Abstract
We have made use of a panel of mouse-hamster somatic cell hybrids and restriction fragment length polymorphisms between two mouse species (Mus musculus and Mus spretus) to determine the chromosomal localization of genes encoding the alpha and beta subunits of the Na,K-ATPase (Na+,K+-activated ATP phosphohydrolase, EC 3.6.1.3). DNA probes for three distinct isoforms of the Na,K-ATPase alpha subunit mapped to three different mouse chromosomes: the alpha 1 gene (Atpa-1) cosegregated with the Egf gene on chromosome 3; alpha 2 (Atpa-2) with the cytochrome P-450PB gene family/coumarin hydroxylase locus on chromosome 7; alpha 3 (Atpa-3) with the alpha-spectrin gene on chromosome 1. The Na,K-ATPase beta-subunit gene (Atpb) mapped to the same region of chromosome 1, but it was not tightly linked to the Atpa-3 gene. These results indicate that three isoforms of the Na,K-ATPase alpha subunit are encoded by three distinct genes. The dispersion of Na,K-ATPase genes suggests that their expression is not likely to be controlled by a common cis-acting regulatory element.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Emanuel J. R., Garetz S., Schneider J., Ash J. F., Benz E. J., Jr, Levenson R. Amplification of DNA sequences coding for the Na,K-ATPase alpha-subunit in ouabain-resistant C+ cells. Mol Cell Biol. 1986 Jul;6(7):2476–2481. doi: 10.1128/mcb.6.7.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kasper C. B. Cloning of DNA complementary to rat liver NADPH-cytochrome c (P-450) oxidoreductase and cytochrome P-450b mRNAs. Evidence that phenobarbital augments transcription of specific genes. J Biol Chem. 1982 May 25;257(10):5962–5968. [PubMed] [Google Scholar]
- Heidmann O., Buonanno A., Geoffroy B., Robert B., Guénet J. L., Merlie J. P., Changeux J. P. Chromosomal localization of muscle nicotinic acetylcholine receptor genes in the mouse. Science. 1986 Nov 14;234(4778):866–868. doi: 10.1126/science.3022377. [DOI] [PubMed] [Google Scholar]
- Huebner K., Palumbo A. P., Isobe M., Kozak C. A., Monaco S., Rovera G., Croce C. M., Curtis P. J. The alpha-spectrin gene is on chromosome 1 in mouse and man. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3790–3793. doi: 10.1073/pnas.82.11.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Fournier R. E., Leinwand L. A., Ruddle F. H. Assignment of the gene governing cellular ouabain resistance to Mus musculus chromosome 3 using human/mouse microcell hybrids. Biochem Genet. 1979 Feb;17(1-2):23–34. doi: 10.1007/BF00484471. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Francke U., Minna J. D. Homologous genes for enolase, phosphogluconate dehydrogenase, phosphoglucomutase, and adenylate kinase are syntenic on mouse chromosome 4 and human chromosome 1p. Proc Natl Acad Sci U S A. 1978 May;75(5):2382–2386. doi: 10.1073/pnas.75.5.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer R. W., Schneider J. W., Savitz A., Emanuel J., Benz E. J., Jr, Levenson R. Rat-brain Na,K-ATPase beta-chain gene: primary structure, tissue-specific expression, and amplification in ouabain-resistant HeLa C+ cells. Mol Cell Biol. 1986 Nov;6(11):3884–3890. doi: 10.1128/mcb.6.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Azizkhan J. C., Greisen K. S., Hamlin J. L. Organization of a Chinese hamster ovary dihydrofolate reductase gene identified by phenotypic rescue. Mol Cell Biol. 1983 Jul;3(7):1266–1273. doi: 10.1128/mcb.3.7.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Pech I. V., Stahl W. L. Immunoreactivity of subunits of the (Na+ + K+)-ATPase. Cross-reactivity of the alpha, alpha + and beta forms in different organs and species. Biochim Biophys Acta. 1981 Dec 21;649(3):691–700. doi: 10.1016/0005-2736(81)90173-5. [DOI] [PubMed] [Google Scholar]
- Schneider J. W., Mercer R. W., Caplan M., Emanuel J. R., Sweadner K. J., Benz E. J., Jr, Levenson R. Molecular cloning of rat brain Na,K-ATPase alpha-subunit cDNA. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6357–6361. doi: 10.1073/pnas.82.18.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Siegel G. J., Desmond T., Ernst S. A. Immunoreactivity and ouabain-dependent phosphorylation of (Na+ + K+)-adenosinetriphosphatase catalytic subunit doublets. J Biol Chem. 1986 Oct 15;261(29):13768–13776. [PubMed] [Google Scholar]
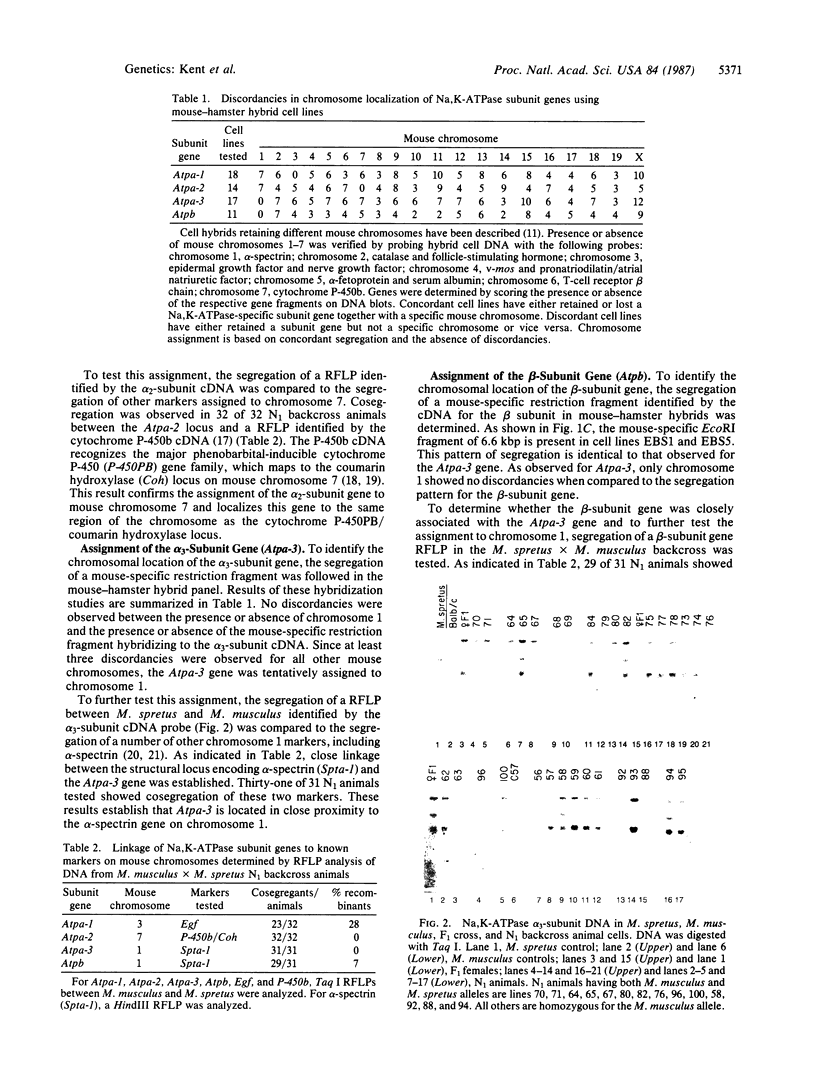
- Simmons D. L., Lalley P. A., Kasper C. B. Chromosomal assignments of genes coding for components of the mixed-function oxidase system in mice. Genetic localization of the cytochrome P-450PCN and P-450PB gene families and the nadph-cytochrome P-450 oxidoreductase and epoxide hydratase genes. J Biol Chem. 1985 Jan 10;260(1):515–521. [PubMed] [Google Scholar]
- Specht S. C., Sweadner K. J. Two different Na,K-ATPases in the optic nerve: cells of origin and axonal transport. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1234–1238. doi: 10.1073/pnas.81.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J Biol Chem. 1979 Jul 10;254(13):6060–6067. [PubMed] [Google Scholar]
- Urayama O., Nakao M. Organ secificity of rat sodium- and potassium-activated adenosine triphosphatase. J Biochem. 1979 Nov;86(5):1371–1381. doi: 10.1093/oxfordjournals.jbchem.a132654. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Taylor B. A. Genetic regulation of coumarin hydroxylase activity in mice. Evidence for single locus control on chromosome. J Biol Chem. 1979 Jul 10;254(13):5647–5651. [PubMed] [Google Scholar]
- Young R. M., Shull G. E., Lingrel J. B. Multiple mRNAs from rat kidney and brain encode a single Na+,K+-ATPase beta subunit protein. J Biol Chem. 1987 Apr 5;262(10):4905–4910. [PubMed] [Google Scholar]
- Zabel B. U., Eddy R. L., Lalley P. A., Scott J., Bell G. I., Shows T. B. Chromosomal locations of the human and mouse genes for precursors of epidermal growth factor and the beta subunit of nerve growth factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):469–473. doi: 10.1073/pnas.82.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]