Abstract
Placental insufficiency-induced intrauterine growth restriction (IUGR) fetuses have chronic hypoxaemia and elevated plasma catecholamine concentrations. In this study, we determined whether adrenergic responsiveness becomes desensitized in the perirenal adipose tissue of IUGR fetuses and lambs by measuring adrenergic receptor (AR) mRNA and protein levels. We also tested the ability of adrenaline to mobilize non-esterified fatty acids (NEFAs) in young lambs. Perirenal adipose tissue was collected from IUGR and control fetuses at 133 days of gestational age (dGA) and lambs at 18 days of age (dA). β2-AR mRNA concentrations were 59% and 74% lower (P < 0.05) in IUGR fetuses and lambs compared to controls, respectively, which also resulted in lower protein levels (P < 0.05). No treatment differences were detected for α1A-, α1B-, α1D-, α2A-, α2B-, α2C-, β1- and β3-AR expression. mRNA concentrations were also determined for hormone sensitive lipase (HSL), perilipin (lipid droplet-associated protein), and two adipokines, leptin and adiponectin. Adiponectin and HSL were not different between treatments at either age. Compared to controls, perilipin and leptin mRNA concentrations were lower (P < 0.05) in IUGR fetuses but not in lambs. Because of the β2-AR results, we challenged a second cohort of lambs with exogenous adrenaline at 21 dA. The ability of adrenaline to mobilize NEFA was 55 ± 15% lower (P < 0.05) in IUGRs than controls. Collectively, our findings indicate that elevated catecholamine exposure in utero causes desensitization of adipose tissue by down-regulation of β2-AR, and this persists in lambs. This impairment in adrenergic stimulated lipolysis might partially explain early onset obesity in IUGR offspring.
Introduction
Placental insufficiency restricts fetal nutrient and oxygen supply and slows growth, requiring nutrient sparing by the fetus (Barker et al. 1993). This could result in an adaptive response in utero that is mismatched to postnatal nutrient availability, increasing the risk for obesity later in life (Ravelli et al. 1976; Gluckman et al. 2005; Fernandez-Twinn & Ozanne, 2006; Yliharsila et al. 2008). For example, small for gestational age children exhibit catch-up growth and are more likely to develop central adiposity at 5 years of age compared to children born with body weight appropriate for gestational age (Hokken-Koelega et al. 1995; Ong et al. 2000). Similarly, lambs that were subjected to intrauterine growth restriction (IUGR) exhibit greater visceral and perirenal adipose mass by 45 days (De Blasio et al. 2007). Together, these findings demonstrate an association between IUGR and obesity, but explanations for this relationship are lacking. Endocrine factors, like catecholamines, may play a major role in fetal nutrient redistribution and could impact postnatal metabolism (Green et al. 2010; Leos et al. 2010). Previous data from our laboratory show that fetal hypoxaemia, as may occur during IUGR, chronically elevates catecholamine concentrations (Limesand et al. 2006; Leos et al. 2010). We postulate that this response causes adrenergic desensitization in adipose tissue, lowering adrenergic stimulated lipolysis, which might increase an individual's risk for childhood obesity.
The catecholamines, adrenaline and noradrenaline, act through G-protein coupled receptors (Altan et al. 2007; Schaak et al. 2007). There are three main types of adrenergic receptor (AR), α1-AR, α2-AR and β-AR, and each has multiple subtypes, which include α1A-, α1B- and α1D-AR, α2A-, α2B- and α2C-AR, and β1-, β2- and β3-AR. In adipocytes, β-AR activates hormone sensitive lipase (HSL) to stimulate lipolysis through a cAMP-dependent process, resulting in accelerated triglyceride hydrolysis into non-esterified fatty acids (NEFAs) and glycerol. At the same time, phosphorylation of perilipin by protein kinase A causes perilipin to facilitate the interaction between the fat droplet and HSL to promote lipolysis (Tansey et al. 2004; Carmen & Victor, 2006). Chronic exposure to high catecholamine concentrations has been shown to down-regulate AR by reducing mRNA synthesis and increasing protein degradation (Benovic et al. 1990; Hausdorff et al. 1990; Santala et al. 1990; Dohlman et al. 1991; Collins et al. 1992; Garcia-Higuera & Mayor, 1994). Therefore, if AR desensitization occurs in the fetus and persists postnatally, it could lead to dyslipidaemia and impaired lipid mobilization, which could partially explain the increased incidence of obesity in IUGR offspring. In the present study, we examined AR expression in perirenal adipose tissue, the major fat depot in fetal sheep (Symonds et al. 2003), in a model of placental insufficiency-induced IUGR created by exposing pregnant ewes to hyperthermic conditions (Bell et al. 1987; Thureen et al. 1992; de Vrijer et al. 2004). Expression levels were determined in near-term fetuses and young lambs prior to weaning to encompass the postnatal transition. In addition, we determined adrenaline's ability to mobilize NEFAs in young IUGR lambs to identify adrenergic desensitization during the perinatal period.
Methods
Ethical approval
The Institutional Animal Care and Use Committee at the University of Arizona approved all animals protocols used in these experiments. All animal experiments were conducted at the William J. Parker Agricultural Research Complex (Tucson, AZ, USA), which is accredited by the American Association for Accreditation of Laboratory Animal Care. All experimental procedures comply with the outlined policies and regulations of The Journal of Physiology (Drummond, 2009).
Animal preparation
Time-mated, pregnant Columbia–Rambouillet crossbred ewes were purchased from Nebekar Ranch (Lancaster, CA, USA). Control ewes were maintained in a thermoneutral environment throughout pregnancy, and placental insufficiency intrauterine growth restriction (IUGR) was induced by exposing pregnant ewes to environmental hyperthermia (40°C for 12 h and 35°C for 12 h with a relative humidity of 35 ± 8%) (Bell et al. 1987; Regnault et al. 1999). Control ewes were pair-fed with hyperthermic ewes. Whenever ewes were carrying twins, only one offspring was randomly included in the study. The IUGR fetus group was generated from nine pregnant ewes (n = 9 fetuses), six carrying singletons and three carrying twins, which were exposed to hyperthermia from 39 ± 2 to 96 ± 5 days gestational age (dGA, term = 150 dGA). IUGR fetuses were compared to control fetuses from ewes carrying singletons (n = 6) and twins (n = 5). Fetal weight, placental weight, plasma noradrenaline concentrations and other parameters for these fetuses were described previously (Limesand et al. 2006; Leos et al. 2010). For the lamb treatment groups, 10 ewes (six carrying singletons and four carrying twins) were exposed to hyperthermic conditions from 40 ± 2 to 85 ± 3 dGA. We chose to shorten their exposure to lessen the severity of IUGR in order to produce viable offspring (Galan et al. 1999). Control lambs (six single and five twins) were from ewes maintained in a constant thermoneutral environment (25 ± 2°C; ∼25% humidity). Lambs were delivered naturally and housed in individual pens. All lambs received four to six feedings of colostrum before being placed solely on milk replacer with ad libitum access (Advance® Lamb Milk Replacer, Milk Specialties Co., Dundee, IL, USA). Birth weights were measured within 3 h of birth, usually prior to suckling. Body weights were measured daily at 08.00 h and immediately before each adrenaline challenge study. Singleton lambs were used for gene and protein expression experiments, and twin lambs were used for in vivo experiments. Pregnant ewes (133.4 ± 0.7 dGA) and singleton lambs (17.6 ± 0.7 dA) were killed with an intravenous overdose of sodium pentobarbital (86 mg kg−1) and phenytoin sodium (11 mg kg−1; Euthasol, Virbac Animal Health, Fort Worth, TX, USA). Perirenal adipose, brain, liver, skeletal muscle, spleen, kidney, lung and heart tissues were dissected and weighed. All tissues were snap frozen in liquid nitrogen and stored at −80°C until RNA and protein were extracted.
RNA extraction and cloning
RNA was extracted from perirenal adipose tissue and reverse transcribed as described previously (Cole et al. 2009). Synthetic oligonucleotide primers were designed with the aid of OligoPerfect Designer software (Invitrogen Life Technologies, Carlsbad, CA, USA; Tables 1 and 2) and purchased from Eurofins MWG Operon (Huntsville, AL, USA). Ovine perirenal adipose cDNA was used as a template to amplify PCR products for ovine α1(A,B,D)-AR, α2(A,B,C)-AR, β(1,2,3)-AR, HSL, perilipin, leptin and adiponectin for cloning and nucleotide sequencing as described previously (Cole et al. 2009). Nucleotide sequences were compared to Bos taurus orthologues with Basic Local Alignment Search Tool and submitted to GenBank (Table 1).
Table 1.
Oligonucleotide primer sequences and description of PCR products cloned for ovine genes
| Gene name | Forward (5′–3′) | Reverse (5′–3′) | Product size (bp) | GenBank accession no. | Identity Bos taurus |
|---|---|---|---|---|---|
| α1A-AR | CTCTGGGCTCCTTCTACGTG | CGCAGACACTGGATTCTCAA | 475 | EU723257 | 98% |
| α1B-AR | CGTGGGCAACATTCTAGTCAT | AGTAGCGCACCCCAATGTAG | 269 | EU851039 | 97% |
| α1D-AR | CTTCTCCTCCCTGTGCTCTTT | AGCAGGGGTAGATGAGTGGAT | 449 | EU723258 | 96% |
| α2A-AR | AACGAGGTCATGGGCTACTG | CTCGACGAGATGACGTACCA | 326 | EU726635 | 97% |
| α2B-AR | AGAAGGAAGGGGTCTGTGGT | ACCCAGGCTGTAGCTGAAGA | 317 | EU741650 | 94% |
| α2C-AR | ACACTGGTCATGCCCTTCTC | TGTACCAGGTCTCGTCGTTG | 328 | EU723259 | 99% |
| β1-AR | ATCGAGACCCTGTGTGTCATC | TGTCGATCTTCTTCACCTGCT | 343 | AF072433 | 98% |
| β2-AR | TCTTCACGAACCAACCCTATG | GATCTTCTGGAGCTGCCTTTT | 122 | EU723260 | 97% |
| β3-AR | TCTATCTTCCGCTTCTGGTGA | GAGTGAAGGTTCCCATGATGA | 270 | AF314205.1 | 92% |
| HSL | AGCAGTGACACAACAGACACG | CAGATCCATCCTCAGATCCAA | 106 | EU741652 | 92% |
| Perilipin | CCTCTGAGCTGAAGGACACC | ACCATCTTCTCGACACCACC | 226 | NM_001113773.1 | 96% |
| Leptin | AGAGGGTCACTGGTTTGGACT | TTGGATCACATTTCTGGAAGG | 125 | OAU84247 | 98% |
| ADIPOQ | TGGAACCTCCTATCTACCCAGA | GCACAGTCATCCCTAACCTCA | 166 | HM149341 | 97% |
AR, adrenergic receptor; HSL, hormone sensitive lipase; ADIPOQ, adiponectin.
Table 2.
Quantitative PCR parameters for ovine genes
| Gene name | Forward (5′–3′) | Reverse (5′–3′) | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| α1A-AR | ACGCACTTCTCCGTGAGACT | ACCCAATGGGCATCACTAAG | 124 | 60.0 |
| α1B-AR | CGTGGGCAACATTCTAGTCAT | AGTAGCGCACCCCAATGTAG | 269 | 60.0 |
| α1D-AR | CTTCTCCTCCCTGTGCTCTTT | AGCAGGGGTAGATGAGTGGAT | 449 | 60.0 |
| α2A-AR | AACGAGGTCATGGGCTACTG | CTCGACGAGATGACGTACCA | 326 | 60.0 |
| α2B-AR | AGGGAGAAGAGGGCTGTGAG | GCCAGCACGAAGGTGAAC | 208 | 60.0 |
| α2C-AR | TCATGGCCTACTGGTACTTCG | AGTAGCGGTCCAGGCTGAT | 114 | 60.0 |
| β1-AR | ATCGAGACCCTGTGTGTCATC | TGTCGATCTTCTTCACCTGCT | 343 | 62.0 |
| β2-AR | TCTTCACGAACCAACCCTATG | GATCTTCTGGAGCTGCCTTTT | 122 | 60.0 |
| β3-AR | TCTATCTTCCGCTTCTGGTGA | GAGTGAAGGTTCCCATGATGA | 270 | 60.0 |
| HSL | AGCAGTGACACAACAGACACG | CAGATCCATCCTCAGATCCAA | 106 | 60.0 |
| Perilipin | CCTCTGAGCTGAAGGACACC | ACCATCTTCTCGACACCACC | 226 | 60.0 |
| Leptin | AGAGGGTCACTGGTTTGGACT | TTGGATCACATTTCTGGAAGG | 125 | 60.4 |
| ADIPOQ | TGGAACCTCCTATCTACCCAGA | GCACAGTCATCCCTAACCTCA | 166 | 60.4 |
| S15 | ATCATTCTGCCCGAGATGGTG | TGCTTTACGGGCTTGTAGGTG | 134 | 60.0 |
AR, adrenergic receptor; HSL, hormone sensitive lipase; ADIPOQ, adiponectin.
Quantitative real-time PCR (qPCR)
The relative expression of α1(A,B,D)-AR, α2(A,B,C)-AR, β(1,2,3)-AR, HSL, perilipin, leptin and adiponectin mRNA transcripts was measured by quantitative PCR using SYBR Green (Qiagen, Valencia, CA, USA) in an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). After the initial denaturation (95°C for 15 min), all reactions went through 40 cycles of 96°C (30 s), annealing temperature (30 s; Table 2), and 72°C (10 s), at which point the fluorescence intensity was measured. Melt curve analysis, starting at 60°C with an increase of 0.2°C every 6 s to 96°C, was performed at the end of the amplification to confirm product homogeneity. Optimal annealing temperature for each primer set was determined using a temperature gradient (54–62°C), and products were examined on an agarose gel for specificity.
PCR efficiency was determined with gene-specific plasmid DNA, for which threshold cycles (Ct) were linear over concentrations varying by eight orders of magnitude. Samples were run in triplicate. The results were normalized to the reference gene (ribosomal protein S15) using the comparative ΔCt method (Ct gene of interest –Ct reference gene), and fold change was determined by Pfaffl's and Livak's method (Pfaffl, 2001; Livak & Schmittgen, 2001).
Western immunoblot analysis
Plasma membrane fractions were enriched from perirenal adipose tissue of control and IUGR fetuses and lambs as describe previously (Limesand et al. 2004). Briefly, pulverized perirenal adipose was homogenized in 0.25 mol l−1 sucrose, 50 mmol l−1 Hepes (pH 7.7), 0.5 mmol l−1 EDTA, 2 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, 0.2 mmol l−1 pheylmethylsulfonyl fluoride (PMSF), and 3 mmol l−1 DTT. Three successive centrifugations at 1200 g (10 min), 9000 g (15 min), and 25,000 g (20 min) were performed on the supernatant before the plasma membranes were pelleted with a 90 min centrifugation at 100,000 g. Plasma membrane pellets were washed once and resuspended in 0.25 mol l−1 sucrose, 50 mmol l−1 Hepes (pH 7.7), 100 mmol l−1 KCl, 5 mmol l−1 MgCl2, 2 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, 0.2 mmol l−1 PMSF, and 3 mmol l−1 DTT. Protein concentrations were determined with the bicinchoninic acid protein assay (Thermo Fisher Scientific Inc., Rockford, IL, USA). Purity and enrichment of plasma membrane proteins from perirenal adipose tissue were confirmed by evaluating Na+/K+ ATPase (anti-Na+/K+ ATPase α-1 clone C464.6, Millipore, Temecula, CA, USA).
Plasma membrane enriched perirenal adipose proteins (100 μg) were separated by 10% SDS-PAGE along with Precision Plus Protein Standards (Bio-Rad Laboratories). The electrophoresed proteins were transferred to polyvinylidene fluoride membrane (Bio-Rad Laboratories) and blocked in 5% non-fat dry milk with Tris-buffered saline (10 mmol l−1 Tris-HCl, 150 mmol l−1 NaCl, pH 8) with 0.05% Tween 20 (TBST) at room temperature for 1 h. Immunoblot detection of β2-AR and perilipin were accomplished, respectively, with a rabbit anti-β2-AR and anti-perilipin affinity-purified polyclonal antibody (β2-AR, sc-569; perilipin, sc-67164, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1 μg ml−1 in TBST containing 5% non-fat dry milk at 4°C for 16 h. The membrane was then washed 3 times for 10 min in TBST buffer. Binding of the rabbit antiserum was detected with anti-rabbit immunoglobulin G horseradish peroxidase conjugated secondary antibody (1:10,000; Bio-Rad Laboratories) in TBST for 1 h at room temperature and detected using SuperSignal West Pico (Thermo Fisher Scientific) exposed to Kodak x-ray film. The antibody specificity was tested in competition experiments with the β2-AR blocking peptide (Santa Cruz; 10 μg ml−1), which was added to the diluted rabbit anti-β2-AR antiserum (1 μg ml−1) and incubated at 4°C overnight (Limesand et al. 2004). Equality of loading was determined with Ponceau S staining (Sigma-Aldrich, St Louis, MO, USA). Densitometry analysis was completed with ImageJ software v. 1.41 (National Institutes of Health, USA), and data are presented as the mean ±s.e.m. Multiple exposure times were analysed to confirm that films were not saturated.
Adrenaline challenge in lambs
An adrenaline challenge was performed on the twin lambs at 21.1 ± 1.1 days of age (dA). Catheters were placed in a jugular vein of lambs 1–2 days prior to study. Catheters were stored in a 4 cm × 4 cm elastic pouch secured to the neck with Vetrap bandaging tape (3M Animal Care Products, St Paul, MN, USA) and glue (Tag Cement, The Ruscoe Co., Akron, OH, USA). All challenges were performed on lambs in the fed state. During the adrenaline challenge, eight venous blood samples from control (23.0 ± 0.7 dA; n = 5) and IUGR (21.5 ± 2.1 dA; n = 4) lambs were collected in syringes lined with EDTA (Sigma-Aldrich) at −40, −20, 2.5, 5, 10, 15, 30 and 50 min relative to the adrenaline bolus. Three different adrenaline doses (0.4, 0.8, 1.6 μg kg−1) were administered in random order as a bolus to each lamb at time 0. The three independent studies were separated by at least 6 h. Plasma was separated by centrifuging (16,000 g) for 2 min at 4°C and then aspirated from the pelleted red blood cells and stored at −80°C for subsequent determination of insulin and NEFA concentrations. Samples collected at −40 and −20 min were considered baseline measurements and the responsiveness to adrenaline (from 2.5 min to 50 min) was determined by calculating the area under the curve (AUC) from baseline (Prism 5.01, GraphPad Software, La Jolla, CA, USA).
Biochemical analysis
Blood pH, oxygen saturation and haemoglobin concentrations were measured in whole blood collected in syringes lined with dried heparin (Elkins-Sinn, Inc., Cherry Hill, NJ, USA) on an ABL 725 (Radiometer, Copenhagen, Denmark). The body temperature of each lamb was measured prior to the study and used for temperature correction of pH and calculations. Plasma glucose and lactate concentrations were also measured immediately using a YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin concentrations were measured by an ovine insulin enzyme-linked immunosorbent assay (ALPCO Diagnostics, Windham, NH, USA) as previously reported (Leos et al. 2010). The inter- and intra-assay coefficients were 5.2% and 4.9%, respectively.
Plasma NEFA concentrations were measured by enzymatic colorimetric analysis using a commercial kit (Wako Chemicals USA, Richmond, VA, USA) as modified by Baumgard et al. (2000). The reactions were conducted in 96-well microplates (Thermo Fisher Scientific) and absorbance read on a microplate spectrophotometer (SpectraMax M2; Molecular Devices, Sunnyvale, CA, USA). Briefly, the method relies upon the acylation of coenzyme A (CoA) by NEFAs in the presence of acyl-CoA synthetase. The acyl-CoA thus produced is oxidized by acyl-CoA oxidase with generation of hydrogen peroxide. Hydrogen peroxide, in the presence of peroxidase, permits the oxidative condensation of 3-methyl-N-ethyl-N-(β-hydroxyethyl)-aniline with a peak absorbance at 550 nm. The inter- and intra-assay coefficients were 4.6% and 4.5%, respectively.
Statistical analysis
Statistical analysis of the body and organ weights, qPCR (ΔCt), and western immunoblot data were subjected to a one-way ANOVA, using the general linear model procedure in SAS Proc GLM (SAS v. 9.1, SAS Institute Inc., Cary, NC, USA), and differences were determined by Student's t test. Statistical analysis of qPCR data was performed on the ΔCt values. In order to avoid a lack of independence between twin pairs, only one lamb from each ewe was evaluated. Biochemical, haematological and hormone measurements during the adrenaline challenge were analysed with a SAS Proc MIXED method that accounted for lamb as a random variable (SAS/STAT User's Guide, v. 6, 4th edn, SAS Institute Inc.). Values for dGA are presented as the mean ±s.d.; all other values are expressed as the mean ±s.e.m.
Results
Morphometric characteristic of fetuses and lambs
In IUGR fetuses at 133 dGA, the average body weight was lower than controls (singles 1.44 ± 0.15 kg versus 3.22 ± 0.18 kg, P < 0.01; twins 1.51 ± 0.11 kg versus 3.02 ± 0.17 kg, P < 0.05). Placental weight was also reduced in IUGR fetuses (singles 117 ± 14 g versus 285 ± 20 g, P < 0.01; twins 119 ± 20 g versus 187 ± 20 g, P < 0.05). In the independent lamb cohorts, IUGR birth weights and body weights at 16 and 21 dA (Table 3) were not significantly less than controls (P < 0.1), but when lamb birth weights were combined and pregnancy type (single or twin) was used as a covariate in the statistical model, the consistent 20% reduction in birth weight in IUGR lambs was significant (P < 0.05). The length of gestation was approximately 3 days shorter (P < 0.05) in both IUGR singletons and twins than controls. In singletons, IUGR and control lambs grew at the same rate of 0.16 ± 0.02 kg day−1 during the first 16 days of life. In twins during the first 21 days, growth rate was also similar; control lambs gained 0.22 ± 0.02 kg day−1 and IUGR lambs gained 0.17 ± 0.02 kg day−1, neither of which were different from the singleton average daily gain. When the singleton and twin average daily gain measurements were combined, no differences were found between control (n = 11) and IUGR (n = 10) treatments (Fig. 1). The average daily dry matter intake (DMI) was also not different between treatments (Table 3).
Table 3.
Lamb biometry measurements, gestational length, and dry matter intake
| 16 days of age | 21 days of age | |||
|---|---|---|---|---|
| Control (6) | IUGR (6) | Control (5) | IUGR (4) | |
| Gestation (days) | 152.2 ± 0.5 | 149.3 ± 1.0* | 151.2 ± 0.9 | 148.5 ± 0.3* |
| Birth weight (kg) | 4.40 ± 0.39 | 3.48 ± 0.47 | 3.66 ± 0.33 | 2.88 ± 0.46 |
| Study weight (kg) | 7.43 ± 0.64 | 5.86 ± 1.22 | 8.28 ± 0.76 | 6.65 ± 0.82 |
| Average DMI (g) | 168.72 ± 13.58 | 157.97 ± 17.18 | 245.01 ± 18.73 | 237.12 ± 9.08 |
Values are means ±s.e.m.; IUGR, intrauterine growth restriction; DMI, dry matter intake
P < 0.05.
Figure 1. Growth rates in lambs.
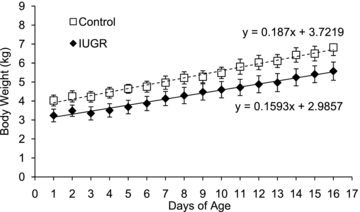
Body weights for control (□, n = 11) and IUGR lambs (♦, n = 10) are presented for their first 16 days of postnatal life. Values are means ± s.e.m.
mRNA expression in perirenal adipose tissue
Adrenergic receptor cDNA for all the genes of interest were cloned from fetal sheep or lamb perirenal adipose mRNA. Nucleotide sequences for these cloned PCR products shared greater than 92% identity with the Bos taurus orthologues (Table 1).
The β2-AR mRNA concentrations were 59% lower (P < 0.01) in IUGR singleton fetuses and 74% lower (P < 0.05) in IUGR lambs compared to the respective controls (Fig. 2A and B). In singleton IUGR fetuses, perilipin mRNA concentrations were 43% less (P < 0.05) than in control fetuses, but there was no treatment effect in lambs. Perilipin protein concentrations were not different between treatments in fetuses or lambs (data not shown). In singleton IUGR fetuses, leptin mRNA concentrations were 49% lower (P < 0.05) than control fetuses and also tended to be lower (P < 0.1) in IUGR lambs. No differences in mRNA expression were observed for HSL, adiponectin and all other ARs (Fig. 2). The findings were similar for ARs in twins; β2-AR mRNA concentration were lower (P < 0.05) in IUGR twins compared to their controls. However, in twin fetuses, leptin and perilipin mRNA expression levels were not different between IUGR and control treatments (data not shown). In control twin fetuses, leptin mRNA concentrations were less (P < 0.05) than singletons control.
Figure 2. mRNA profiles in perirenal adipose tissue.
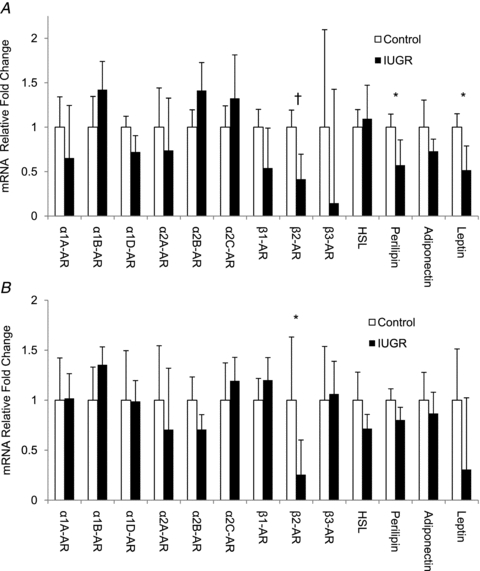
mRNA concentrations for the genes listed on the abscissa are normalized to ribosomal protein S15 and expressed as the fold change from control calculated by the 2−ΔΔCt in perirenal adipose tissue collected from singleton fetuses (A) and lambs (B). Mean ±s.e.m. values for control (▪) and IUGR (□) animals are presented. Significance is indicated by * (P < 0.05) and † (P < 0.01).
β2-AR protein levels in perirenal adipose tissue
The antibody against β2-AR detected a single protein with an apparent molecular mass of 68 kDa, and this interaction was blocked with the immunizing peptide (data not shown). The average β2-AR protein level was 56 ± 14% (P < 0.05) lower in IUGR fetuses (Fig. 3A) and 48 ± 12% (P < 0.05) lower in IUGR lambs compared to controls (Fig. 3B).
Figure 3. Immunoblot analysis for β2-AR in perirenal adipose tissue.
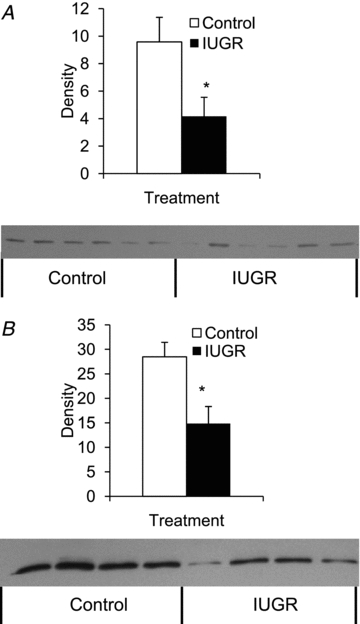
β2-AR protein concentrations were measured in fetuses (A) and lambs (B). Representative immunoblots for plasma membrane enriched fractions from control (□) and IUGR (▪) perirenal adipose tissue are presented below the bar graphs, which summarize the 68 kDa band intensities from each treatment group. Significance from Student's t test is depicted with an * (P < 0.05) for each age.
Adrenaline challenge in lambs
To determine adrenergic responsiveness, adrenaline was administrated to control and IUGR lambs. The pH, oxygen content, haematocrit, glucose, insulin and lactate concentrations are presented for the highest dose (1.6 μg kg−1 adrenaline; Tables 4 and 5). Haematological values were unchanged by adrenaline (Table 4). The baseline plasma glucose and lactate concentrations were similar between treatments; however, plasma glucose and lactate concentrations increased in response to the adrenaline challenge to a greater extent (P < 0.05) in the IUGR lambs compared with control lambs (Table 5).
Table 4.
Haematological values for lambs prior to and after the adrenaline challenge studies
| Treatment condition | Control | IUGR |
|---|---|---|
| Prior to the adrenaline challenge | ||
| pH | 7.41 ± 0.02 | 7.43 ± 0.02 |
| O2 (mmol l−1) | 3.30 ± 0.18 | 3.35 ± 0.65 |
| Haematocrit (%) | 31.21 ± 1.32 | 33.63 ± 3.08 |
| After adrenaline challenge | ||
| pH | 7.39 ± 0.01 | 7.42 ± 0.02 |
| O2 (mmol l−1) | 3.33 ± 0.24 | 3.58 ± 0.43 |
| Haematocrit (%) | 31.39 ± 1.29 | 32.7 ± 3.35 |
Values are means ±s.e.m.; IUGR, intrauterine growth restriction.
Table 5.
Plasma metabolite and insulin concentrations in lambs prior to and after the adrenaline challenge studies
| Treatment condition | Control | IUGR |
|---|---|---|
| Prior to the adrenaline challenge | ||
| Glucose (mmol l−1) | 6.84 ± 0.20 | 6.98 ± 0.44 |
| Lactate (mmol l−1) | 1.31 ± 0.10 | 1.33 ± 0.19 |
| Insulin (μg l−1) | 0.76 ± 0.19 | 1.34 ± 0.22 |
| Response after adrenaline challenge (AUC) | ||
| Glucose (mmol min l−1) | 25.01 ± 6.67 | 51.83 ± 3.41* |
| Lactate (mmol min l−1) | 8.81 ± 2.91 | 17.67 ± 1.82* |
| Insulin (μg min l−1) | 25.12 ± 16.31 | 13.23 ± 4.87 |
Values are means ± s.e.m.; IUGR, intrauterine growth restriction; AUC, area under the curve
P < 0.05.
All three adrenaline doses caused an elevation in plasma NEFA concentrations, and AUC values were not different between doses (data not shown). Therefore, the NEFA results from all three doses were combined to provide three replicate measurements per lamb. Baseline NEFA concentrations were similar between IUGR (468 ± 81 μEq l−1) and control (338 ± 33 μEq l−1) lambs (Fig. 4). However, the NEFA AUC was 55 ± 15% lower in IUGR lambs (P < 0.05) than in controls (Fig. 4).
Figure 4. Adrenergic responsiveness in lambs at 21 days of age.
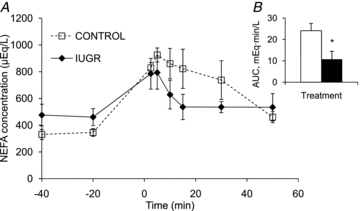
During the adrenaline challenge, mean plasma NEFA concentrations (y-axis) for control (□) and IUGR (♦) lambs are presented for the duration of the study in the line graph. Time (x-axis) is presented relative to the administration of adrenaline, which occurred at time 0. Baseline values were taken as the average of samples prior to giving adrenaline, and area under the curve (AUC) post-adrenaline was calculated. AUC values are presented in the inserted bar graph with * indicating difference with P < 0.05.
Discussion
In the present study, we demonstrate that adrenergic desensitization occurs in response to placental insufficiency-induced IUGR and can persist throughout the perinatal transition, causing impaired adrenaline induced lipolysis in lambs. Declines in β2-adrenergic receptor mRNA and protein concentrations in perirenal adipose tissue appear to be the primary cause for the impaired adrenergic responsiveness. Previously, we showed that plasma noradrenaline concentrations are elevated in IUGR singleton and twin fetuses above threshold for all physiological aspects and are negatively associated with blood oxygen content (Padbury et al. 1987; Limesand et al. 2006; Leos et al. 2010). This indicates that hypoxaemia chronically raises fetal catecholamine concentrations during placental insufficiency, thus redistributing nutrients by suppressing insulin (Bassett & Hanson, 2000; Leos et al. 2010). Previous reports have shown that prolonged administration of catecholamines to fetal sheep causes adrenergic desensitization because several metabolite and hormone values returned to normal (Bassett & Hanson, 1998; Bassett & Symonds, 1998). Adrenergic desensitization is known to result from reductions in receptor mRNA concentration in a cAMP-dependent fashion due to either a suppression of receptor gene transcription or selective degradation of receptor mRNA (Bouvier et al. 1989; Collins et al. 1991; Hosoda et al. 1995; Kirigiti et al. 2001). Our current study in perirenal adipose tissue supports this assertion (Figs 2 and 3). The impairment in lipid mobilization is likely to leave IUGR offspring at greater risk for obesity and dyslipidaemia in postnatal life.
Catecholamines serve as regulators of intermediary metabolism and stimulate the mobilization of energy stores. IUGR lambs challenged with adrenaline at 21 dA had a lower NEFA response (Fig. 4), showing that lipid mobilization is impaired. In adipose tissue, adrenaline acts primarily through the β2-AR to stimulate HSL to hydrolyse stored triglycerides into free fatty acids and glycerol (Fig. 5; Carmen & Victor 2006). Signalling for lipolysis occurs via a cAMP-dependent protein kinase A pathway, where HSL and perilipin are activated by phosphorylation after catecholamines bind to the β2-AR. HSL is the rate-limiting enzyme for lipolysis, and perilipin undergoes conformational changes to facilitate HSL localization with the lipid droplet surface (Tansey et al. 2004). Therefore, reductions in β2-AR in the perirenal adipose tissue (Figs 2 and 3) will lead to decreased activation of downstream signalling events. Thus, the reduced adrenergic responsiveness observed in the present study is most likely due to lower mRNA and protein concentrations of β2-AR, because HSL and perilipin mRNA concentrations were not different between control and IUGR lambs.
Figure 5. β2-AR signalling pathway of lipolysis in adipocytes.
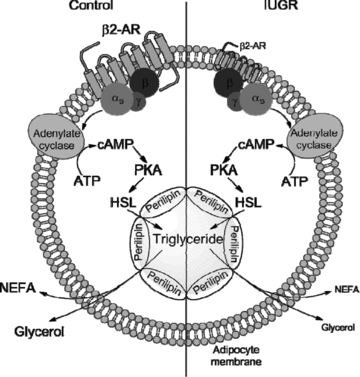
In control fetuses and lambs, stimulation of the β2-AR increases cAMP production to activate PKA to phosphorylate HSL and perilipin, which hydrolyses intracellular triglyceride stored in the lipid droplet. IUGR fetuses and lambs have lower β2-AR concentrations, which desensitizes them to catecholamine stimulate lipolysis, resulting in less NEFA and glycerol released from adipocytes. In the diagram, reductions in the IUGR animals are portrayed by smaller font and receptor size.
Placental restricted lambs with IUGR created by uterine carunclectomy exhibited postnatal catch-up growth by 45 days (De Blasio et al. 2007). Although body weights were not different between controls and placental restriction lambs at autopsy, the ratios of visceral and perirenal adipose tissues relative to body weight were greater in placental restricted lambs (De Blasio et al. 2007). In our study, the weight of perirenal adipose tissue relative to body weight was not different between treatments in lambs at 18 dA. This may be too early to distinguish any treatment effects for fat mass, because differences in rate of gain and DMI were not observed (Fig. 1).
Global energy deficiency during hypoxia increases cAMP concentrations, which stimulates glycolysis and synthesis of pyruvate in muscle, leading to the accumulation of cytoplasmic pyruvate and NADH. Because oxygen is lacking, lactate dehydrogenase converts pyruvate to lactate, which can be released into circulation and used as a substrate for hepatic gluconeogenesis. Interestingly, we have previously reported that IUGR fetuses have approximately twice as much skeletal muscle glycogen as controls (Limesand et al. 2007). Therefore, if elevated glycogen persists in lambs, it would promote glycogenolysis and lactate production. In IUGR lambs, a greater lactate response was found during the adrenaline challenge (Table 5). Thus, we postulate that increased lactate generation is caused by increased glycogenolysis in skeletal muscle. In addition to lowering NEFA mobilization, lower β2-AR will also reduce the efficiency of free fatty acid utilization as an energy source. Similar attributes in glycogenolysis would be expected in skeletal muscle, which also has lower β2-AR expression (X. Chen and S. W. Limesand, unpublished observation), but the increased glycogen content might partially compensate for the deficiencies in catabolic efficiency. Therefore, it appears that in order for IUGR lambs to appropriately meet metabolic demands, they may shift substrate metabolism from fat to glucose by increasing glycolysis and glycogenolysis. This shift is further supported by the observed elevation in plasma glucose concentrations post-adrenaline challenge in IUGR lambs (Table 5).
In addition to lipid storage, adipose tissue also has endocrine functions; it synthesizes and secretes adiponectin and leptin. These adipokines regulate glucose and fatty acids to control energy balance and metabolism. In our study, adiponectin expression was not different between IUGR and control fetuses or lambs, similar to findings in the uterine carunclectomy model (Muhlhausler et al. 2007a,b;). Leptin expression, however, was reduced in perirenal adipose tissue of IUGR fetuses and tended to be lower in IUGR lambs. Interestingly, lower leptin expression was not specific to placental insufficiency-induced IUGR because twin fetuses, which are growth restricted compared to singletons, also had lower leptin mRNA concentrations. In near-term fetal sheep, leptin has been infused chronically to raise leptin concentrations for 5 days, where it was found to inhibit gluconeogenic enzymes and endogenous glucose production (Forhead et al. 2008). Furthermore, in rat hepatocyte cultures, exposure to higher leptin concentrations lowers gluconeogenesis by inhibiting phosphoenolpyruvate carboxykinase (PEPCK) (Anderwald et al. 2002). However, in our model of IUGR, hepatic gluconeogenesis is occurring, and gluconeogenic enzymes, glucose-6-phophatase and PEPCK, are elevated (Limesand et al. 2007). Therefore, the reduction in leptin may also be playing an important role in IUGR nutrient redistribution by promoting gluconeogenesis. In the lamb, adequate nutrients are available, but if glucose rather than fat utilization is occurring, lower leptin concentrations might prevail albeit to a lesser extent (Fig. 2).
In summary, we have shown that placental insufficiency-induced IUGR leads to β2-AR down-regulation in the perirenal adipose tissue of sheep fetuses, which most likely is caused by the chronic elevation of catecholamines in late gestation (Limesand et al. 2006; Leos et al. 2010). Furthermore, we show that reductions in β2-AR persist in IUGR lambs, causing AR desensitization that impairs adrenaline-stimulated lipolysis. Interestingly, plasma lactate and glucose concentrations were elevated in IUGR lambs following the adrenaline challenge, indicating that carbohydrate metabolism is enhanced while fat metabolism is spared. These data begin to explain how endocrine factors, like catecholamines, may facilitate prenatal adaptations to benefit survival but ultimately result in impaired metabolic function after birth, leading to dyslipidaemia and obesity.
Acknowledgments
The project described was supported by award number R01DK084842 (to S.W.L.) from the National Institute of Diabetes and Digestive and Kidney Diseases and an Endocrine Society Bridge Grant (to S.W.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. A.S.G. was supported by F32 DK088514 and T32 HL7249 from the National Institutes of Health. We thank Jessica B. Wheelock and Mandie M. Dunham for their technical assistance.
Glossary
Abbreviations
- AR
adrenergic receptor
- AUC
area under the curve
- dA
days of age
- dGA
days of gestational age
- DMI
dry matter intake
- HSL
hormone sensitive lipase
- IUGR
intrauterine growth restriction
- NEFA
non-esterified fatty acid
- PEPCK
phosphoenolpyruvate carboxykinase
Author contributions
X.C. and S.W.L. designed the experiment. All authors participated in data collection, analysis, and interpretation. X.C. and S.W.L. wrote the manuscript. All authors revised the manuscript and approved the final version. The experiments were conducted at the William J. Parker Agricultural Research Complex, Department of Animal Sciences, University of Arizona, Tucson AZ, USA.
References
- Altan VM, Arioglu E, Guner S, Ozcelikay AT. The influence of diabetes on cardiac beta-adrenoceptor subtypes. Heart Fail Rev. 2007;12:58–65. doi: 10.1007/s10741-007-9005-6. [DOI] [PubMed] [Google Scholar]
- Anderwald C, Muller G, Koca G, Furnsinn C, Waldhausl W, Roden M. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol Endocrinol. 2002;16:1612–1628. doi: 10.1210/mend.16.7.0867. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1536–R1545. doi: 10.1152/ajpregu.1998.274.6.R1536. [DOI] [PubMed] [Google Scholar]
- Bassett JM, Hanson C. Prevention of hypoinsulinemia modifies catecholamine effects in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1171–R1181. doi: 10.1152/ajpregu.2000.278.5.R1171. [DOI] [PubMed] [Google Scholar]
- Bassett JM, Symonds ME. β2-Agonist ritodrine, unlike natural catecholamines, activates thermogenesis prematurely in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 1998;275:R112–R119. doi: 10.1152/ajpregu.1998.275.1.R112. [DOI] [PubMed] [Google Scholar]
- Baumgard LH, Corl BA, Dwyer DA, Saebo A, Bauman DE. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R179–R184. doi: 10.1152/ajpregu.2000.278.1.R179. [DOI] [PubMed] [Google Scholar]
- Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9:17–29. [PubMed] [Google Scholar]
- Benovic JL, Onorato JJ, Caron MG, Lefkowitz RJ. Regulation of G protein-coupled receptors by agonist-dependent phosphorylation. Soc Gen Physiol Ser. 1990;45:87–103. [PubMed] [Google Scholar]
- Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Collins S, O’Dowd BF, Campbell PT, de Blasi A, Kobilka BK, MacGregor C, Irons GP, Caron MG, Leftowitz RJ. Two distinct pathways for cAMP-mediated down-regulation of the β2-adrenergic receptor. J Biol Chem. 1989;264:16786–16792. [PubMed] [Google Scholar]
- Cole L, Anderson M, Antin PB, Limesand SW. One process for pancreatic β-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol. 2009;203:19–31. doi: 10.1677/JOE-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Caron MG, Lefkowitz RJ. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu Rev Physiol. 1991;53:497–508. doi: 10.1146/annurev.ph.53.030191.002433. [DOI] [PubMed] [Google Scholar]
- Collins S, Caron MG, Lefkowitz RJ. From ligand binding to gene expression: new insights into the regulation of G-protein-coupled receptors. Trends Biochem Sci. 1992;17:37–39. doi: 10.1016/0968-0004(92)90425-9. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology. 2007;148:1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287:E1114–1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seventransmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav. 2006;88:234–243. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forhead AJ, Lamb CA, Franko KL, O’Connor DM, Wooding FB, Cripps RL, Ozanne S, Blache D, Shen QW, Du M, Fowden AL. Role of leptin in the regulation of growth and carbohydrate metabolism in the ovine fetus during late gestation. J Physiol. 2008;586:2393–2403. doi: 10.1113/jphysiol.2007.149237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, Battaglia FC. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–1282. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I, Mayor F., Jr Rapid desensitization of neonatal rat liver β-adrenergic receptors. A role for β-adrenergic receptor kinase. J Clin Invest. 1994;93:937–943. doi: 10.1172/JCI117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–24. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den HH, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res. 1995;38:267–271. doi: 10.1203/00006450-199508000-00022. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Fitzgerald LR, Vaidya VA, Feussner GK, Fishman PH, Duman RS. Regulation of β2-adrenergic receptor mRNA and gene transcription in rat C6 glioma cells: effects of agonist, forskolin, and protein synthesis inhibition. Mol Pharmacol. 1995;48:206–211. [PubMed] [Google Scholar]
- Kirigiti P, Bai Y, Yang YF, Li X, Li B, Brewer G, Machida CA. Agonist-mediated down-regulation of rat β1-adrenergic receptor transcription: role of potential post-transcriptional degradation factors. Mol Pharmacol. 2001;60:1308–1324. doi: 10.1124/mol.60.6.1308. [DOI] [PubMed] [Google Scholar]
- Leos RA, Anderson MJ, Chen X, Pugmire JP, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–778. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand SW, Regnault TR, Hay WW., Jr Characterization of glucose transporter 8 (GLUT8) in the ovine placenta of normal and growth restricted fetuses. Placenta. 2004;25:70–77. doi: 10.1016/j.placenta.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2007;293:E1716–E1725. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition increases leptin expression in perirenal and subcutaneous adipose tissue in the postnatal lamb. Endocrinology. 2007a;148:6157–6163. doi: 10.1210/en.2007-0770. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007b;148:878–885. doi: 10.1210/en.2006-1115. [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padbury J, Agata Y, Ludlow J, Ikegami M, Baylen B, Humme J. Effect of fetal adrenalectomy on catecholamine release and physiologic adaptation at birth in sheep. J Clin Invest. 1987;80:1096–1103. doi: 10.1172/JCI113166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV. Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol. 1999;162:433–442. doi: 10.1677/joe.0.1620433. [DOI] [PubMed] [Google Scholar]
- Santala M, Saarikoski S, Castren O, Parviainen M. Lymphocyte β-2-adrenoceptors and plasma catecholamines in fetal hypoxia. Gynecol Obstet Invest. 1990;30:150–154. doi: 10.1159/000293246. [DOI] [PubMed] [Google Scholar]
- Schaak S, Mialet-Perez J, Flordellis C, Paris H. Genetic variation of human adrenergic receptors: from molecular and functional properties to clinical and pharmacogenetic implications. Curr Top Med Chem. 2007;7:217–231. doi: 10.2174/156802607779318163. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol. 2003;179:293–299. doi: 10.1677/joe.0.1790293. [DOI] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol Regul Integr Comp Physiol. 1992;263:R578–585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008;87:1769–1775. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]


