Abstract
KCNQ1 osmosensitivity is of physiological and pathophysiological relevance in epithelial and cardiac cells, but the mechanism involved remains elusive. In COS-7 cells expressing the KCNE1–KCNQ1 fusion protein, extracellular hypoosmolarity and hyperosmolarity modify the channel biophysical parameters. These changes are consistent with hypoosmolarity increasing the level of membrane phosphatidylinositol-4,5-bisphosphate (PIP2), which in turn upregulates KCNE1–KCNQ1 channels. We showed that increasing PIP2 levels with a water-soluble PIP2 analogue prevented channel upregulation in hypoosmotic condition, suggesting a variation of the channel–PIP2 interaction during channel osmoregulation. Furthermore, we showed that polyamines and Mg2+, already known to tonically inhibit KCNQ channels by screening PIP2 negative charges, are involved in the osmoregulatory process. Indeed, intracellular Mg2+ removal and polyamines chelation inhibited the channel osmoregulation. Thus, the dilution of those cations during cell swelling might decrease channel inhibition and explain the channel upregulation by hypoosmolarity. To support this idea, we quantified the role of Mg2+ in the osmodependent channel activity. Direct measurement of intracellular [Mg2+] variations during osmotic changes and characterization of the channel Mg2+ sensitivity showed that Mg2+ participates significantly to the osmoregulation. Using intracellular solutions that mimic the variation of Mg2+ and polyamines, we were able to recapitulate the current amplitude variations in response to extracellular osmolarity changes. Altogether, these results support the idea of a modulation of the channel–PIP2 interactions by Mg2+ and polyamines during cell volume changes. It is likely that this mechanism applies to other channels that are sensitive to both osmolarity and PIP2.
Introduction
Many channels are osmosensitive, a property essential to allow cells to survive changes in extracellular osmolarity. This adaptive mechanism is necessary for bacteria (Perozo, 2006) and for eukaryotic cells (Hoffmann et al. 2009). A major mechanism underlying osmosensitivity seems to emerge for prokaryotes with a direct coupling between membrane tension and channel opening (Perozo et al. 2002). For eukaryotic cells, many mechanisms exist, including (i) a direct coupling between membrane tension and channel opening such as in prokaryotes (Kloda et al. 2007), (ii) an interaction with the cytoskeleton which pulls the channel open when the cell is swelling (Hamill, 2006), (iii) new channel recruitment at the stretched plasma membrane (Lan et al. 2006; Lim et al. 2006), or (iv) channel regulation by an osmodependent factor (Zhang et al. 2004).
Interestingly, channel osmoregulation is linked to several physiological and pathophysiological processes. In hepatocytes and mammary epithelial cells, the potassium channel subunit KCNQ1 (alias KvLQT1 or Kv7.1) is implicated in the regulatory volume decrease (Lan et al. 2006; vanTol et al. 2007). The voltage-dependent cardiac IKs current, generated by co-assembly of KCNQ1 and KCNE1 (alias IsK or minK) to form the voltage-activated K+ channel (Barhanin et al. 1996; Sanguinetti et al. 1996), is also osmosensitive (Sasaki et al. 1994). Cardiac ischaemia leads to an increase by 35 mm of intracellular osmolites such as lactate (Tranum-Jensen et al. 1981) which provokes cell swelling, which is further increased upon reperfusion, when the hypertonic extracellular fluid is washed out by normotonic blood. Cell-swelling induced current such as IKs may promote the apparition of re-entrant arrhythmias during ischaemia and reperfusion (De Mello, 2009) and limits the effect of anti-arrhythmic agents (Groh et al. 1996).
Even if KCNQ1 osmosensitivity does not seem to imply membrane stretch, the mechanism underlying this osmosensitivity is still unknown (Hammami et al. 2009). At least three of the aforementioned mechanisms have been suggested for KCNQ1 osmoregulation: (i) an interaction of the N-terminus with the cytoskeleton (Grunnet et al. 2003), (ii) the recruitment of new channels via phospholipase C (PLC) stimulation (Lan et al. 2006), and (iii) KCNQ1 osmoregulation through a transduction pathway, involving a tyrosine kinase (Missan et al. 2006, 2008). However, each of these observations depends on the model used. The involvement of the cytoskeleton described in Xenopus oocytes (Grunnet et al. 2003) does not appear in cardiac cells (Wang et al. 1996; Kubota et al. 2002). The contribution of PLC observed in hepatocytes does not appear in cardiac cells either (Zankov et al. 2009). Tyrosine kinase activation has been observed in the osmoregulation of IKs in both cardiac cells (Zhou et al. 1997) and BHK cells transfected with KCNQ1 and KCNE1 (Missan et al. 2006) but not in transfected COS-7 cells (Kubota et al. 2002). Moreover, a phosphotyrosyl phosphatase inhibitor, applied to maintain tyrosine phosphorylation, failed to prevent IKs osmoregulation, inconsistent with tyrosine kinase implication in the channel osmoregulation (Missan et al. 2008). These results, as much as the apparent discrepancies enumerated above, suggest that a key element is missing.
Phosphatidylinositol-4,5-bisphosphate (PIP2), which regulates the KCNE1–KCNQ1 complex (Loussouarn et al. 2003), has already been shown to be implicated in GIRK channel osmoregulation (Zhang et al. 2004). We thus hypothesized that PIP2 could be a key element in KCNE1–KCNQ1 osmoregulation. The present study indicates that channel–PIP2 interactions are controlled by Mg2+ and polyamines in an osmodependent manner.
Methods
Cell culture and transfection
The African green monkey kidney-derived cell line COS-7 was obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (Gibco, Paisley, UK). Cells were transfected with the plasmids (2 μg per 35 mm plate) complexed with Fugene-6 (Roche Molecular Biochemicals, Indianapolis, IN, USA) for giant-patch experiments or Jet-PEI (Polyplus-tranfection, Strasbourg, France) for whole-cell and perforated-patch experiments, according to the standard protocols recommended by the manufacturers. pCDNA3.1-KCNE1–KCNQ1 was a kind gift of Dr Robert S. Kass (Columbia University, New York). The plasmid coding for the green fluorescent protein (pEGFP) that we used to identify transfected cells was purchased from Clontech (Palo Alto, CA, USA). For giant-patch experiments, relative DNA composition was 80% pCDNA3.1–KCNE1–KCNQ1 and 20% pEGFP. For whole-cell and perforated-patch experiments, relative DNA composition was 40% pCDNA3.1–KCNE1–KCNQ1 and 60% pEGFP.
Electrophysiology
Patch-clamp experiments were performed on COS-7 cells, 24–48 h post-transfection, as previously described (Loussouarn et al. 2006).
For KCNE1–KCNQ1 osmoregulation, a single-step protocol (1 s pulse at +40 mV, 0.5 s tail at −40 mV, holding potential at −80 mV) was applied every 5 s to monitor current variation. Once steady state was reached, a multi-step protocol was applied (cf. legend of Fig. 2). For other whole-cell and perforated-patch experiments, a single-step protocol (1 s pulse at +80 mV, 0.5 s tail at −40 mV, holding potential at −80 mV) was applied every 2 or 5 s to monitor current variation.
Figure 2. A decrease in osmolarity stabilizes the channel open state (37°C).
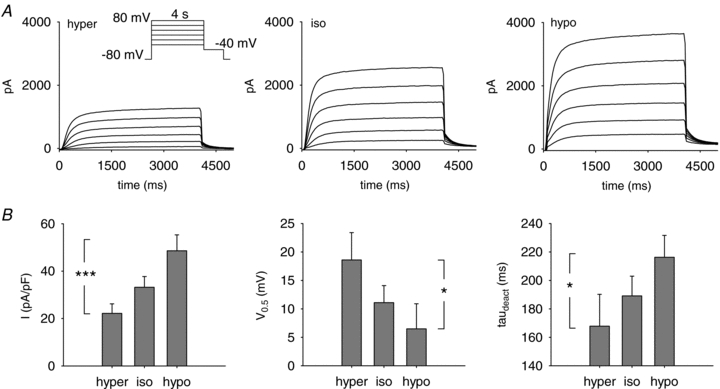
A, representative perforated-patch recordings of the KCNE1–KCNQ1 current measured in hyper- (hyper), iso- (iso) and hypoosmolar (hypo) solutions, in the same cell. Inset: voltage-clamp protocol used. Voltage was stepped from a holding potential of −80 mV to various voltages (4 s) between −20 mV and +80 mV in 20 mV increments, and then to −40 mV (1 s). B, effect of osmolarity on the mean tail-current density (left), the half-activation potential (V0.5; middle) and the deactivation time constant at −40 mV (τdeact; right) of KCNE1–KCNQ1 (n = 10). Tail current was measured at −40 mV after a 4 s pulse to +80 mV. V0.5 was calculated by a Boltzmann fit of the activation curve. *P < 0.05, ***P < 0.005 (one-way ANOVA for repeated measures).
In cell-stretch experiments, negative pressure was applied to the interior of the patch pipette with a 20 ml syringe and controlled by a water manometer. Pressures in the range of 0–20 cmH2O could be applied with this system.
Activation curves were fitted by a Boltzmann equation. The KCNE1–KCNQ1 activation kinetics were obtained by single-exponential fits of the first second of the activating current at +80 mV. KCNE1–KCNQ1 deactivation kinetics were obtained by single-exponential fits of the tail current at −40 mV after a step at +80 mV. Patch-clamp data are presented as means ± s.e.m. Statistical analysis was performed with Student's t test and one-way and two-way ANOVA completed by a Student–Newman–Keuls (SNK) test when needed. Off-line analysis was performed using Acquis1 Bio-logic Science Instruments, Claix, France and Microsoft Excel programs. Microsoft Solver was used to fit data by a least-square algorithm.
Microfluorometry
Recording conditions
Experiments were performed on an inverted microscope (Nikon Diaphot 300, Tokyo, Japan) equipped with an ×40 oil-immersion objective (1.3 NA). The fluorescent dye mag-indo-1 (Invitrogen, Carlsbad, CA, USA) was excited with a 100 W xenon lamp at a wavelength of 345 nm and the fluorescence emissions at 405 nm and 485 nm were measured with two photomultiplier tubes. The ratio of fluorescence emission at these two wavelengths (R = F405/F485) was calculated after background fluorescence subtraction. Data recording was performed by Felix software (Photon Technology International, Monmouth Junction, Birmingham, NJ, USA) at an acquisition frequency of 1 Hz.
Monitoring of intracellular [Mg2+] in COS-7 cells
Free intracellular Mg2+ concentration ([Mg2+]i) was monitored using the acetoxy-methyl ester form of mag-indo-1 (mag-indo-1 AM). Cells were loaded by a 10 min incubation at 35°C of 6.84 μmol l−1 of mag-indo-1 AM together with 40 μmol l−1 pluronic prepared by dilution in the control extracellular solution of a 500× stock solution in DMSO. Cells were bathed in a standard Tyrode solution before iso-, hypo- and hyperosmolar solutions were locally applied (see. Solutions and drugs). Experiments were performed at 35 ± 1°C.
For calibration, cells were bathed in solutions containing 10 mmol l−1 Hepes and various concentrations of KCl and MgCl2 for a total of 310 mosmol l−1 and saponin (0.02%). Figure 1A shows a typical fluorescence recording measured from a COS-7 cell bathed in 10 mmol l−1 Mg2+ calibration solution. Saponin permeabilization rapidly induced a transient increase in F405, while F485 started to decrease, then the fluorescence strongly decreased at both wavelengths (upper panel). Membrane permeabilization by saponin is responsible for the dye leaking out of the cell and the consequent loss of fluorescence. However, the ratio at the peak of F405 (Fig. 1A, lower panel) is a reliable indicator of [Mg2+]i (Csernoch et al. 1998). Figure 1B shows the obtained calibration curve. The following equation, optimized to fit the calibration data points, was used to calculate the free [Mg2+]i from the measured ratio: [Mg2+]i = 3.03 (R− 0.21)/(0.74 −R) (Grynkiewicz et al. 1985).
Figure 1. Calibration curve of mag-indo-1 fluorescence ratio (37°C).
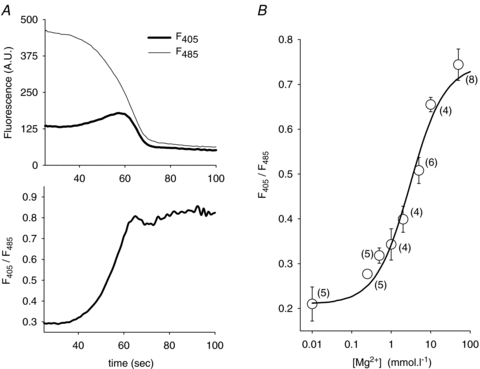
A, effect of applying saponin, in the presence of 10 mmol l−1 extracellular Mg2+. COS-7 cells were loaded with mag-indo-1 (Methods), and then bathed in a 10 mmol l−1 MgCl2 calibrating solution. Saponin (0.02%) was added at time 0. Top, fluorescence emission measured during Mg2+ diffusion, at 405 nm (thick line) and 485 nm (thin line). Bottom, ratio of F405/F485, calculated from the data shown above. B, calibration curve of mag-indo-1 fluorescence. Datapoints correspond to the mean values of F405/F485 for each of the calibration solutions used. Experimental data points were fitted (lines) with the equation described in Methods. Each point represents the mean ± s.e.m.; numbers of experiments are shown in parentheses.
Solutions and drugs
For giant-patch experiments, the cells were perfused with a solution containing (in mmol l−1): 145 KCl, 10 Hepes, 1 EGTA (pH 7.3 with KOH). The same solution was used in the upper part of the patch pipette, for electrical connection with the Ag–AgCl electrode. The following solution was used to perfuse the cell during K+ currents measurement and to fill the lower part of the patch pipette (in mmol l−1): 145 potassium gluconate, 10 Hepes, 1 EGTA (pH 7.3 with KOH).
For perforated-patch experiments, the pipette (intracellular) solution used had the following composition (in mmol l−1): 145 KCl, 10 Hepes, 1 EGTA and 0.85 amphotericin B (pH 7.2 with KOH). For diC8-PIP2 experiments, the pipette solution used had the following composition (in mmol l−1): 145 KCl, 10 Hepes and 1 EGTA (pH 7.2 with KOH). In Mg2+ experiments, the pipette solution used had the following composition (in mmol l−1): 145 KCl, 10 Hepes, 1 EGTA plus various concentration of MgCl2 or 3 mm EDTA (pH 7.2 with KOH). The standard Tyrode perfusion solution contained (in mmol l−1): 145 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes (pH 7.4 with NaOH). The extracellular control solution (334 mosmol l−1) contained (in mmol l−1): 145 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, 5 glucose, 20 mannitol (pH 7.4 with NaOH).
Hypoosmotic and hyperosmotic challenges were prepared with a variation of 60 mmol l−1 of NaCl concentration (Lan et al. 2006; Bessac & Fleig, 2007). For an optimized local perfusion of the cell assayed, densities of the different solutions were made identical by the modulation of the mannitol concentration. Hypoosmotic challenge (234 mosmol l−1) was induced by a decrease of NaCl from 145 to 85.5 mmol l−1 and an increase of mannitol from 20 to 39.1 mmol l−1. In hyperosmotic solution (434 mosmol l−1) NaCl was increased to 204.5 mmol l−1 and mannitol was decreased to 0.9 mmol l−1.
In experiments simulating the variation of Mg2+ and polyamine concentration during osmotic shocks, we used the Mg2+ concentration measured in the microfluorometry experiments. Assuming that the cells are poorly permeable to polyamines, we applied the same relative variation of concentration to polyamines. Thus, the isoosmolar solution contained (in mmol l−1): 150 KCl, 10 Hepes, 1 EGTA, 0.34 Mg2+, 0.035 spermine and 0.01 spermidine. Spermine and spermidine concentrations were based on a previous study on other cell types (Watanabe et al. 1991). The hypoosmolar solution contained (in mmol l−1): 0.11 Mg2+, 0.012 spermine and 0.003 spermidine. The hyperosmolar solution contained (in mmol l−1): 0.5 Mg2+, 0.051 spermine and 0.015 spermidine.
In the study of the potential role of the cytoskeleton, a 1 h pretreatment with 1 mmol l−1 cytochalasin D was used.
Free activities were calculated using software designed by G. L. Smith (University of Glasgow, UK). DiC8-PIP2 (Cayman Chemicals Co., Ann Arbor, MI, USA) was directly dissolved in the microperfusion solution. All other products were purchased from Sigma (St Louis, MO, USA).
Results
A decrease in osmolarity stabilizes the channel open state
PIP2 effects on KCNE1–KCNQ1 current biophysical parameters were previously characterized (Loussouarn et al. 2003). Using the perforated-patch configuration of the patch clamp technique, we first tested if the effect of osmolarity on KCNE1–KCNQ1 current biophysical parameters was compatible with a role of PIP2 in osmoregulation. To limit the variability in the biophysical parameters due to various KCNE1–KCNQ1 stoichiometries, the KCNE1–KCNQ1 concatemer was used (Park et al. 2005).
We measured KCNE1–KCNQ1 current in isoosmolar (334 mosmol l−1), hypoosmolar (234 mosmol l−1, 70% of control osmolarity) and hyperosmolar extracellular solutions (434 mosmol l−1, 130% of control osmolarity; cf. Methods). Tail current density after a depolarization to +80 mV was measured (Fig. 2). Extracellular hypoosmolarity induced a 48% increase in current density, from 33 ± 5 pA pF−1 to 49 ± 7 pA pF−1 (Fig. 2B; n = 10; SNK test: P < 0.05). Conversely, extracellular hyperosmolarity decreased current density to 22 ± 4 pA pF−1, i.e. by about 33% (Fig. 2B; n = 10; SNK test: P < 0.05).
Switching from the extracellular hyperosmolar solution to the hypoosmolar solution also induced a shift of the half-activation potential toward negative values and slowed deactivation (Fig. 2B). Activation was accelerated by switching from the hyper- to the isoosmolar condition. The time constant of activation, τact decreased from 298 ± 34 ms to 188 ± 18 ms (n = 10; SNK test: P < 0.05). But activation kinetics was not changed further by switching from the isoosmolar (τact = 188 ± 18 ms) to the hypoosmolar condition (225 ± 19 ms; n = 10; SNK test: not significant). Interestingly, such changes are very close to those described for an increase in membrane PIP2 (Loussouarn et al. 2003): increased current amplitude, negative shift of the half-activation potential, slowed deactivation, no change in activation. Thus, a decrease in osmolarity led to changes in KCNE1–KCNQ1 channel biophysical parameters that were consistent with an increase in membrane PIP2.
Increasing [diC8-PIP2] decreases KCNE1–KCNQ1 osmoregulation
If KCNE1–KCNQ1 osmoregulation implicates channel–PIP2 interaction, manipulating the intracellular concentration of PIP2 should have an impact on channel osmoregulation. To vary the phosphoinositide concentration, 1,2-dioctanoyl-phosphatidylinositol-4,5-bisphosphate (diC8-PIP2), a water-soluble PIP2 analogue with short acyl chains, was added to the pipette solution in the whole-cell configuration. Moreover, in order to maximize the effect of diC8-PIP2, Mg2+ ions, which are known to screen PIP2 negative charges, were omitted throughout these experiments. Hence, the pipette solution used should not prevent the MgATP-dependent rundown (Loussouarn et al. 2003). Because of the complexity of the KCNE1–KCNQ1 current rundown, due to at least two different mechanisms (Loussouarn et al. 2003), analysis of kinetics of deactivation was preferred over that of current amplitude as an index of diC8-PIP2 and channel interaction. The dose-dependent effect of diC8-PIP2 on KCNE1–KCNQ1 current deactivation demonstrated the interaction of diC8-PIP2 with the channel. The time constant of deactivation (τdeact) measured 75 s after patch rupture was 101 ± 9 ms at 10 μmol l−1 (n = 12), 137 ± 17 ms at 60 μmol l−1 (n = 10) and 131 ± 14 ms at 100 μmol l−1 of diC8-PIP2 (n = 11; P < 0.05, one-way-ANOVA).
Figure 3A shows a representative effect of osmolarity with 10 μmol l−1 diC8-PIP2 in the pipette. The current density presented a slow rundown probably due to the lack of MgATP (Loussouarn et al. 2003). The effect of osmolarity was maintained at this concentration approaching the physiological levels of PIP2 (Li et al. 2005). This was also observed at 60 μmol l−1 concentrations of diC8-PIP2 in the pipette (Fig. 3B). At 100 μmol l−1 diC8-PIP2, however, the effect of hypoosmolarity was abolished but the channel remained sensitive to hyperosmolarity. This suggests that at high diC8-PIP2 concentration, the channel–PIP2 interaction was increased, leading to a maximal open probability (Fig. 3C) and preventing further current increase in the hypoosmotic condition. This indicates that PIP2 is directly or indirectly implicated in channel osmoregulation. However, high diC8-PIP2 concentration could not reduce channel regulation by hyperosmolarity, which is inconsistent with PIP2 synthesis or degradation as the basis of osmoregulation. If it was the case, application of high diC8-PIP2 concentration should have prevented or reduced the decrease in current levels in hyperosmotic conditions, as is observed for other PIP2-dependent regulations (Bian et al. 2001).
Figure 3. KCNE1–KCNQ1 response to osmolarity in presence of diC8-PIP2 in the patch pipette (37°C).
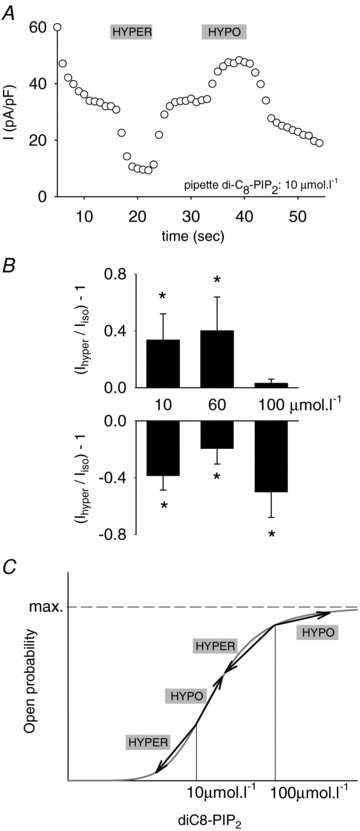
A, representative effect of hyperosmolar and hypoosmolar solutions on the amplitude of the KCNE1–KCNQ1 tail current in a COS-7 cell exposed to 10 μmol l−1 intracellular diC8-PIP2, in the whole-cell configuration. KCNE1–KCNQ1 tail currents (−40 mV) were measured after a 1 s depolarization at +80 mV, from a holding potential of −80 mV, every 5 s. B, mean relative response of the KCNE1–KCNQ1 tail currents to hyperosmolar and hypoosmolar conditions with various pipette concentrations of diC8-PIP2 (n = 10–12), normalized to the current amplitude in an isoosmotic condition. *P < 0.05 versus isoosmotic condition (t test). C, theoretical dose–response curves of KCNE1–KCNQ1 for diC8-PIP2 (with 0 Mg2+ in the pipette). This illustrates the maximum open probability (and consequent absence of response to hypoosmolarity) reached by the channel with 100 μmol l−1 diC8-PIP2.
Removing Mg2+ and polyamines prevents KCNE1–KCNQ1 osmoregulation
From the previous conclusions, we hypothesized that PIP2 levels are constant but that channel–PIP2 interaction is controlled by cytosolic cations such as Mg2+ and polyamines. This hypothesis was based on results demonstrating that cytosolic Mg2+ and polyamines electrostatically interact with membrane PIP2 and inhibit about half of the current generated by KCNQ2/KCNQ3 channels (Suh & Hille, 2007). Cell volume changes that occur during osmotic stress may lead to the dilution or to the concentration of these intracellular cations. It is then possible that the hypoosmolarity-induced dilution of cations is responsible for the observed increase in KCNE1–KCNQ1 activity. Conversely, the hyperosmolarity-induced increase in the cation concentration may be responsible for the current decrease.
In experiments with diC8-PIP2, channel osmoregulation was maintained, despite the absence of Mg2+. This suggests that in addition to Mg2+, other intracellular cations such as polyamines (Suh & Hille, 2007) also interact with the channel and play a role in its osmoregulation. Poorly diffusible polycations, likely to be long polyamines, may remain in the cytosol in the whole-cell configuration, since they also remain in inside-out patches (Lopatin et al. 1995). To test this hypothesis, 3 mmol l−1 EDTA was added in the pipette in the absence of Mg2+ to chelate the polyamines remaining in the cell, as shown previously (Guo & Lu, 2002). This led to a loss of channel osmoregulation (Fig. 4A–B), confirming the role of cytosolic cations such as polyamines in the channel osmosensitivity.
Figure 4. Intracellular polycations, including Mg2+, participate to the KCNE1–KCNQ1 osmoregulation.
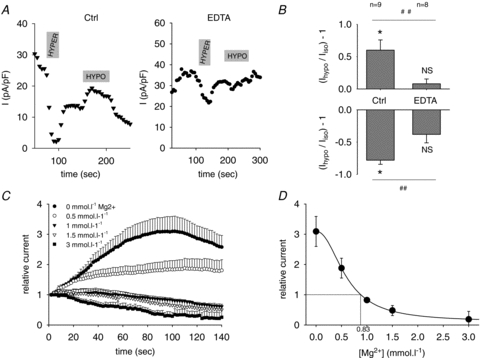
A, representative KCNE1–KCNQ1 tail currents response to hyperosmotic and hypoosmotic conditions in the presence of 1 mmol l−1 cytosolic Mg2+ (left) or 3 mmol l−1 EDTA (right) in the pipette (test operated after an initial rise as shown in C). Same voltage protocol as in Fig. 3A, experiment performed at 37°C. B, mean relative response of the tail currents to hyperosmotic and hypoosmotic conditions in the presence of 1 mmol l−1 cytosolic Mg2+ (Ctrl, n = 9) or 3 mmol l−1 EDTA (EDTA, n = 8) in the pipette. *P < 0.05 versus isoosmotic condition (t test). NS: non-significant difference versus isoosmotic condition (t test). ##P < 0.01 in EDTA versus control solutions (t test). C, time course of KCNE1–KCNQ1 current amplitude for various pipette [Mg2+] in the whole-cell configuration. Peak current was normalized to the first pulse after patch rupture. Same voltage protocol as in Fig. 3A, except stimulation frequency: 2 s. Experiment realized at room temperature. D, steady-state KCNE1–KCNQ1 current calculated as in C, vs. pipette [Mg2+] (n = 9–12). Data points are fitted by a modified Hill equation, giving an IC50 of 0.66 mmol l−1, a slope constant of 2.44, and an estimation of cytosolic Mg2+ of 0.83 mmol l−1.
KCNE1–KCNQ1 sensitivity to [Mg2+]i suggests a role for Mg2+ in channel osmoregulation
To quantify the role of intracellular Mg2+ ([Mg2+]i) in osmoregulation, it is necessary to determine both the channel sensitivity to Mg2+ and the variations of [Mg2+]i during channel osmoregulation.
To look at the channel sensitivity to Mg2+, we varied the pipette [Mg2+] in the whole-cell configuration as already done for KCNQ2/KCNQ3 channels (Suh & Hille, 2007), and recorded the evolution of the tail current after a depolarization at +80 mV, recorded every 2 s (Fig. 4C). Patch rupture with pipette [Mg2+] of 0 and 0.5 mmol l−1 led to a current increase, followed by a rundown most probably due to the absence of MgATP (Loussouarn et al. 2003). Conversely, patch rupture with [Mg2+] of 1, 1.5 and 3 mmol l−1 led to a current decrease. If Mg2+ diffusion is fully responsible for the current variation in this time scale, this indicates that [Mg2+]i is between 0.5 and 1 mmol l−1. The dose–response curve of relative steady-state current (or maximal current with [Mg2+] of 0 and 0.5 mmol l−1) as a function of pipette [Mg2+] is shown in Fig. 4D. The IC50 obtained from the fitted Hill equation was 0.59 mmol l−1 and the slope was 2.2. Using this curve and the initial current, and assuming that only Mg2+ is responsible for channel inhibition, we estimated COS-7 [Mg2+]i at around 0.83 mmol l−1 (Fig. 4D). This value is consistent with previous studies (Maughan, 1983; Gupta et al. 1984; White & Hartzell, 1989; London, 1991; Romani & Scarpa, 2000). The estimated [Mg2+]i is close to the IC50, suggesting that Mg2+ plays a role in the channel osmoregulation, since a small variation in [Mg2+]i will then induce a significant change in the current level. But this hypothesis needed confirmation by measurement of [Mg2+]i in the different conditions.
[Mg2+]i variations contribute to KCNE1–KCNQ1 osmoregulation
We performed microfluorometry experiments to measure the variations of cytosolic Mg2+ concentration in COS-7 cells during osmotic stress, using the Mg2+-sensitive dye mag-indo-1 AM (Fig. 5). The mean fluorescence ratio (F405/F485) measured in COS-7 cells bathed in isoosmotic extracellular solution during osmotic stress experiments was 0.26 ± 0.01 (n = 17). Using the calibration curve (Fig. 1B), the ratio gave a mean resting [Mg2+]i of 0.34 ± 0.08 mmol l−1. This is almost a third of the value estimated in the patch-clamp experiments (cf. above). The normal [Mg2+]i estimated by microfluorometry is thus too low to account for the tonic channel inhibition relieved when Mg2+ was omitted in the patch pipette (Fig. 4C). As suggested above, other intracellular cations, such as polyamines, most probably participate to the tonic inhibition.
Figure 5. [Mg2+]i variation accounts for a part of KCNE1–KCNQ1 osmoregulation (37°C).
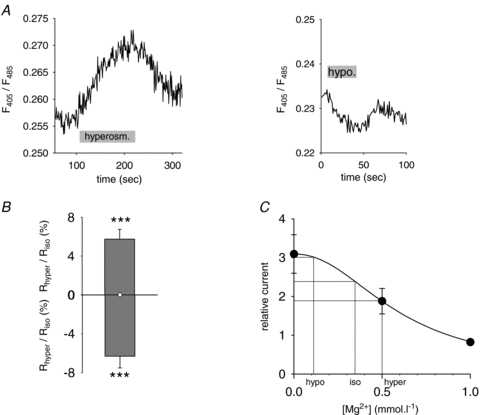
A, representative recordings of the fluorescence ratio during osmotic challenges, indicated by a grey bar. B, mean relative response of the fluorescence ratio to osmotic changes. ***P < 0.005 versus isoosmotic condition (t test). C, reproduction of Fig. 4D, on which measured Mg2+ concentrations have been added, to calculate current amplitudes in the hyperosmolar (hyper), isoosmolar (iso) and hypoosmolar (hypo) conditions.
The value of the fluorescence ratio measured in cells during osmotic stress was also converted to [Mg2+]i. When cells were perfused with the hyperosmolar solution, the ratio increased by 5.8 ± 1% (Fig. 5B) giving a [Mg2+]i of 0.5 mmol l−1 (n = 9; P < 0.005). The hypoosmolar solution decreased the fluorescence ratio by 6.3 ± 1.2% (n = 8; P < 0.005), giving a [Mg2+]i of 0.11 mmol l−1.
Knowing the channel sensitivity to cytosolic Mg2+ (Fig. 4D) and the variations of cytosolic Mg2+ during osmotic stress (above) we could estimate the role of [Mg2+]i variations in KCNE1–KCNQ1 osmoregulation (Fig. 5C). This calculation predicted a 25% increase in the channel activity by hypoosmolarity, which is lower than the 48% current increase observed in the perforated-patch experiments (Fig. 2B). The effect of hyperosmolarity on channel inhibition by Mg2+ predicted a 23% decrease in channel activity, as compared to the 33% decrease observed in the perforated-patch experiments.
Intracellular Mg2+ and polyamine variations determine KCNE1–KCNQ1 osmoregulation
Four observations suggest that polyamines play also a role in channel osmoregulation. First, we have mentioned above that the measured concentration of Mg2+ is much lower than the concentration necessary to explain the tonic inhibition of KCNQ1 currents, suggesting that other cations may participate in the tonic inhibition of KCNQ1. Second, the measured variation of Mg2+ explains only a part of the current variation during hypo- and hyperosmotic shocks. Third, intracellular application of EDTA in the absence of Mg2+ inhibited the channel osmodependency, and EDTA has been shown to chelate polyamines (Guo & Lu, 2002). Fourth, polyamines have been shown to inhibit KCNQ channels (Suh & Hille, 2007). In order to test if the variation of the current amplitude during osmoregulation is solely due to a variation of Mg2+ and polyamine concentration, we prepared pipette solutions to mimick the variation of Mg2+ and polyamine concentrations during osmotic shocks. We used the Mg2+ concentration measured in the microfluorometry experiments in the three osmolarity conditions. Spermine and spermidine concentrations in isoosmolar condition were based on a previous study on other cell types (Watanabe et al. 1991). Assuming that the cells are poorly permeable to polyamines, we applied the relative variations of Mg2+ concentration to polyamines. Figure 6A shows the time course of KCNE1–KCNQ1 current amplitude recorded with the intracellular solution mimicking the control Mg2+ and polyamine concentration (isoosmolar condition). In this condition, KCNE1–KCNQ1 current did not vary, suggesting that the pipette contains similar concentrations of Mg2+, spermine and spermidine as in the cytosol. On the contrary, whole-cell currents measured with the pipette solution mimicking the hypotonic shock showed a gradual increase up to 50% of the basal level, before running down. This 50% increase is consistent with the 48% increase observed during the hypotonic shock. In Fig. 6B, whole-cell currents measured with the pipette solution mimicking the hypotonic shock showed a gradual decrease, slightly higher but in the range of the 33% decrease observed during the hypotonic shock. Altogether, these data strongly suggest that the intracellular variation of Mg2+ and the most abundant polyamines can explain the variation of current caused by extracellular hypo- and hyperosmolarity.
Figure 6. Intracellular Mg2+ and polyamine variation fully accounts for KCNE1–KCNQ1 osmoregulation (room temperature).
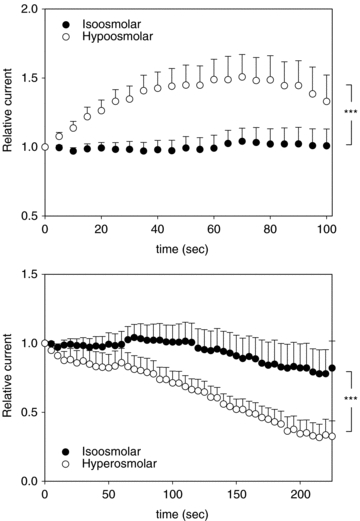
A, time course of KCNE1–KCNQ1 current amplitude for pipette solution mimicking the concentration of Mg2+ and polyamines in iso- and hypoosmotic conditions, recorded in the whole-cell configuration. Peak current was normalized to the first pulse after patch rupture. Same voltage protocol as in Fig. 3A. ***P < 0.005 (two-way ANOVA). B, time course of KCNE1–KCNQ1 current amplitude for pipette solution mimicking the concentration of Mg2+ and polyamines in iso- and hyperosmotic conditions, recorded in the whole-cell configuration. Peak current was normalized to the first pulse after patch rupture. Same voltage protocol as in Fig. 3A. ***P < 0.005 (two-way ANOVA).
KCNE1–KCNQ1 osmoregulation does not involve its mechanosensitivity
The native IKs has been shown to be both osmosensitive and mechanosensitive (Sasaki et al. 1994; Wang et al. 1996). These properties do not seem to be linked because only the osmosensitivity was observed in Xenopus oocytes expressing KCNQ1 (Hammami et al. 2009). We aimed at determining if COS-7 is a model closer to cardiac cells, with recombinant KCNE1–KCNQ1 being volume sensitive and stretch sensitive. If it is the case, we wanted to test a potential link between membrane stretch and cell volume sensitivities in this model.
In the cell-attached configuration, voltage steps generated a typical slowly activating/deactivating current (Fig. 7A). The membrane patch was stretched by suction (from 5 to 20 cmH2O) applied through the patch pipette. Such a manipulation allows stretching the membrane patch without altering the cytosolic ions concentrations. The channel was upregulated by stretch, as shown in Fig. 7A and B. Noteworthy, and contrary to the effect of osmolarity, the activation kinetics were drastically modified (Fig. 7C), and the deactivation kinetics were unchanged (Fig. 7D), suggesting that the two processes are controlled by distinct mechanisms.
Figure 7. KCNE1–KCNQ1 activation by membrane stretch (room temperature).
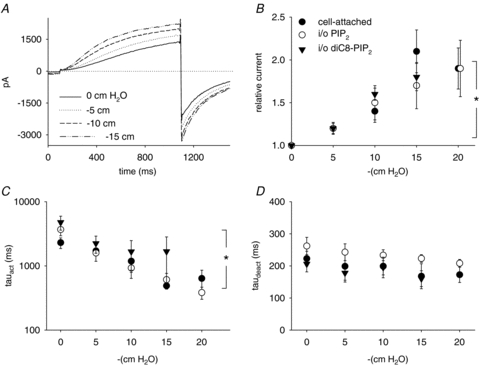
A, representative recording of KCNE1–KCNQ1 current response to membrane stretch, in the giant-patch configuration, in the presence of 10 μmol l−1 diC8-PIP2+ 1.4 mmol l−1 MgATP. Same voltage protocol as in Fig. 3A. B, mean relative KCNE1–KCNQ1 current in response to negative pressure, normalized to the non-stretched condition. Mean relative currents in the cell-attached configuration (cell-attached), in inside-out before rundown (i/o), and after rundown was counteracted by diC8-PIP2 and MgATP (i/o diC8-PIP2) (n = 4–12). C and D, mean time constants of activation (τact, n = 3–10) and deactivation (τdeact, n = 3–12). *P < 0.05 (two-way ANOVA for repeated measures).
To test if the same mechanism as in osmoregulation (variation in the concentration of KCNQ1 inhibitors) applies to the channel stretch sensitivity, we also looked at the effect of suction in the inside-out configuration, in which there is no change of the intracellular medium (giant-patch solution) during stretch. The test was done right after patch excision or after rundown was reversed by application of diC8-PIP2. We obtained similar results (Fig. 7B–D) as in the cell-attached configuration, suggesting that, in contrast to channel osmosensitivity, no cytosolic components are implicated in the channel stretch-sensitivity. Altogether, these results showed that KCNE1–KCNQ1 osmosensitivity and mechanosensitivity are distinct phenomena.
The cytoskeleton does not participate in the KCNE1–KCNQ1 osmosensitivity
Cytoskeleton integrity may also influence channel osmoregulation even though we had already shown that microtubules are not implicated in KCNE1–KCNQ1 osmoregulation (Nicolas et al. 2008). We thus tested the potential role of another major component of the cytoskeleton, the actin filaments. The response to the osmolarity variations of cells treated with 1 mmol l−1 cytochalasin D, an inhibitor of actin filaments polymerization (pretreatment 1 h at 37°C), was identical to that of the untreated cells, suggesting that actin filaments do not participate to the KCNE1–KCNQ1 channel osmoregulation (Fig. 8). Thus, in COS7 cells as in cardiac cells, the channel osmosensitivity seems to be independent of the cytoskeleton (Sasaki et al. 1994).
Figure 8. The cytoskeleton does not participate in the KCNE1–KCNQ1 osmoregulation (37°C).
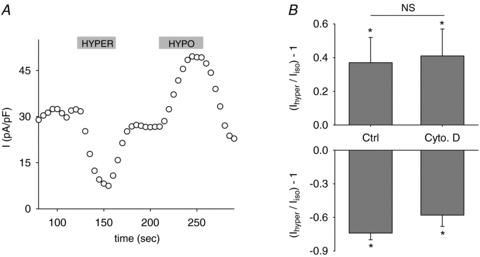
A, representative evolution of the KCNE1–KCNQ1 tail current in a cell pre-treated with cytochalasin D, and bathed in iso-, hyper- and hypoosmolar solutions. Same voltage protocol as in Fig. 3A. B, mean relative response of the KCNE1–KCNQ1 tail currents to hypo- and hyperosmolar conditions in control conditions (Ctrl) or after a cytochalasin D pretreatment (Cyto. D, 1 mmol l−1, pretreatment 1 h at 37°C) (n = 6–7). *P < 0.05 versus isoosmotic condition (t test). NS: non-significant difference between Ctrl and Cytochalasin D conditions.
Discussion
Our study shows that intracellular Mg2+ and polyamines act as mediators of the KCNE1–KCNQ1 osmoregulation via modulation of the channel–PIP2 interaction. Switching from hyperosmolar to hypoosmolar extracellular solution led to an increase of the KCNE1–KCNQ1 current density, a shift of the activation curve towards negative potential and slower deactivation kinetics. It is worth noting that an increase of membrane PIP2 has strictly the same effects. Moreover, both intracellular application of 100 μmol l−1 short-chain PIP2 and decrease in Mg2+ and polyvalent cations drastically decreased KCNE1–KCNQ1 osmoregulation. Such cations partially inhibit KCNQ channel activity by screening the PIP2 negative charges, as shown for KCNQ1 (Park et al. 2005) and KCNQ2/3 channels (Suh & Hille, 2007). This inhibition may be osmodependent, intracellular Mg2+ and polyamines being concentrated during cell shrinking and diluted during cell swelling. Based on the measured variations of Mg2+ in hypo- and hyperosmolar conditions, we mimicked the variation of intracellular Mg2+ and the most abundant polyamines, spermine and spermidine. We could reproduce the effect of hypo- and hyperosmolarity, suggesting that KCNE1–KCNQ1 channel osmodependency is mediated by variation of the intracellular concentration of these cations. Such a modulation of channel–PIP2 electrostatic interactions has already been suggested in the pH regulation of a TRP channel (Kozak et al. 2005). Thus, we propose that Mg2+ and polyamines act as modulators of PIP2 availability in the KCNE1–KCNQ1 response to osmotic stress.
Channel osmoregulation thus appears to be a consequence of the KCNQ channel sensitivity to intracellular Mg2+ and polyamines suggested previously for KCNQ1 (Loussouarn et al. 2003; Zhang et al. 2003), and then described in details for KCNQ2/KCNQ3 channels (Suh & Hille, 2007). Importantly, such a regulation by Mg2+ has also been observed in guinea pig cardiomyocytes. Indeed, the initial increase in IKs at low pipette [Mg2+] and the decrease at high [Mg2+] show that the native current is also inhibited by Mg2+ (Hirahara et al. 1998). The mechanism of channel osmoregulation in cardiac cells may thus be the same as in the recombinant system studied here. Consistent with that, the effects of hypoosmolarity on the cardiac IKs and on the recombinant KCNE1–KCNQ1 share a similar biophysical pattern: an increase in current amplitude by 65% (Rees et al. 1995) to 75% (Sasaki et al. 1994), no change in activation and a slowed deactivation (Rees et al. 1995). Interestingly, this comparison also holds for stretch dependency of IKs and the recombinant KCNE1–KCNQ1 with an effect on activation kinetics and no effect on deactivation (Wang et al. 1996 and this study).
In conclusion, the present study indicates that Mg2+ and polyamine modulation of channel–PIP2 interaction plays a major role in the osmoregulation of KCNE1–KCNQ1 channel in COS-7 cells, and probably of the native IKs current. Neither a direct membrane stretch nor the cytoskeleton is implicated, for both the recombinant channel (present study) and the cardiac channel (Sasaki et al. 1994; Wang et al. 1996). Since many channels are regulated by PIP2, this mechanism may also apply to channels that are sensitive to small variations of PIP2 levels (Gamper et al. 2005). For instance, atrial KATP channels are upregulated by both osmolarity and PIP2, suggesting a similar mechanism for the osmoregulation of these channels (Van Wagoner, 1993; Shyng & Nichols, 1998). In the case of GIRK channels, these channels are upregulated by PIP2 but downregulated by hypoosmolarity (Zhang et al. 2004), suggesting a different mechanism like a local decrease in PIP2, but this needs confirmation.
Acknowledgments
We thank Béatrice Leray, Marie-Joseph Louérat and Agnès Carcouët for expert technical assistance. This work was supported by grants from the Agence Nationale de la Recherche to G.L. (ANR-05-JCJC-0160-01) and to I.B. (ANR-05-PCOD-034-01). J.P. was supported by the Association Française contre les Myopathies, and F.S.C. by Génavie, the Fondation pour la Recherche Médicale, and the Fédération Française de Cardiologie.
Glossary
Abbreviations
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PLC
phospholipase C
Author contributions
Conception and design of the experiments: G.L., I.B. and N.R. Collection, analysis and interpretation of data J.P., F.S.C. and M.Y.A. Drafting the article or revising it critically for important intellectual content F.C., J.M., I.B. and G.L. All authors approved the final version of the manuscript.
Author's present address
J. Piron: INSERM UMRS U930, CNRS FRE2448, CHRU Bretonneau, 2 boulevard Tonnellé, 37044 Tours, France.
References
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Fleig A. TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol. 2007;582:1073–1086. doi: 10.1113/jphysiol.2007.130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J, Cui J, McDonald TV. HERG K+ channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ Res. 2001;89:1168–1176. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- Csernoch L, Bernengo JC, Szentesi P, Jacquemond V. Measurements of intracellular Mg2+ concentration in mouse skeletal muscle fibers with the fluorescent indicator mag-indo-1. Biophys J. 1998;75:957–967. doi: 10.1016/S0006-3495(98)77584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello WC. Cell swelling, impulse conduction, and cardiac arrhythmias in the failing heart. Opposite effects of angiotensin II and angiotensin (1–7) on cell volume regulation. Mol Cell Biochem. 2009;330:211–217. doi: 10.1007/s11010-009-0135-0. [DOI] [PubMed] [Google Scholar]
- Gamper N, Li Y, Shapiro MS. Structural requirements for differential sensitivity of KCNQ K+ channels to modulation by Ca2+/calmodulin. Mol Biol Cell. 2005;16:3538–3551. doi: 10.1091/mbc.E04-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh WJ, Gibson KJ, Maylie JG. Hypotonic-induced stretch counteracts the efficacy of the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Cardiovasc Res. 1996;31:237–245. [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, MacAulay N, Jorgensen NK, Schmitt N, Pongs O, Olesen SP, Klaerke DA. KCNQ1 channels sense small changes in cell volume. J Physiol. 2003;549:419–427. doi: 10.1113/jphysiol.2003.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guo D, Lu Z. IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J Gen Physiol. 2002;120:539–551. doi: 10.1085/jgp.20028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Gupta P, Moore RD. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- Hammami S, Willumsen NJ, Olsen HL, Morera FJ, Latorre R, Klaerke DA. Cell volume and membrane stretch independently control K+ channel activity. J Physiol. 2009;587:2225–2231. doi: 10.1113/jphysiol.2008.163550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Matsubayashi T, Matsuura H, Ehara T. Intracellular Mg2+ depletion depresses the delayed rectifier K+ current in guinea pig ventricular myocytes. Jpn J Physiol. 1998;48:81–89. doi: 10.2170/jjphysiol.48.81. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Kloda A, Lua L, Hall R, Adams DJ, Martinac B. Liposome reconstitution and modulation of recombinant N-methyl-d-aspartate receptor channels by membrane stretch. Proc Natl Acad Sci U S A. 2007;104:1540–1545. doi: 10.1073/pnas.0609649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Horie M, Takano M, Yoshida H, Otani H, Sasayama S. Role of KCNQ1 in the cell swelling-induced enhancement of the slowly activating delayed rectifier K+ current. Jpn J Physiol. 2002;52:31–39. doi: 10.2170/jjphysiol.52.31. [DOI] [PubMed] [Google Scholar]
- Lan WZ, Wang PY, Hill CE. Modulation of hepatocellular swelling-activated K+ currents by phosphoinositide pathway-dependent protein kinase C. Am J Physiol Cell Physiol. 2006;291:C93–103. doi: 10.1152/ajpcell.00602.2005. [DOI] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CH, Schoonderwoerd K, Kleijer WJ, de Jonge HR, Tilly BC. Regulation of the cell swelling-activated chloride conductance by cholesterol-rich membrane domains. Acta Physiol (Oxf) 2006;187:295–303. doi: 10.1111/j.1748-1716.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- London RE. Methods for measurement of intracellular magnesium: NMR and fluorescence. Annu Rev Physiol. 1991;53:241–258. doi: 10.1146/annurev.ph.53.030191.001325. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J Gen Physiol. 1995;106:923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn G, Baro I, Escande D. KCNQ1 K+ channel-mediated cardiac channelopathies. Methods Mol Biol. 2006;337:167–183. doi: 10.1385/1-59745-095-2:167. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D. Diffusible magnesium in frog skeletal muscle cells. Biophys J. 1983;43:75–80. doi: 10.1016/S0006-3495(83)84325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Role of kinases and G-proteins in the hyposmotic stimulation of cardiac IKs. Biochim Biophys Acta. 2006;1758:1641–1652. doi: 10.1016/j.bbamem.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Missan S, Linsdell P, McDonald TF. Involvement of tyrosine kinase in the hyposmotic stimulation of IKs in guinea-pig ventricular myocytes. Pflugers Arch. 2008;456:489–500. doi: 10.1007/s00424-007-0424-y. [DOI] [PubMed] [Google Scholar]
- Nicolas CS, Park KH, El Harchi A, Camonis J, Kass RS, Escande D, Merot J, Loussouarn G, Le Bouffant F, Baro I. IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules. Cardiovasc Res. 2008;79:427–435. doi: 10.1093/cvr/cvn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Piron J, Dahimene S, Merot J, Baro I, Escande D, Loussouarn G. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ Res. 2005;96:730–739. doi: 10.1161/01.RES.0000161451.04649.a8. [DOI] [PubMed] [Google Scholar]
- Perozo E. Gating prokaryotic mechanosensitive channels. Nat Rev Mol Cell Biol. 2006;7:109–119. doi: 10.1038/nrm1833. [DOI] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Rees SA, Vandenberg JI, Wright AR, Yoshida A, Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J Gen Physiol. 1995;106:1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:D720–D734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Mitsuiye T, Wang Z, Noma A. Increase of the delayed rectifier K+ and Na+-K+ pump currents by hypotonic solutions in guinea pig cardiac myocytes. Circ Res. 1994;75:887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Electrostatic interaction of internal Mg2+ with membrane PIP2 Seen with KCNQ K+ channels. J Gen Physiol. 2007;130:241–256. doi: 10.1085/jgp.200709821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J, Janse MJ, Fiolet WT, Krieger WJ, D’Alnoncourt CN, Durrer D. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ Res. 1981;49:364–381. doi: 10.1161/01.res.49.2.364. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- vanTol BL, Missan S, Crack J, Moser S, Baldridge WH, Linsdell P, Cowley EA. Contribution of KCNQ1 to the regulatory volume decrease in the human mammary epithelial cell line MCF-7. Am J Physiol Cell Physiol. 2007;293:C1010–C1019. doi: 10.1152/ajpcell.00071.2007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Mitsuiye T, Noma A. Cell distension-induced increase of the delayed rectifier K+ current in guinea pig ventricular myocytes. Circ Res. 1996;78:466–474. doi: 10.1161/01.res.78.3.466. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991;266:20803–20809. [PubMed] [Google Scholar]
- White RE, Hartzell HC. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem Pharmacol. 1989;38:859–867. doi: 10.1016/0006-2952(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Zankov DP, Toyoda F, Omatsu-Kanbe M, Matsuura H, Horie M. Angiotensin II type 1 receptor mediates partially hyposmotic-induced increase of IKs current in guinea pig atrium. Pflugers Arch. 2009;458:837–849. doi: 10.1007/s00424-009-0669-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lee JK, John SA, Uozumi N, Kodama I. Mechanosensitivity of GIRK channels is mediated by protein kinase C-dependent channel-phosphatidylinositol 4,5-bisphosphate interaction. J Biol Chem. 2004;279:7037–7047. doi: 10.1074/jbc.M307323200. [DOI] [PubMed] [Google Scholar]
- Zhou YY, Yao JA, Tseng GN. Role of tyrosine kinase activity in cardiac slow delayed rectifier channel modulation by cell swelling. Pflugers Arch. 1997;433:750–757. doi: 10.1007/s004240050341. [DOI] [PubMed] [Google Scholar]


