1 mice, characterized by an upregulation of HRAS signalling, showed a LTP deficit only when a TBS protocol was used, but not with HFS stimulation. The differential behaviour of TBS and HFS in NF1 mice can be explained by the complexity of the LTP phenomenon that has multiple forms involving multiple electrophysiological components of neurons (Kim & Linden, 2007). The same is true for rTMS after-effects, although the several rTMS techniques induce similar changes in the cortex, they exert their effects through different mechanisms (Ridding & Ziemann, 2010). Thus, a strict parallelism between findings in NF1 animals and PAS effects in Costello patients is not possible even from an electrophysiological point of view. It could be that the presence of a constitutional alteration in the synaptic and intracellular signalling pathway, like that observed in CS and in other syndromes affecting the Ras–MAPK system, could result in heterogeneous after-effects induced by the different rTMS protocols.
It should be also considered that it is unlikely that there is a strict correspondence between mechanisms underlying in vivo PAS-induced LTP-like plasticity and those underlying experimental LTP because of the non-invasive nature of PAS studies at the system level (Ziemann et al. 2004).
Finally, it should also be considered that Ras signalling not only affects LTP but is also likely to change basal synaptic transmission (Stornetta & Zhu, 2010), and thus the enhancement of PAS might originate from changes in the intrinsic excitability properties of the central synapses involved in PAS. Because cortical excitability as evaluated by threshold and MEP evaluation was within normal limits, aberrant Ras may have a more profound impact on intrinsic properties on neurons different from those activated by TMS such as those recruited by afferent stimulation.
In any case, the discrepancy between the LTP enhancement in our study and the impairment of synaptic plasticity predicted by the ‘happy-medium’ theory about Ras signalling and synaptic plasticity (Stornetta & Zhu, 2010) indicates that further studies are needed to elucidate the role of HRAS signalling in PAS related synaptic plasticity in CS patients.
In agreement with previous studies (Cesarini et al. 2009), all our patients presented a moderate mental retardation. It is of note, however, that when we used a specific test for long term memory, the results showed a marked impairment. This cannot be simply explained by the overall global mental retardation as the low results persisted even when the scores were calculated according to mental age. The concomitant finding of a very high level of LTP-like activity and poor memory in our patients might appear to be in contrast with extensive evidence supporting a positive correlation between LTP and mammalian learning and memory (Malenka & Bear, 2004). The reasons for these findings are still unclear and further ad hoc studies are needed to evaluate any correlation between LTP-like activity level and learning and memory in Costello patients.
A pathological increase in PAS-induced after-effects was observed also in focal and generalized dystonia (Quartarone et al. 2003, 2009; Weise et al. 2006). Interestingly, there are signs of mild dystonic symptoms in CS patients and a deficit in motor learning in dystonic patients carrying DYT1 mutations (Ghilardi et al. 2003). This suggests, that both dystonia and impaired motor learning might be related to excessive plasticity that results in deleterious effects (Quartarone et al. 2006). However, there are some differences between electrophysiological findings of CS patients and dystonic patients because in the latter PAS abnormality is associated with SICI abnormality while in the former SICI is completely normal.
Study limitations and possible confounders
The number of patients included in this study is relatively small but it should be considered that the number of CS sufferers reported in the literature is very limited, about 250 individuals worldwide (Gripp & Lin, 2009). Moreover, since, in adherence with rTMS safety guidelines (Rossi et al. 2009), we studied the oldest CS patients available in our hospital, it is possible that some components of our findings will not generalize well to a broader CS population.
Again we should consider that PAS-induced MEP increase is just a surrogate of experimental and synaptic LTP so that our results could not perfectly correspond to future experimental data about the link between H-Ras and LTP.
Conclusions
We observed extremely pronounced LTP-like activity with a loss of topographical specificity in patients with a single mutation in the HRAS gene and cognitive impairment, providing the first demonstration in humans of the role of HRAS in LTP-like mechanisms and possibly in memory and learning. Furthermore, the present study could provide the basis for further and more extensive investigations into the relationship between LTP-like activity and memory in CS and in other disorders also due to mutations in genes of the Ras cascade, including the more recurrent Noonan syndrome.
Acknowledgments
We wish to thank the patients and families who participated in the study. This study was supported in part by the Associazione Italiana Sindromi di Costello e CFC (to G.Z.) and the ERA-Net for research programmes on rare diseases 2009 (to M.T.).
Glossary
Abbreviations
- AMT
active motor threshold
- APB
abductor pollicis brevis
- CS
Costello syndrome
- FDI
first dorsal interosseus muscle
- ICF
intracortical facilitation
- ISI
interstimulus interval
- MEP
motor evoked potentials
- PAS
paired associative stimulation
- pMT
pheripheral motor threshold
- RMT
resting motor threshold
- SEP
somatosensory evoked potential
- SICI
short interval intracortical inhibition
- SPT
sensory perceptual threshold
- TMS
transcranial magnetic stimulation
Author contributions
M.D. interpreted the data, performed neurophysiological experiments and wrote the paper; P.P., F.P., R.D.I. performed neurophysiological experiments and drafted the manuscript. P.A., L.C. and R.H. performed neuropsychological evaluation of CS patients and drafted the manuscript. C.L. enrolled CS patents and performed clinical evaluation of them. G.Z. and V.D.L. conceived and designed the study and drafted and edited the manuscript. All authors approved the final version of the manuscript. The work was done in the Department of Neurosciences, Università Cattolica of Roma.
References
- Alpar A, Naumann N, Hartig W, Arendt T, Gartner U. Enhanced Ras activity preserves dendritic size and extension as well as synaptic contacts of neurons after functional deprivation in synRas mice. Eur J Neurosci. 2008;27:3083–3094. doi: 10.1111/j.1460-9568.2008.06313.x. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S, Matsubara Y. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The Ras/MAPK syndromes: novel roles of the Ras pathway in human genetic disorders. Hum Mutat. 2008;29:992–1006. doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- Arendt T, Gartner U, Seeger G, Barmashenko G, Palm K, Mittmann T, Yan L, Hummeke M, Behrbohm J, Bruckner MK, Holzer M, Wahle P, Heumann R. Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci. 2004;19:2953–2966. doi: 10.1111/j.0953-816X.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- Axelrad ME, Glidden R, Nicholson L, Gripp KW. Adaptive skills, cognitive, and behavioral characteristics of Costello syndrome. Am J Med Genet A. 2004;128A:396–400. doi: 10.1002/ajmg.a.30140. [DOI] [PubMed] [Google Scholar]
- Axelrad ME, Nicholson L, Stabley DL, Sol-Church K, Gripp KW. Longitudinal assessment of cognitive characteristics in Costello syndrome. Am J Med Genet A. 2007;143A:3185–3193. doi: 10.1002/ajmg.a.31968. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, Mariotti P, Leoni C, Ricci D, Vicari S, Selicorni A, Tartaglia M, Mercuri E, Zampino G. Cognitive profile of disorders associated with dysregulation of the Ras/MAPK signaling cascade. Am J Med Genet A. 2009;149A:140–146. doi: 10.1002/ajmg.a.32488. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Costello JM. A new syndrome: mental subnormality and nasal papillomata. Aust Paediatr J. 1977;13:114–118. doi: 10.1111/j.1440-1754.1977.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Der Kaloustian VM, Moroz B, McIntosh N, Watters AK, Blaichman S. Costello syndrome. Am J Med Genet. 1991;41:69–73. doi: 10.1002/ajmg.1320410118. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex. 2009;19:2326–2330. doi: 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, Ghez C, Eidelberg D. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Lin AE. GeneReviews at GeneTests: Medical Genetics Information Resource. Seattle: University of Washington; 2009. Costello syndrome; pp. 1997–2009. [Google Scholar]
- Guilding C, McNair K, Stone TW, Morris BJ. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. Eur J Neurosci. 2007;25:99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, Zhu JJ. Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools. Neuron. 2009;62:84–101. doi: 10.1016/j.neuron.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, Pozzo-Miller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Leon AM, Holler I, Vila JF, Siebner HR, Della Marca G, Di Lazzaro V, Alvarez JT. Reduced sensorimotor inhibition in the ipsilesional motor cortex in a patient with chronic stroke of the paramedian thalamus. Clin Neurophysiol. 2005;116:2592–2598. doi: 10.1016/j.clinph.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant’angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N, Girlanda P, Bhatia KP, Rothwell JC. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you. Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–631. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher AJ, Guerra C, Sauzeau V, Canamero M, Bustelo XR, Barbacid M. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest. 2008;118:2169–2179. doi: 10.1172/JCI34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Zhu JJ. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2010 doi: 10.1177/1073858410365562. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res. 2009;193:555–563. doi: 10.1007/s00221-008-1658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-d-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem. 2003;278:23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S. Prove di memoria ed apprendimento per età evolutiva. Giunti O. S. Organizzazioni Speciali; 2007. [Google Scholar]
- Viosca J, Schuhmacher AJ, Guerra C, Barco A. Germline expression of H-Ras(G12V) causes neurological deficits associated to Costello syndrome. Genes Brain Behav. 2009;8:60–71. doi: 10.1111/j.1601-183X.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J. The two sides of associative plasticity in writer's cramp. Brain. 2006;129:2709–2721. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C, Pogna EA, De Feo E, Delogu A, Sarkozy A, Atzeri F, Selicorni A, Rauen KA, Cytrynbaum CS, Weksberg R, Dallapiccola B, Ballabio A, Gelb BD, Neri G, Tartaglia M. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–272. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Activity level-dependent synapse-specific AMPA receptor trafficking regulates transmission kinetics. J Neurosci. 2009;29:6320–6335. doi: 10.1523/JNEUROSCI.4630-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


