Abstract
Recent animal studies indicate that insulin increases arterial baroreflex control of lumbar sympathetic nerve activity; however, the extent to which these findings can be extrapolated to humans is unknown. To begin to address this, muscle sympathetic nerve activity (MSNA) and arterial blood pressure were measured in 19 healthy subjects (27 ± 1 years) before, and for 120 min following, two common methodologies used to evoke sustained increases in plasma insulin: a mixed meal and a hyperinsulinaemic euglycaemic clamp. Weighted linear regression analysis between MSNA and diastolic blood pressure was used to determine the gain (i.e. sensitivity) of arterial baroreflex control of MSNA. Plasma insulin was significantly elevated within 30 min following meal intake (Δ34 ± 6 uIU ml−1; P < 0.05) and remained above baseline for up to 120 min. Similarly, after meal intake, arterial baroreflex-MSNA gain for burst incidence and total MSNA was increased and remained elevated for the duration of the protocol (e.g. burst incidence gain: −3.29 ± 0.54 baseline vs.−5.64 ± 0.67 bursts (100 heart beats)−1 mmHg−1 at 120 min; P < 0.05). During the hyperinsulinaemic euglycaemic clamp, in which insulin was elevated to postprandial concentrations (Δ42 ± 6 μIU ml−1; P < 0.05), while glucose was maintained constant, arterial baroreflex-MSNA gain was similarly enhanced (e.g. burst incidence gain: −2.44 ± 0.29 baseline vs.−4.74 ± 0.71 bursts (100 heart beats)−1 mmHg−1 at 120 min; P < 0.05). Importantly, during time control experiments, with sustained fasting insulin concentrations, the arterial baroreflex-MSNA gain remained unchanged. These findings demonstrate, for the first time in healthy humans, that increases in plasma insulin enhance the gain of arterial baroreflex control of MSNA.
Introduction
The arterial baroreflex modulates beat-to-beat oscillations in arterial blood pressure via the control of efferent sympathetic outflow to the vasculature. Due to the importance of the arterial baroreflex in the regulation of blood pressure a large body of research has been dedicated to understanding alterations in baroreflex sensitivity (i.e. gain) in physiological (e.g. exercise) as well as pathophysiological conditions (Fadel et al. 2003; Joyner, 2006; Skrapari et al. 2006). With regard to the latter, numerous studies have indicated that hypertensive conditions are typically associated with reductions in arterial baroreflex sensitivity (Bristow et al. 1969; Gribbin et al. 1971; Eckberg, 1979; Matsukawa et al. 1991). Another common risk factor shown to be present in hypertensive patients is insulin resistance (Ferrannini et al. 1987; Reaven et al. 1996). Interestingly, a number of recent studies have indicated that several conditions exhibiting reductions in insulin sensitivity may also demonstrate impairments in arterial baroreflex function and hypertension, including obesity (Grassi et al. 1995; Schreihofer et al. 2007; Huber & Schreihofer, 2010), and the metabolic syndrome (Grassi et al. 2005). Although not causative, the concomitant occurrence of insulin resistance with reductions in arterial baroreflex function clearly suggests a link between the two that warrants investigation.
Emerging evidence from studies in experimental animal models has begun to establish a role for insulin in the central control of the arterial baroreflex. Recently, Pricher et al. (2008) demonstrated that increases in insulin within the brain, via lateral ventricular infusion, enhanced the gain of arterial baroreflex control of lumbar sympathetic nerve activity in rats. Other studies have indicated that neurons in central baroreflex regulatory regions (i.e. nucleus tractus solitarius) are responsive to insulin (Ruggeri et al. 2001). Collectively, these findings indicate that insulin can directly influence arterial baroreflex function within central neural cardiovascular control pathways. However, to date, no work has been performed in humans evaluating the potential influence of insulin on arterial baroreflex control of sympathetic nerve activity, and therefore the extent to which these findings in experimental animals can be translated to humans remains unknown.
Therefore, the purpose of the current study was to examine a role for insulin in arterial baroreflex control of sympathetic nerve activity in humans. Although, insulin is not produced in significant amounts within the brain, increases in circulating insulin gain access to the central nervous system via transport-mediated uptake across the blood–brain barrier (Woods et al. 2003; Banks, 2004). Thus, we utilized two common methodologies to raise plasma insulin concentrations. First, a mixed meal was used as a physiological method to evoke increases in plasma insulin. In addition, to further isolate the influence of insulin on arterial baroreflex control we also performed hyperinsulinemic euglycemic clamps, in which insulin concentrations were elevated to a similar extent as postprandial conditions, without concomitant increases in plasma glucose. We hypothesized that increases in plasma insulin would enhance the gain of arterial baroreflex control of muscle sympathetic nerve activity in healthy humans.
Methods
General procedures
Nineteen healthy male subjects (age, 27 ± 1 years; height, 180 ± 1 cm; weight, 80 ± 3 kg) volunteered for participation in these studies. No subject had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease and none were taking medication. Subjects were instructed to abstain from caffeinated beverages and food for 12 h, alcohol for 24 h, and physical activity for 48 h prior to the experimental sessions. After receiving a detailed verbal and written explanation of the intended experimental protocol and measurements, each subject provided written informed consent. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri Health Sciences Institutional Review Board.
Experimental measurements
Subjects were studied in the supine position at a constant ambient room temperature of 22–23°C. Heart rate was continuously monitored using a lead II electrocardiogram. Arterial blood pressure was measured on a beat-to-beat basis using servo-controlled finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). In addition, arterial blood pressure was measured with an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY, USA) to confirm the Finometer measurements of absolute blood pressure. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position around the abdomen (Pneumotrace, UFI, Morro Bay, CA, USA).
Multiunit recordings of postganglionic muscle sympathetic nerve activity (MSNA) were obtained by inserting unipolar tungsten microelectrodes percutaneously through the intact, unanaesthetized skin and positioned into muscle nerve fascicles of the peroneal nerve near the fibular head. The nerve signal was processed by a pre-amplifier and an amplifier (Dept of Bioengineering, University of Iowa, Iowa City, IA, USA), band pass filtered (bandwidth 700–2000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. MSNA recordings were identified by their characteristic pulse-synchronous burst pattern and increased neural activity in response to an end-expiratory apnoea or Valsalva manoeuvre, without any response to arousal stimuli or stroking of the skin. MSNA was first identified by visual inspection and was then analysed using custom-designed software (MatLab, The Math Works, Natick, MA, USA), as previously described (Hamner & Taylor, 2001; Young et al. 2010). The amplitude of the largest burst at baseline was assigned a value of 1000 (arbitrary units; AU) and all other bursts within a trial were normalized with respect to this value. All variables were sampled at 1000 Hz and stored for off-line analysis (Chart v5.2 and Powerlab, ADInstruments, Bella Vista, NSW, Australia).
Plasma insulin and glucose were measured from venous blood samples drawn from an antecubital (Protocol 1) or a hand (Protocol 2 and 3) intravenous catheter. Insulin was determined using chemiluminescent enzyme immunoassay (Immulite 1000 Analyzer, Diagnostic Products Corp., Los Angeles, CA, USA) and glucose was determined using the glucose oxidase method (Thermo, Waltham, MA, USA or Beckman Instruments, Brea, CA, USA).
Experimental protocols
Protocol 1: arterial baroreflex control of MSNA following a mixed meal
A mixed meal was utilized as a physiological method to evoke sustained increases in plasma insulin (n = 12). MSNA, arterial blood pressure, heart rate and respiration were measured before, and for 120 min following, ingestion of a liquid mixed meal (Ensure Plus, Abbott Laboratories, Columbus, OH, USA; 57% carbohydrate, 28% fat, 15% protein), corresponding to 20% of the subject's estimated energy expenditure calculated from body weight (Mifflin et al. 1990). All neuro-cardiovascular variables were measured at baseline for 20 min and for a 5 min period, every 30 min, following consumption of the mixed meal. Venous blood samples were similarly collected from an antecubital intravenous catheter and the resulting plasma was stored at −80°C for later analysis of plasma insulin and glucose.
Protocol 2: arterial baroreflex control of MSNA during a hyperinsulinaemic euglycaemic clamp
To further isolate the influence of insulin on arterial baroreflex control of MSNA, hyperinsulinaemic euglycaemic clamps were performed (n = 8) in which insulin was elevated to postprandial concentrations while glucose was maintained constant (DeFronzo et al. 1979). Intravenous catheters were placed in a left antecubital vein and a right-hand vein for the infusion of insulin/glucose and blood sampling, respectively. The right hand was placed in a heated box (50°C) for determination of arterialized venous blood samples (Liu et al. 1992). Insulin (Humulin, Eli Lilly, Indianapolis, IN, USA) was diluted in 0.9% saline with 5 ml of the subject's blood and a 10 min priming insulin infusion was followed by a constant infusion at 30 mU m−2 min−1, for a total of 120 min. Glucose was maintained at euglycaemic concentrations throughout via a variable 20% dextrose infusion. Plasma glucose was determined every 5 min and plasma was stored at −80°C for later insulin analysis. MSNA, arterial blood pressure, heart rate and respiration were collected for 20 min at baseline and for a 5 min period, every 30 min, during the hyperinsulinaemic euglycaemic clamp. The glucose infusion rate during the last 30 min of the clamp was used as an index of each subject's insulin sensitivity (DeFronzo et al. 1979).
Protocol 3: arterial baroreflex control of MSNA during time control experiments
In a subset of subjects (n = 4) time control experiments were also performed in which 0.9% saline was infused to match the volume administered during the hyperinsulinaemic euglycaemic clamp, while insulin was sustained at fasting concentrations.
Data analysis
Baseline MSNA, arterial blood pressure and heart rate were calculated as mean values over a 6 min period. Following consumption of the mixed meal and during the hyperinsulinaemic euglycaemic clamp or time controls, 3 min averages were calculated from the 5 min data segments collected every 30 min. The same segments were used to evaluate arterial baroreflex control of MSNA by analysing the relationship between spontaneously occurring fluctuations in diastolic blood pressure and MSNA, as previously described (Sundlof & Wallin, 1978; Kienbaum et al. 2001; Keller et al. 2006; Ogoh et al. 2007). Briefly, the diastolic blood pressure for each cardiac cycle within a data collection period was grouped into 3 mmHg pressure bins. The burst incidence within each pressure bin was calculated by determining the percentage of heart beats that were associated with a burst of MSNA and expressed as bursts (100 heart beats)−1. In addition, total MSNA was determined for each pressure bin by calculating the total area of all MSNA bursts, relative to the number of cardiac cycles, and expressed as arbitrary units (AU) beat−1. The slope of the relationship between MSNA variables and diastolic blood pressure was identified using linear regression analysis (SPSS v17.0, SPSS Inc., Chicago, IL, USA), with a minimum r value of 0.5 used as a criteria for accepting slopes. The mean r value for all of the time points during each protocol was: Protocol 1, −0.88 ± 0.01 (range: −0.56 to −0.99); Protocol 2, −0.90 ± 0.01 (range: −0.78 to −0.99); and Protocol 3, −0.86 ± 0.21 (range: −0.74 to −0.98), indicating adequate fit of the linear regression analyses. All data were weighted to account for the number of cardiac cycles within each pressure bin; thus removing bias due to bins containing a small number of cardiac cycles. The diastolic blood pressure range used for the linear regression analyses was approximately 20 mmHg under resting conditions (mixed meal, 21 ± 1; hyperinsulinaemic euglycaemic clamp, 21 ± 2; time control, 19 ± 1) and importantly the range was the same at all time points examined.
The slope of the relationship between spontaneous fluctuations in MSNA burst incidence and diastolic blood pressure was recently shown to be highly correlated with slopes derived using the more invasive modified Oxford approach for assessing arterial baroreflex-MSNA gain (i.e. bolus sodium nitroprusside and phenylephrine) (Hart et al. 2010). In addition, van Schelven et al. (2008) have reported that arterial baroreflex burst incidence gain was not altered from baseline during steady-state nitroprusside infusions in which MSNA was robustly increased, demonstrating the stability of these measures. Furthermore, during a variety of manoeuvres, including exercise (Fadel et al. 2001; Keller et al. 2004; Ogoh et al. 2007), postexercise ischaemia (Cui et al. 2001; Kamiya et al. 2001; Ichinose et al. 2004; Ogoh et al. 2009) and increases in plasma osmolality (Charkoudian et al. 2005; Wenner et al. 2007), similar changes in MSNA baroreflex gain have been demonstrated using the spontaneous analysis method and traditional baroreflex approaches. Overall, there is a wealth of recent data supporting and validating the use of the burst incidence-diastolic blood pressure measures to assess arterial baroreflex control of MSNA, providing the rationale for using these measures in the current study. In addition, a priori we reasoned that the serial measurements needed to address our question precluded repetitive application of the modified Oxford for such an extended period of time (2 h). Also, given the vasodilatory actions of insulin, comparisons of responses to nitroprusside and phenylephrine between baseline and the mixed meal or clamps might prove difficult.
Because insulin may also affect the baroreflex control of heart rate (Daubert et al. 2007; Pricher et al. 2008; Brooks et al. 2010), at baseline and every 30 min following meal intake or during the hyperinsulinaemic euglycaemic clamp, 3 min beat-to-beat time series of systolic blood pressure and heart rate or R–R interval were analysed using the sequence technique for estimating cardiac baroreflex sensitivity (Nevrokard, Izola, Slovenia). Briefly, sequences of three or more consecutive beats where blood pressure and heart rate changed in the opposite direction or blood pressure and R–R interval changed in the same direction were identified as arterial baroreflex sequences. A linear regression was applied to each individual sequence, and only those sequences in which R2 was >0.85 were accepted. The slopes of the systolic blood pressure–heart rate (or R–R interval) relationships were calculated as measures of spontaneous cardiac baroreflex sensitivity. Adequate sequences could not be obtained for one subject during the mixed meal protocol and therefore, this subject was not included in the presented data.
Statistical analysis
Univariate repeated measures ANOVA was used and significant main effects were evaluated with Bonferroni post hoc analyses when appropriate. Statistical significance was set at P < 0.05. Results are presented as mean ± standard error of the mean (s.e.m.).
Results
MSNA and cardiovascular parameters
As anticipated, following the mixed meal and during the hyperinsulinaemic euglycaemic clamp, MSNA burst frequency, burst incidence and total MSNA were increased within 30 min and remained above baseline for the remainder of the protocol (Fig. 1). Systolic and diastolic blood pressures, as well as heart rate, were slightly, but significantly increased following consumption of the mixed meal. Arterial blood pressure and heart rate remained unchanged during the hyperinsulinaemic euglycaemic clamp and time control experiments (Table 1).
Figure 1.
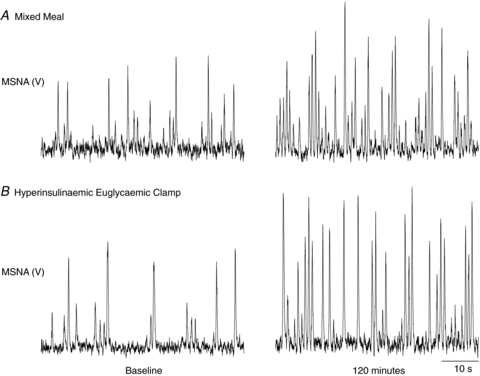
Original records illustrating muscle sympathetic nerve activity (MSNA) at baseline and at 120 min following the mixed meal (A) and during the hyperinsulinaemic euglycaemic clamp (B). V, volts.
Table 1.
Sympathetic nerve activity and cardiovascular responses to a mixed meal, hyperinsulinaemic euglycaemic clamp and time control experiments
| Time (min) | ||||||
|---|---|---|---|---|---|---|
| Baseline | 30 | 60 | 90 | 120 | ||
| Mixed meal | MSNA burst frequency (bursts min−1) | 12 ± 2 | 19 ± 2* | 22 ± 2* | 23 ± 2* | 23 ± 2* |
| MSNA burst incidence (bursts (100 Hb)−1) | 21 ± 3 | 29 ± 3* | 34 ± 3* | 34 ± 4* | 32 ± 3* | |
| Total MSNA (AU beat−1) | 32 ± 4 | 49 ± 5* | 64 ± 6* | 66 ± 11* | 64 ± 10* | |
| Heart rate (beats min−1) | 59 ± 2 | 67 ± 3* | 66 ± 2* | 68 ± 2* | 70 ± 3* | |
| Systolic blood pressure (mmHg) | 116 ± 2 | 119 ± 3 | 119 ± 3 | 118 ± 2 | 120 ± 3* | |
| Diastolic blood pressure (mmHg) | 69 ± 2 | 70 ± 2 | 70 ± 3 | 71 ± 3 | 72 ± 2* | |
| Hyperinsulinaemic euglycaemic clamp | MSNA burst frequency (bursts min−1) | 11 ± 2 | 17 ± 2* | 19 ± 2* | 20 ± 2* | 23 ± 2* |
| MSNA burst incidence (bursts (100 Hb)−1) | 18 ± 3 | 26 ± 3* | 31 ± 3* | 32 ± 3* | 36 ± 3* | |
| Total MSNA (AU beat−1) | 30 ± 5 | 45 ± 6* | 55 ± 7* | 54 ± 7* | 74 ± 8* | |
| Heart rate (beats min−1) | 61 ± 3 | 65 ± 3 | 62 ± 3 | 64 ± 3 | 65 ± 4 | |
| Systolic blood pressure (mmHg) | 114 ± 3 | 114 ± 3 | 115 ± 3 | 112 ± 3 | 113 ± 3 | |
| Diastolic blood pressure (mmHg) | 65 ± 2 | 64 ± 1 | 64 ± 1 | 62 ± 3 | 61 ± 2 | |
| Time Control | MSNA burst frequency (bursts min−1) | 12 ± 2 | 13 ± 1 | 11 ± 2 | 10 ± 2 | 12 ± 2 |
| MSNA burst incidence (bursts (100 Hb)−1) | 21 ± 2 | 24 ± 3 | 19 ± 4 | 19 ± 6 | 20 ± 3 | |
| Total MSNA (AU beat−1) | 34 ± 5 | 35 ± 5 | 30 ± 8 | 27 ± 9 | 31 ± 6 | |
| Heart rate (beats min−1) | 57 ± 4 | 56 ± 3 | 57 ± 5 | 57 ± 4 | 57 ± 3 | |
| Systolic blood pressure (mmHg) | 111 ± 2 | 110 ± 2 | 110 ± 2 | 112 ± 1 | 112 ± 1 | |
| Diastolic blood pressure (mmHg) | 63 ± 2 | 64 ± 5 | 63 ± 3 | 64 ± 4 | 62 ± 1 | |
Hb, heart beats; AU, arbitrary units. Values are mean ±s.e.m.
P < 0.05 vs. baseline.
Increased arterial baroreflex-MSNA gain following a mixed meal
Mixed meal intake induced a significant rise in plasma insulin, as well as plasma glucose (Fig. 2). The arterial baroreflex gain of MSNA burst incidence was significantly enhanced (i.e. more negative) within 30 min (Δ−1.91 ± 0.53 bursts (100 heart beats)−1 mmHg−1) and remained elevated for the duration of the study (e.g. 120 min: Δ−1.82 ± 0.37 bursts (100 heart beats)−1 mmHg−1) (Fig. 3). Similarly, arterial baroreflex control of total MSNA was increased at 30 min, and thereafter remained above baseline up to 120 min.
Figure 2.
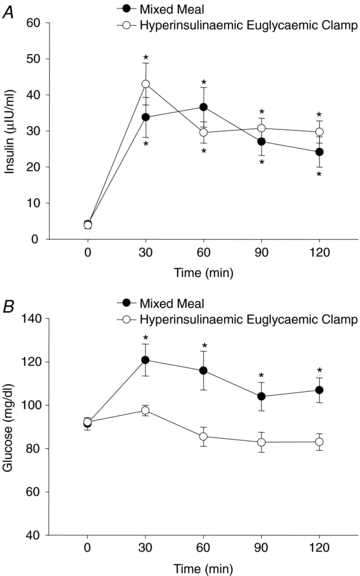
Mean plasma insulin (A) and glucose concentrations (B) at baseline (time 0) and for 120 min following consumption of the mixed meal and during the hyperinsulinaemic euglycaemic clamp. *P < 0.05 vs. baseline.
Figure 3.
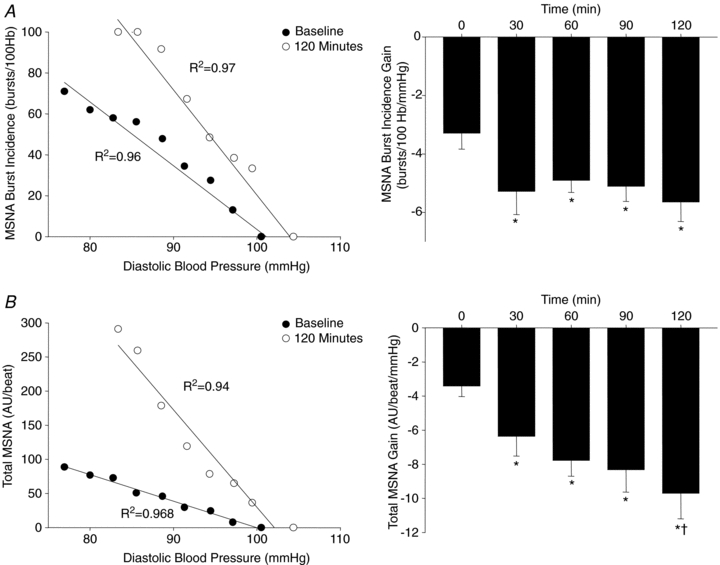
Linear relationships between MSNA and diastolic blood pressure from one subject (left), as well as group summary data (right) illustrating the gain of arterial baroreflex control of MSNA burst incidence (A) and total MSNA (B) at baseline (time 0) and for 120 min following consumption of the mixed meal. Hb, heart beats; AU, arbitrary units; R2, coefficient of determination. *P < 0.05 vs. baseline. †P < 0.05 vs. 30 min.
Increased arterial baroreflex-MSNA gain during a hyperinsulinaemic euglycaemic clamp
During the hyperinsulinaemic euglycaemic clamp, plasma insulin was increased similarly to that after the mixed meal; however, euglycaemia was maintained throughout at fasting concentrations (Fig. 2). Importantly, in line with the findings from the mixed meal, similar and robust increases in the gain of arterial baroreflex control of MSNA burst incidence (e.g. 120 min: Δ−2.30 ± 0.62 bursts (100 heart beats)−1 mmHg−1) and total MSNA were observed with the concomitant increase in plasma insulin (Fig. 4). In contrast, during the time control experiments, in which plasma insulin and glucose were not changed (data not shown), the gain of the arterial baroreflex was not different from baseline at any time point (Fig. 4). Interestingly, the increase in arterial baroreflex-MSNA gain during the hyperinsulinaemic euglycaemic clamp was inversely correlated to the glucose infusion rate, such that the subjects with the highest glucose infusion rate (i.e. highest insulin sensitivity) demonstrated the largest increase in the gain of arterial baroreflex control of MSNA (Fig. 5).
Figure 4.
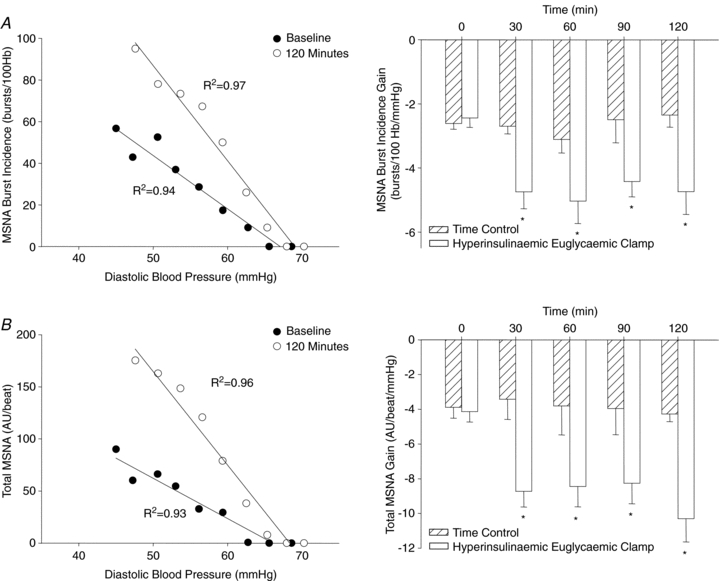
Left panels show linear relationships between MSNA and diastolic blood pressure illustrating the gain of arterial baroreflex control of MSNA burst incidence (A) and total MSNA (B) at baseline (time 0) and 120 min of the hyperinsulinaemic euglycaemic clamp. Right panels present group summary data during the hyperinsulinaemic euglycaemic clamp and time control experiments. Hb, heart beats; AU, arbitrary units; R2, coefficient of determination. *P < 0.05 vs. baseline.
Figure 5.
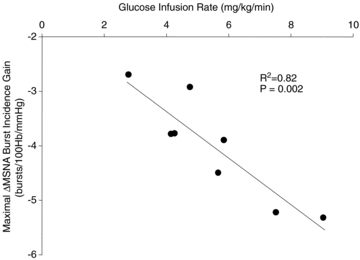
Relationship between the glucose infusion rate (measure of insulin sensitivity) and the maximal increase in the gain of arterial baroreflex control of MSNA burst incidence during the hyperinsulinaemic euglycaemic clamp. R2, coefficient of determination.
Cardiac baroreflex sensitivity
The spontaneous relationship between systolic blood pressure and heart rate remained unchanged following consumption of the mixed meal (baseline, −1.13 ± 0.09; 60 min, −1.22 ± 0.15; 120 min, −1.19 ± 0.16 beats min−1 mmHg−1; P > 0.05) and during the hyperinsulinaemic euglycaemic clamp (baseline, −1.16 ± 0.12; 60 min, −1.17 ± 0.11; 120 min, −1.12 ± 0.11 beats min−1 mmHg−1; P > 0.05). Similar results were found when R–R interval was used as the dependent variable (data not shown).
Discussion
The primary novel finding of this investigation is that increases in plasma insulin enhanced the gain of arterial baroreflex control of MSNA. Indeed, physiological elevations in plasma insulin following the mixed meal were associated with an increased slope of the relationship between diastolic blood pressure and MSNA. Furthermore, during the hyperinsulinaemic euglycaemic clamp, in which insulin was increased to postprandial concentrations, while glucose was maintained at euglycaemia, arterial baroreflex control of MSNA burst incidence was also augmented. Overall, these findings strongly support a role for insulin in the modulation of the sympathetic arterial baroreflex in healthy humans.
In addition to the well-described sympathoexcitatory effect(s) of insulin (Muntzel et al. 1995; Landsberg, 2001), recent findings obtained in animals illustrate a role for insulin in the central modulation of the arterial baroreflex (Pricher et al. 2008). During lateral ventricular infusion of insulin in rats, robust increases in the gain of arterial baroreflex control of lumbar sympathetic nerve activity have been demonstrated, illustrating that acute increases in insulin within the brain modulate arterial baroreflex control of sympathetic outflow (Pricher et al. 2008). The findings from the current study support and extend these findings by demonstrating for the first time in healthy humans that acute increases in insulin are associated with an enhanced arterial baroreflex-MSNA gain. Importantly, the enhanced sympathetic arterial baroreflex control was noted during modest increases in plasma insulin under both postprandial (mixed meal) and experimental (insulin clamp) conditions, supporting a role for insulin within a normal physiological range in the modulation of the arterial baroreflex.
Insulin is not produced in large quantities in the central nervous system; however, plasma insulin gains access to the brain via saturable transport-mediated uptake across the blood–brain barrier (Woods et al. 2003; Banks, 2004). As such, circulating insulin can influence arterial baroreflex control via central neural pathways and indeed, insulin receptors are present in numerous, yet distinct cardiovascular regulatory regions, including the hypothalamus and brainstem (Werther et al. 1987; Schulingkamp et al. 2000). In this context, Schwartz et al. (1991) have previously demonstrated in dogs that cerebrospinal fluid concentrations of insulin are increased within 30 min after systemic infusion of insulin, in line with our finding of an increase in arterial baroreflex-MSNA gain within 30 min after the mixed meal or during the hyperinsulinaemic euglycaemic clamp. Moreover, the half-life of insulin within cerebrospinal fluid has been reported to be approximately 140 min (Schwartz et al. 1990b), which may explain the sustained enhancement in arterial baroreflex control of MSNA demonstrated in the current study.
Interestingly, the increase in arterial baroreflex-MSNA gain was noted for the relationships between diastolic blood pressure and both MSNA burst incidence as well as total MSNA. In this regard, previous investigations have suggested that the occurrence of a sympathetic burst (incidence) and the area of a burst may reflect distinct central sites involved in arterial baroreflex control of MSNA (Kienbaum et al. 2001; Keller et al. 2006). The current data demonstrate that during elevations in insulin, for a given change in diastolic blood pressure there is a greater change in MSNA burst incidence and total MSNA (area/beat), relative to fasting conditions. As such, our findings suggest that insulin may influence central pathways involved in both the occurrence of a burst of MSNA as well as the size of a given burst. Due to the peripheral vasodilatory effect of insulin, the physiological significance of an enhanced arterial baroreflex control over the occurrence and size of a burst of MSNA may provide optimal protection of arterial blood pressure during acute elevations in plasma insulin. Interestingly, it should be noted that arterial blood pressure was well maintained throughout the mixed meal and hyperinsulinaemic euglycaemic clamp in the present study. Furthermore, autonomic failure patients demonstrate robust falls in arterial blood pressure after meal consumption (Robertson et al. 1981), illustrating the importance of the sympathetic nervous system in the maintenance of blood pressure during periods of increased circulating insulin.
Of note, although we found an increase in arterial baroreflex gain for the control of MSNA burst occurrence and area (i.e. total MSNA), methodological considerations of the use of MSNA burst incidence, in comparison to total MSNA, to derive spontaneous baroreflex measures is warranted. In this regard, previous findings have demonstrated that the gain of arterial baroreflex control of MSNA burst incidence was similar to sympathetic baroreflex sensitivities obtained using pharmacological manipulations of arterial blood pressure (van Schelven et al. 2008; Hart et al. 2010), demonstrating the usefulness of these measurements. In contrast, spontaneous arterial baroreflex gains calculated from measurements using MSNA burst area (e.g. total MSNA) have been suggested to be influenced more by non-baroreflex inputs (Kienbaum et al. 2001; Hart et al. 2010), which may limit the interpretation of such measures. Indeed, several studies have reported weak relationships between burst area and diastolic blood pressure, whereas burst incidence consistently demonstrates strong relationships with diastolic blood pressure (Rudas et al. 1999; Kienbaum et al. 2001; Keller et al. 2006; Ogoh et al. 2007; Hart et al. 2010). Thus, although robust increases in arterial baroreflex control of total MSNA were observed following the mixed meal and during the hyperinsulinaemic euglycaemic clamp, we focused our interpretation on baroreflex measures of burst incidence.
From these human studies, although we cannot determine the precise region(s) of insulin action on arterial baroreflex control of MSNA, several areas are worthy of consideration. The aforementioned work in rats using lateral ventricular infusion of insulin suggests a hypothalamic region, such as the paraventricular nucleus, may play a major role (Pricher et al. 2008). In addition, neurons from brainstem regions involved in arterial baroreflex afferent processing (e.g. nucleus of the tractus solitarius) are responsive to insulin (Ruggeri et al. 2001). Furthermore, neural projections from the circumventricular organs, which lack a blood–brain barrier, could also impact arterial baroreflex function during elevations in plasma insulin (Woods et al. 2003). Indeed, the investigation of insulin effects on arterial baroreflex control of sympathetic outflow is in its infancy; however, this area undoubtedly deserves further attention.
Previous animal investigations have suggested that, in addition to an influence on sympathetic baroreflex gain, insulin may also enhance the gain of the cardiac baroreflex (Daubert et al. 2007; Pricher et al. 2008; Brooks et al. 2010). Although not the focus of the current study, we assessed spontaneous cardiac baroreflex sensitivity following consumption of the mixed meal and during the hyperinsulinaemic euglycaemic clamp. Interestingly, we did not find any changes in baroreflex control of heart rate. Although the reason for the differences between these findings and those of previous animal studies is unclear, several points should be considered. First, although the plasma concentration of insulin observed in the current study has been shown to modulate MSNA, this concentration of insulin has not been shown to influence heart rate (Hausberg et al. 1995). In addition, we studied all male subjects and therefore sex-specific effects of insulin's action on baroreflex control of heart rate cannot be excluded. In this regard, although subtle, insulin may have a greater influence on cardiac baroreflex function in female rats (Pricher et al. 2008). Furthermore, a caveat to the use of the sequence technique is the number of sequences that can be obtained. Indeed, only 10–18% of the cardiac cycles within each time segment analysed was associated with a sequence. In contrast, the spontaneous analysis for the sympathetic baroreflex utilized every cardiac cycle (∼180–200 per segment). Overall, further examination of the influence of insulin on the gain of the cardiac baroreflex is warranted.
Perspectives
A number of conditions that are typically characterized by reductions in arterial baroreflex gain, including hypertension (Bristow et al. 1969; Gribbin et al. 1971; Eckberg, 1979; Matsukawa et al. 1991), metabolic syndrome (Grassi et al. 2005) and pregnancy (Daubert et al. 2007; Brooks et al. 2010), also exhibit decreases in insulin sensitivity. Emerging evidence indicates that insulin-resistant states are accompanied by reduced cerebrospinal fluid insulin concentrations, probably emanating from an attenuated transport of insulin into the central nervous system (Schwartz et al. 1990a; Israel et al. 1993; Kaiyala et al. 2000; Kern et al. 2006; Daubert et al. 2007; Brooks et al. 2010). In line with this, Brooks and colleagues have advanced the hypothesis that a certain level of insulin within the brain is essential for normal arterial baroreflex function (Pricher et al. 2008). Therefore, in insulin-resistant conditions, a decrease in brain insulin may reverse the normal effect of insulin to support optimal baroreflex control. The present findings lend support to this growing body of literature by demonstrating for the first time that insulin can modulate the sympathetic arterial baroreflex in healthy humans. However, whether chronic alterations in insulin transport and/or signalling in the central nervous system contribute to a decreased sympathetic arterial baroreflex gain in insulin-resistant conditions remains to be determined.
Interestingly, in the present study, we found a strong relationship between insulin sensitivity and the increase in arterial baroreflex-MSNA gain during the hyperinsulinaemic euglycaemic clamp (Fig. 5). Albeit a small cohort, these data support the concept that, aside from the beneficial peripheral effects to increase glucose uptake, increasing insulin sensitivity in patients, either pharmacologically (Daubert et al. 2007) or with exercise training (Mousa et al. 2008; Thyfault, 2008), may also improve arterial baroreflex control of sympathetic nerve activity. Given the increased prevalence of insulin resistance, future studies, from experimental animals to humans, are warranted to delineate the precise neural pathways and mechanism(s) of insulin action on neurocardiovascular control in health and disease.
In summary, for the first time, we found that physiological increases in plasma insulin following a mixed meal and during a hyperinsulinemic euglycemic clamp enhanced the gain of arterial baroreflex control of MSNA in humans. Collectively, these findings extend recent studies in animals and strongly support a role for insulin in the modulation of the sympathetic arterial baroreflex.
Acknowledgments
The authors thank Dr Jill Kanaley, Dr Lauro Vianna, Charla Jay, Catherine Mikus and Leryn Boyle for their technical assistance. The time and effort expended by all of the volunteer subjects are greatly appreciated. This research is the result of work supported with resources by a University of Missouri Institute for Clinical and Translational Science Grant to C.N.Y. and by National Institute of Health Grants HL-093167 and DK-076636 to P.J.F.
Glossary
Abbreviations
- MSNA
muscle sympathetic nerve activity
Author contributions
C.N.Y. contributed to study design, data acquisition, data analysis, data interpretation and wrote the first draft of the manuscript. S.H.D. contributed to data acquisition and critical review of the manuscript. K.C. provided clinical support and contributed to data acquisition. J.P.T. contributed to study design, data acquisition and critical review of the manuscript. P.J.F. contributed to study design, data acquisition, data analysis, data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript. The experiments of this study were conducted in the Department of Medical Pharmacology and Physiology at the University of Missouri, Columbia, MO, USA.
References
- Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol. 1969;202:45P–46P. [PubMed] [Google Scholar]
- Brooks VL, Mulvaney JM, Azar AS, Zhao D, Goldman RK. Pregnancy impairs baroreflex control of heart rate in rats: role of insulin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2010;298:R419–R426. doi: 10.1152/ajpregu.00441.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol. 2005;289:H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Carotid baroreflex function in young men with borderline blood pressure elevation. Circulation. 1979;59:632–636. doi: 10.1161/01.cir.59.4.632. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol. 2003;88:671–680. doi: 10.1113/eph8802650. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol. 2001;91:1199–1206. doi: 10.1152/jappl.2001.91.3.1199. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2010;298:H816–H822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberg M, Mark AL, Hoffman RP, Sinkey CA, Anderson EA. Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J Hypertens. 1995;13:1015–1021. doi: 10.1097/00004872-199509000-00012. [DOI] [PubMed] [Google Scholar]
- Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol. 2010;588:1515–1525. doi: 10.1113/jphysiol.2009.186387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol. 2004;286:H701–H707. doi: 10.1152/ajpheart.00618.2003. [DOI] [PubMed] [Google Scholar]
- Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D, Jr, Figlewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull. 1993;30:571–575. doi: 10.1016/0361-9230(93)90084-o. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Fu Q, Niimi Y, Iwase S, Mano T, Suzumura A. Static handgrip exercise modifies arterial baroreflex control of vascular sympathetic outflow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1134–R1139. doi: 10.1152/ajpregu.2001.281.4.R1134. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49:2790–2792. doi: 10.1007/s00125-006-0409-y. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) J Hypertens. 2001;19:523–528. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Hasegawa O, Shionoiri H, Tochikubo O, Ishii M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J Hypertens. 1991;9:537–542. doi: 10.1097/00004872-199106000-00009. [DOI] [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J Appl Physiol. 2008;104:616–624. doi: 10.1152/japplphysiol.00601.2007. [DOI] [PubMed] [Google Scholar]
- Muntzel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens. 1995;17:39–50. doi: 10.3109/10641969509087053. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007;293:H2202–H2209. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol. 2009;296:H1416–H1424. doi: 10.1152/ajpheart.01223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- Robertson D, Wade D, Robertson RM. Postprandial alterations in cardiovascular hemodynamics in autonomic dysfunction states. Am J Cardiol. 1981;48:1048–1052. doi: 10.1016/0002-9149(81)90319-2. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Ruggeri P, Molinari C, Brunori A, Cogo CE, Mary DA, Picchio V, Vacca G. The direct effect of insulin on barosensitive neurones in the nucleus tractus solitarii of rats. Neuroreport. 2001;12:3719–3722. doi: 10.1097/00001756-200112040-00023. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293:H2543–H2549. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Jr, Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D., Jr Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990a;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Sipols A, Kahn SE, Lattemann DF, Taborsky GJ, Jr, Bergman RN, Woods SC, Porte D., Jr Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am J Physiol Endocrinol Metab. 1990b;259:E378–E383. doi: 10.1152/ajpendo.1990.259.3.E378. [DOI] [PubMed] [Google Scholar]
- Skrapari I, Tentolouris N, Katsilambros N. Baroreflex function: determinants in healthy subjects and disturbances in diabetes, obesity and metabolic syndrome. Curr Diabetes Rev. 2006;2:329–338. doi: 10.2174/157339906777950589. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1103–R1110. doi: 10.1152/ajpregu.00924.2007. [DOI] [PubMed] [Google Scholar]
- van Schelven LJ, Karemaker JM, Blankestijn PJ, Oey PL. Short-term sympathetic baroreflex sensitivity increases at lower blood pressures. Clin Neurophysiol. 2008;119:869–879. doi: 10.1016/j.clinph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol. 2007;293:H2313–H2319. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- Young CN, Deo SH, Kim A, Horiuchi M, Mikus CR, Uptergrove GM, Thyfault JP, Fadel PJ. Influence of endurance training on central sympathetic outflow to skeletal muscle in response to a mixed meal. J Appl Physiol. 2010;108:882–890. doi: 10.1152/japplphysiol.01174.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


