Abstract
Although methylphenidate (Ritalin) has been used therapeutically for nearly 60 years, the mechanisms by which it acutely modifies behavioral performance are poorly understood. Here we combined intra–lateral amygdala in vivo pharmacology and ex vivo electrophysiology to show that acute administration of methylphenidate, as well as a selective dopamine transporter inhibitor, facilitated learning-induced strengthening of cortico-amygdala synapses through a postsynaptic increase in AMPA receptor–mediated currents, relative to those in saline-treated rats. Furthermore, local administration of methylphenidate in the lateral amygdala enhanced cue-reward learning through dopamine D1 receptor–dependent mechanisms and suppressed task-irrelevant behavior through D2 receptor–dependent mechanisms. These findings reveal critical and distinct roles for dopamine receptor subtypes in mediating methylphenidate-induced enhancements of neural transmission and learning performance.
Although methylphenidate (MPH) is primarily prescribed for the treatment of attention deficit hyperactivity disorder (ADHD)1, the behavioral enhancements of MPH are not limited to those with ADHD, as MPH also improves task performance and decreases motor restlessness in the general population2. ADHD is characterized by inattention, hyperactivity and impulsivity3 and has been linked to impaired learning performance in scholastic settings4. In recent decades, the diagnosis of ADHD and the prescription of MPH have markedly increased1. MPH is a highly effective therapeutic agent for both those with ADHD and those without2, improving scholastic performance in 70% of children and adults5,6.
What cellular and pharmacological mechanisms underlie acute MPH-induced enhancements of behavioral performance in the mammalian brain? Functional abnormalities of the basolateral amygdala (BLA), a brain region critical for learning the emotional and motivational significance of environmental stimuli7–12, have been linked to ADHD13. To identify neural mechanisms underlying MPH effects on learning performance, we tested the effects of MPH in the lateral amygdala, where the relationship between the intrinsic microcircuitry and acute learning performance has been well characterized7,8. The lateral amygdala is an early site of convergence for thalamic and cortical afferents carrying sensory information about environmental cues and primary reinforcers14,15 and is important for the acquisition and retrieval of stimulus-outcome memories16–18. Furthermore, thalamo-amygdala synaptic strength predicts the success of cue-reward learning8, and memory consolidation is facilitated by infusions of MPH in the amygdala19 after training. Thus, the lateral amygdala brain region is well suited for studying the synaptic mechanisms by which MPH alters acquisition. Here we show that local administration of MPH facilitates performance on a sucrose self-administration task and facilitates learning-induced plasticity within a single training session.
RESULTS
Performance is modulated by MPH in the amygdala
To examine the acute effects of MPH on learning performance, we locally administered MPH into the lateral amygdala of rats (Supplementary Figs. 1 and 2), before training in a lateral amygdala–dependent cue-reward learning paradigm8 (Supplementary Figs. 2 and 3). After training, we collected brains for acute slice preparation and used ex vivo electrophysiological recording procedures to evaluate synaptic function (Supplementary Fig. 2).
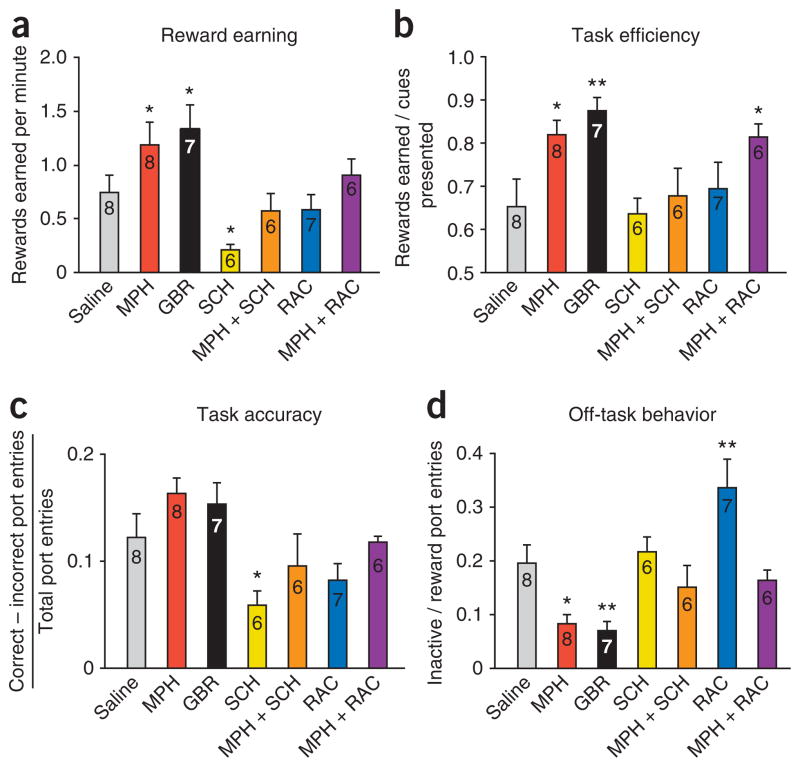
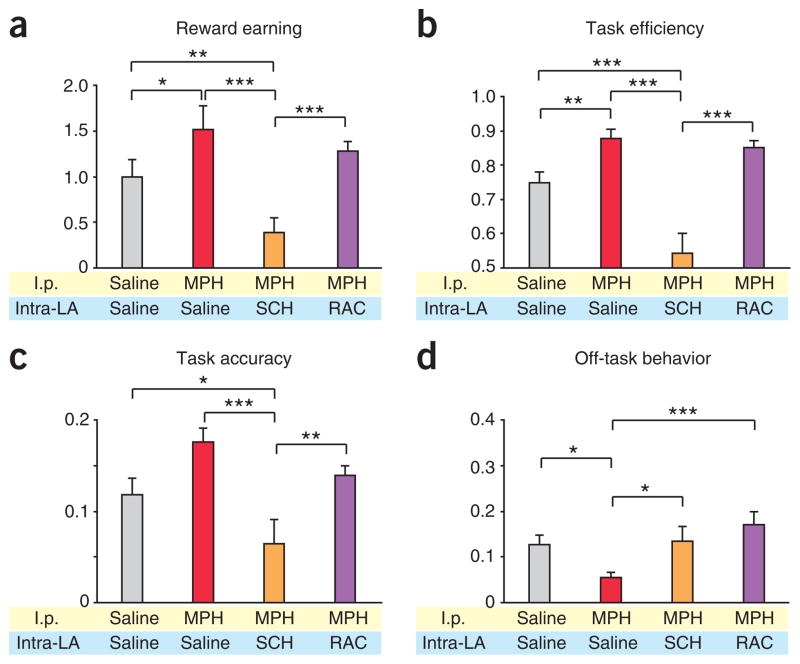
Rats that received intra–lateral amygdala (intra-LA) MPH before training, relative to those that received saline, earned a significantly higher (P = 0.049; Fig. 1) number of rewards per minute (‘reward earning’) but did not differ in overall motor activity (Supplementary Fig. 4). We used a behavioral index, ‘task efficiency’, defined as the number of rewards earned per cue presented8, to quantify the degree to which a rat had learned that the presentation of the cue indicated that sucrose was available. MPH-treated rats performed with a significantly higher task efficiency than saline-treated rats (P = 0.012; Fig. 1b). We also quantified the relative distribution of attention to goal-directed behavior using a behavioral index termed ‘off-task behavior’, defined as the number of inactive port entries per reward port entry. Because sucrose is delivered to the reward port, reward port entries reflect goal-oriented behavior, whereas inactive port entries reflect task-irrelevant behavior. MPH-treated rats showed ~50% less off-task behavior than saline controls (P = 0.010; Fig. 1d). Thus, the enhancement in reward earning seen by MPH rats relative to saline controls was due to a decrease in the amount of task-irrelevant behavior relative to goal-directed behavior, as well as an enhancement in the acquisition of the cue-reward association (Fig. 1a–d).
Figure 1.
MPH enhances task performance by altering different aspects of behavior through distinct D1 and D2 receptor–dependent mechanisms. (a) Intra-LA drug infusion alters reward earning (F6,47 = 5.161, P < 0.001). Relative to saline-treated rats, MPH and GBR groups earned significantly more rewards per minute, whereas SCH-treated rats earned a significantly fewer. MPH+SCH-treated, but not MPH+RAC-treated, rats earned significantly fewer than MPH-treated alone. (b) Task efficiency was altered by intra-LA drug infusion (F6,47 = 3.886, P = 0.004). Relative to saline, MPH, GBR and MPH+RAC-treated groups all showed significantly higher task efficiency. The MPH+SCH group, but not the MPH+RAC, showed lower task efficiency than the group treated with MPH alone. (c) Relative to saline, SCH-treated rats showed significantly lower task accuracy, and MPH+SCH-treated rats showed an attenuation of the enhancements induced by MPH alone, but MPH+RAC-treated rats did not differ from those treated with MPH alone (F6,47 = 3.806, P = 0.019). (d) Relative to the saline-treated group, MPH and GBR-treated groups showed significantly less off-task behavior, whereas RAC-treated rats showed significantly more (F6,47 = 8.024, P < 0.001). In a–d, numbers in bars indicate rats per group. All values are mean ± s.e.m. One-way analysis of variance followed by all-pairwise multiple comparison procedure (Fisher least significant difference method; *P < 0.05, **P < 0.01).
Distinct effects of NET and DAT inhibition
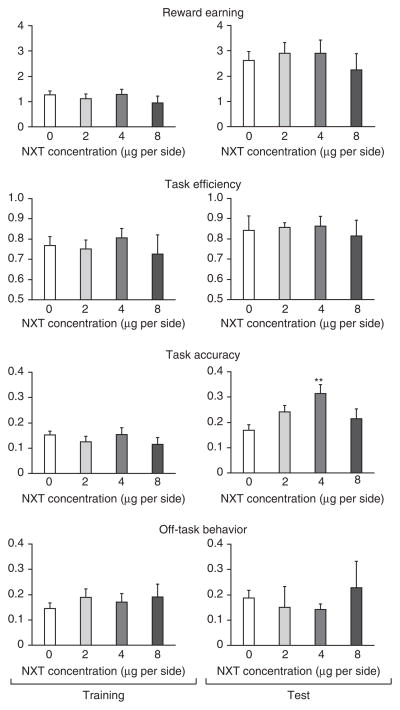
MPH targets multiple pharmacological targets, potently inhibiting both the norepinephrine transporter (NET) and the dopamine transporter (DAT). MPH has a higher binding affinity for the NET than for the DAT20, and an increasingly prescribed alternative treatment for ADHD, atomoxetine (Strattera), preferentially targets the NET. Thus, we investigated the effects of intra-LA administration before training of nisoxetine (NXT), a highly selective NET inhibitor, on learning performance (Supplementary Fig. 5). Consistent with evidence that intra-BLA MPH enhances memory consolidation19, we found that although NXT dose-dependently enhanced memory retention, as measured by the behavioral index ‘task accuracy’ on a subsequent test session in the absence of any drug treatment (P = 0.002), there was no acute effect on learning performance relative to that of saline controls (Fig. 2). Task accuracy quantifies the rat’s ability to recognize that the absence of the cue indicates the absence of sucrose8 and is defined as the difference of the number of port entries in the presence of sucrose and the number of port entries in the absence of sucrose, normalized to the total port entry responses during the session.
Figure 2.
Intra-LA NXT before training enhances memory retention but not acute task performance. Left column, performance on the initial training session; right column, performance during a 20-min memory retrieval test. No significant differences were observed during training, but NXT dose-dependently enhanced task accuracy (F3,26 = 4.209, P = 0.002) during a test session on the next day, on which no infusions were performed, suggesting that NET blockade may enhance memory retention in this task. Saline vehicle, N = 8; NXT 2 μg per side, N = 6; NXT 4 μg per side, N = 7; NXT 8 μg per side, N = 6. **P < 0.01.
Accumulating evidence suggests that MPH inhibition of the DAT20 contributes to its therapeutic effects21. For example, subjects diagnosed with ADHD show significant increases in DAT density22 and presence of certain alleles of the DAT1 (also known as SLC6A3) gene correlates with hyperactivity and impulsivity scores in subjects with ADHD23. Furthermore, therapeutic doses of orally administrated MPH in humans inhibit DAT function (50–75%) and increase extracellular dopamine24,25. Because NET inhibition alone did not yield the same enhancements of acute task performance as did MPH, we hypothesized that the effects of MPH on learning occur by means of DAT inhibition. If so, then intra-LA infusions of the selective DAT blocker GBR-12909 (GBR)26 should mimic the effects of MPH. In contrast to NXT treatment, GBR treatment resulted in a behavioral profile notably similar to that of MPH, with no differences observed between GBR and MPH groups. GBR-treated rats showed higher reward earning (P = 0.013) and task efficiency (P = 0.002), as well as less off-task behavior (P = 0.006), than saline-treated controls (Fig. 1a,b,d).
Thus, MPH inhibition of NET enhances memory retention, whereas MPH inhibition of DAT acutely enhances task performance. If increases in extracellular dopamine caused these enhancements in acute task performance, then dopamine receptor activation is likely to be required for acute MPH-induced performance enhancement.
Distinct contributions of D1 and D2 receptors to performance
To test this hypothesis, we performed intra-LA infusions of the potent dopamine D1 receptor (D1R) antagonist SCH-23390 (SCH). SCH-treated rats showed significantly lower reward earning (P = 0.033; Fig. 1a) and task accuracy (Fig. 1c) than did saline-treated rats. Impairments in reward earning and task accuracy indicate that D1R activation is necessary for general task performance and cue-reward learning. However, the ability to suppress task-irrelevant behavior, as measured by off-task behavior, was spared (Fig. 1d).
If MPH enhances task performance by increasing the activation of D1Rs, then infusion of MPH together with SCH (MPH+SCH) should attenuate MPH-induced enhancements. Rats given intra-LA infusions of MPH+SCH before training showed lower reward earning (P = 0.013) and task efficiency (P = 0.042) than those treated with MPH alone (Fig. 1a,b). However, there was no change in off-task behavior for MPH+SCH compared to the MPH, SCH or saline groups (Fig. 1d). Thus, learning the motivational significance of a reward-predictive cue requires D1R activation.
We next tested the role of D2 receptors (D2Rs) in mediating learning performance by infusing raclopride (RAC), a potent antagonist of D2Rs, before training into the lateral amygdala. Whereas RAC treatment did not change reward earning or task efficiency, there was significantly more off-task behavior (P = 0.003) relative to saline (Fig. 1d). This increase in off-task behavior was due not to a decrease in reward port entries but to a >40% increase in inactive port entries (Supplementary Figs. 6 and 7). Thus, D1R function is critical for cue-reward learning, whereas D2R function is critical for the suppression of task-irrelevant behavior.
If MPH exerts some behavioral effects by increasing the activation of D2Rs, then infusion of MPH together with RAC (MPH+RAC) should attenuate a subset of MPH-induced behavioral enhancements. Rats treated with MPH+RAC showed a task efficiency that was higher (P = 0.023) than that of saline controls but the same as that seen in rats treated with MPH alone (Fig. 1b). Therefore, MPH affects task efficiency independently of D2R activation. In contrast to rats treated with MPH alone, rats treated with MPH+RAC did not show a difference in off-task behavior from that seen in saline controls (Fig. 1d). Although these specific behavioral aspects may be interrelated (Supplementary Figs. 8–10), these findings further support the hypothesis that acquisition of the cue-reward association and suppression of task-irrelevant behavior are mediated by distinct dopamine receptor subtypes.
MPH facilitates cortico-amygdala plasticity via dopamine
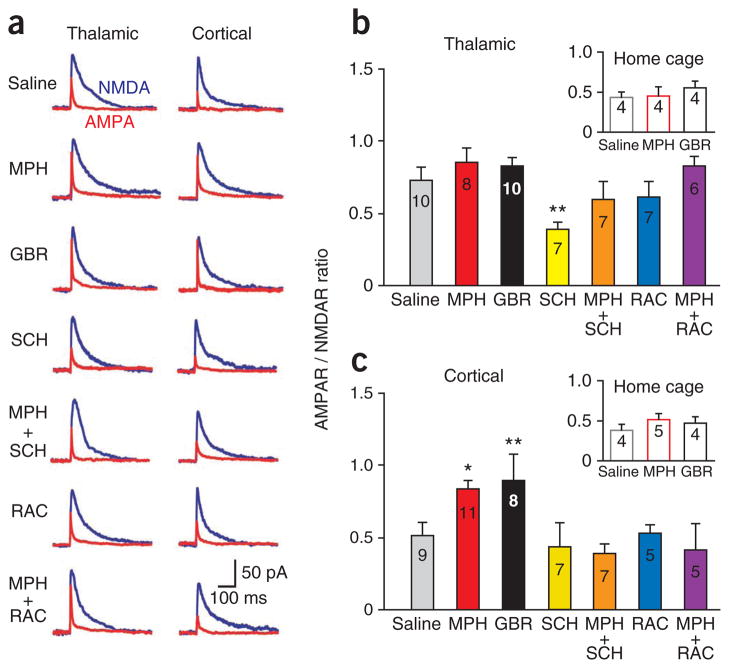
To test whether the MPH-induced enhancement in learning performance is related to changes in excitatory synaptic function, we performed whole-cell patch-clamp recordings within lateral amygdala slices after intra-LA infusions and training (Supplementary Fig. 2) in the same subjects whose behavioral data are presented in Figure 1. We measured the ratio of AMPA receptors (AMPAR) to NMDA receptors (NMDAR) by stimulating thalamic (internal capsule) or cortical (external capsule) afferents to determine the effects of intra-LA administration of MPH, GBR, SCH and RAC on learning-induced glutamatergic synaptic plasticity. Notably, rats that received infusions of saline before training showed significantly higher thalamo- amygdala AMPAR/NMDAR than rats treated with saline in their home cage (not trained; P = 0.05). Treatment with SCH before training yielded significantly lower thalamo-amygdala AMPAR/NMDAR relative to saline treatment (P = 0.008; Fig. 3a,b), in addition to impairing cue-reward learning (Fig. 1c). Thus, D1R blockade may impair cue-reward learning by attenuating the learning-induced increases in thalamo- amygdala synaptic strength27. No other treatment significantly altered synaptic strength in this pathway (Fig. 3b).
Figure 3.
Inhibition of the dopamine transporter gates cortico-amygdala synaptic potentiation. (a) Representative traces of AMPAR/NMDAR ratios evoked from thalamic and cortical afferents for each group. (b,c) AMPAR/NMDAR ratios evoked from thalamic (F8,62 = 3.471, P = 0.003) or cortical (F8,59 = 3.557, P = 0.002) afferents for each drug-treatment group after training were significantly altered. Inset shows rats treated with saline, MPH and GBR that were returned to their home cages in lieu of training. Numbers in bars indicate the number of cells per group. (b) SCH-treated rats show a significant decrease in thalamo-amygdala AMPAR/NMDAR. (c) MPH and GBR groups show significant increases in cortico-amygdala AMPAR/NMDAR. *P < 0.05, **P < 0.01.
In contrast, rats treated before training with saline infusions did not show a difference in cortico-amygdala synaptic strength relative to saline home-cage rats (Fig. 3c), indicating that plasticity at these synapses is not required for learning this task. Only MPH (P = 0.023) and GBR (P = 0.012) groups showed learning-induced increases in cortico-amygdala synaptic strength relative to saline controls (Fig. 3c), suggesting that DAT blockade changes the inhibitory constraints on cortico-amygdala plasticity. The enhancement in cortico-amygdala AMPAR/NMDAR by MPH was reversed by co-infusion with either D1R (MPH+SCH; P = 0.004) or D2R antagonists (MPH+RAC; P = 0.013; Fig. 3c), indicating that this change in synaptic strength requires coactivation of these receptor subtypes.
We then confirmed these synaptic changes were learning induced rather than a result of acute drug exposure alone. Rats that received MPH or GBR infusions in their home cages did not show any increases relative to saline. In contrast, rats infused with MPH or GBR in their home cages were significantly lower in thalamo-amygdala (P = 0.006, P = 0.049, respectively) or cortico-amygdala synaptic strength (P = 0.045, P = 0.012, respectively) relative to rats that received MPH or GBR infusions before training (Fig. 3b,c).
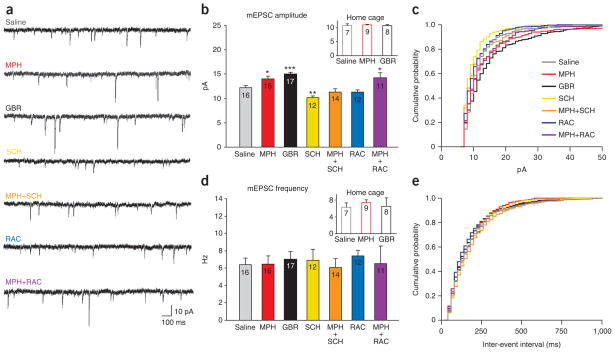
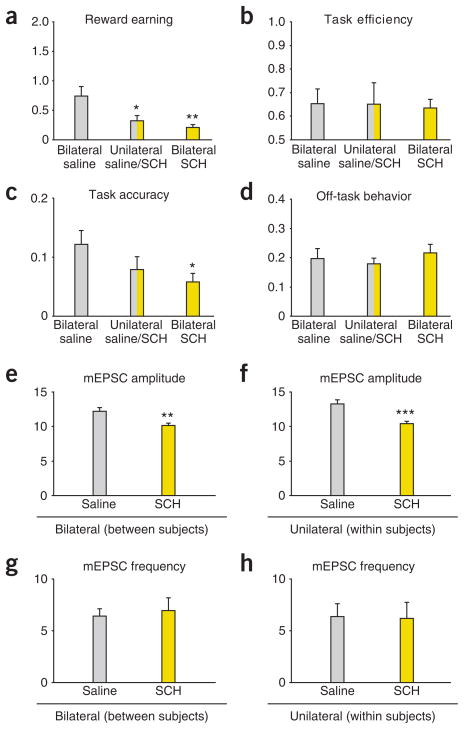
Because a change in AMPAR/NMDAR ratio may reflect a change in either AMPAR- or NMDAR-mediated currents, we examined miniature excitatory postsynaptic currents (mEPSCs), which reflect spontaneously released vesicles of glutamate28. A change in the amplitude of mEPSCs typically reflects a change in the number or function of postsynaptic AMPARs, whereas a change in the frequency of mEPSCs may reflect a change in the probability of release at the presynaptic terminal28. We found that intra-LA MPH (P = 0.012), GBR (P < 0.001) or MPH+RAC (P = 0.016) infusions increased, while SCH infusions decreased (P = 0.007), mEPSC amplitude relative to saline infusions before training (Fig. 4a–c). mEPSC amplitude was not increased by home-cage MPH or GBR treatment relative to saline and was significantly lower than that of rats that received MPH or GBR before training (both P < 0.001; Fig. 4b). Co-infusion of MPH+SCH attenuated (P < 0.001), whereas MPH+RAC spared, MPH-induced facilitation of learning-induced increases in mEPSC amplitude (Fig. 4a–c). We observed no differences in frequency (Fig. 4a,d,e), suggesting that the change in AMPAR/NMDAR was mediated postsynaptically28. A lack of difference among groups for either cortico- or thalamo-amygdala afferents in paired-pulse ratio measurements (Supplementary Fig. 11), which reflect the probability of vesicle release29, further supports the hypothesis that increases in both thalamo- and cortico-amygdala synaptic strength are mediated by postsynaptic increases in AMPAR currents. To confirm that D1R antagonism, rather than the associated impairment in learning performance, was the cause of the attenuation in mEPSC amplitude, we performed unilateral infusions of SCH before training to provide a within-subject control (Fig. 5).
Figure 4.
Dopamine modulates learning-induced increases in mEPSC amplitude but not frequency. (a) Sample mEPSCs from each drug-treatment group. (b) Mean mEPSC amplitude for each group varied (F8,113 = 10.177, P < 0.001) with treatment. MPH, GBR and MPH+RAC groups had higher, whereas SCH-treated rats had lower, mEPSC amplitude than did saline controls. Inset: saline-, MPH- and GBR-treated home-cage controls. (c) Cumulative probability plot of mEPSC amplitude for representative cells from each group; 1 pA bins. (d) No significant change in mEPSC frequency of any groups relative to saline (F8,113 = 0.202, P = 0.990). (e) Cumulative probability plot of mEPSC frequency; 20-ms bins. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
D1R antagonism in the lateral amygdala attenuates learning-induced synaptic changes. (a–h) Rats given, before training, bilateral infusions of SCH compared with rats given unilateral infusions of SCH and contralateral infusions of saline. (a) Unilateral infusion of SCH and saline significantly decreased reward earning (F2,19 = 5.107, P = 0.018) relative to bilateral saline infusion (P = 0.009) and did not differ from bilateral SCH. (b–d) Rats treated with unilateral SCH and saline infusions did not show significant differences from rats treated with bilateral saline in task efficiency, task accuracy or off-task behavior. (f,h) Unilateral infusions of SCH and saline provide a within-subjects (N = 6 rats; n = 11 cells from saline-treated side; n = 9 cells from SCH-treated side) comparison of the effects of D1 receptor antagonism on learning-induced plasticity. (e–h) For both between-subjects and within-subjects comparisons, treatment with SCH significantly attenuated learning-induced increases in mEPSC amplitude (*P < 0.05, **P < 0.01, ***P < 0.001, Student’s t-test) relative to saline (e,f), with no change in mEPSC frequency (g,h).
To probe the relationship between these distinct aspects of behavior and the associated synaptic changes, we examined correlations among AMPAR/NMDAR, mEPSCs and task performance. Both cortico-amygdala and thalamo-amygdala AMPAR/NMDAR ratios were significantly correlated with reward earning and task efficiency (Supplementary Figs. 12 and 13). In contrast, only the cortico- amygdala AMPAR/NMDAR was inversely correlated with off-task behavior (Supplementary Fig. 14), suggesting that cortico-amygdala synapses may selectively modulate the ability to suppress task-irrelevant behavior, consistent with evidence linking ADHD to abnormal cortico- amygdala connectivity13. Studies in drug-naive rats show correlations between learning and AMPAR/NMDAR ratios in thalamo-amygdala, but not cortico-amygdala synapses8, a result we replicated in saline-treated rats (Supplementary Fig. 15). Here the MPH and GBR groups were the only groups to show an increase in cortical AMPAR/NMDAR, and they heavily contributed to the correlation between cortical AMPAR/NMDAR and task efficiency. Additionally, mEPSC amplitude, but not frequency, was correlated with reward earning, task efficiency and off-task behavior (Supplementary Figs. 16–18), suggesting that postsynaptic AMPAR number or function predicts success in a cue-reward learning task.
MPH-induced enhancements require lateral amygdala dopamine
To test whether dopamine signaling in the amygdala is required for mediating MPH-induced behavioral enhancements, we systemically administered, using intraperitoneal (i.p.) injection, saline or a low dose of MPH along with intra-LA saline, SCH or RAC before training (Fig. 6 and Supplementary Fig. 19). Systemic MPH significantly increased reward earning and task efficiency, while reducing off-task behavior (P = 0.037, 0.008 and 0.04, respectively; Fig. 6a,b,d), relative to systemic saline. Intra-LA SCH attenuated systemic MPH–induced enhancements in all behavioral measures tested, and these rats were impaired relative to rats treated with systemic saline in reward earning, task efficiency and task accuracy (Fig. 6a–c; P = 0.013, 0.001 and 0.042, respectively).
Figure 6.
Dopamine signaling in the amygdala is necessary for mediating enhancements of learning performance induced by systemic administration of MPH. Behavioral measures of four groups of rats treated before training with (1) i.p. saline and intra-LA saline (N = 8 rats), (2) i.p. MPH and intra-LA saline (N = 8 rats), (3) i.p. MPH and intra-LA SCH (N = 7 rats) and (4) i.p. MPH and intra-LA RAC (N = 9 rats). (a–c) Intra-LA infusion of SCH significantly attenuated, whereas RAC spared, enhancements induced by systemic MPH in reward earning (F3,31 = 8.568, P < 0.001), task efficiency (F3,31 = 20.194, P < 0.001) and task accuracy (F3,31 = 6.004, P = 0.003). (d) Intra-LA infusion of RAC or SCH significantly attenuated reductions induced by systemic MPH in off-task behavior (F3,31 = 4.48, P = 0.011). In a–d, *P < 0.05, **P < 0.01,***P < 0.001.
In contrast, intra-LA RAC selectively attenuated MPH-induced reduction of off-task behavior (Fig. 6d; P = 0.001). Thus, D1R activation in the amygdala is necessary for mediating MPH-induced enhancements in cue-reward learning performance, and D2R activation in the amygdala is required for mediating MPH-induced reductions in off-task behavior. Therefore, dopamine signaling in the amygdala is critical in MPH-mediated learning performance enhancement.
DISCUSSION
Using a combination of in vivo pharmacology and ex vivo electrophysiology, we show that MPH enhances a lateral amygdala–dependent form of cue-reward learning and provide evidence suggesting that the learning enhancement depends upon dopaminergic modulation of excitatory synaptic plasticity within the lateral amygdala. These results extend previous findings that demonstrated an important role for dopamine within the lateral amygdala in the formation of both appetitive30–34 and aversive35–40 associations by identifying a potential mechanism whereby increases in dopamine in the amygdala modulate excitatory synaptic plasticity. Specifically, we found that MPH, and the dopamine uptake blocker GBR, enhanced the AMPAR/NMDAR at cortico-amygdala synapses. We also found that different dopamine receptor subtypes contribute to distinct aspects of learning performance, such that cue-reward learning depends upon dopamine D1 receptor–dependent mechanisms, and the suppression of task-irrelevant behavior depends upon D2 receptor–dependent mechanisms. Together, these findings indicate a specific synaptic mechanism whereby MPH may enhance associative learning through actions in the lateral amygdala.
How does DAT blockade in the amygdala enhance learning performance? At basal dopamine levels, GABAergic activity keeps the firing rates of pyramidal neurons low, while D2Rs on pyramidal neurons influence the responsiveness of the cell by modulating input resistance41. When dopamine levels are elevated, spontaneous background inhibition from local interneurons increases42,43, while feed-forward inhibition mediated by intercalated cell masses decreases owing to D1R activation27,43. Thus, lateral amygdala pyramidal neurons will become less responsive to weaker, background inputs but much more responsive to stronger, coordinated excitatory inputs carrying sensory information39,44. We hypothesize that by elevating extracellular dopamine24, MPH or GBR release inhibitory constraints on cortico-amygdala plasticity and alter the responsiveness of amygdala neurons driving different aspects of behavior through mechanisms dependent on distinct D1 and D2 receptors. An extension of this hypothesis is that whereas elevated dopamine is required for cortico-amygdala potentiation, only basal levels of dopamine are required for thalamo-amygdala potentiation. Thus, at basal and elevated levels of dopamine, thalamo-amygdala synapses are readily potentiated with learning, but when dopamine receptors are antagonized, bringing the level of dopaminergic signaling below the basal level, potentiation in these synapses may be attenuated.
Therefore, we suggest that increased activation of dopamine receptors enhances the ability to acquire cue-reward associations and to suppress task-irrelevant behavior. Specifically, treatment with MPH or GBR enhances task efficiency, likely owing to the dopamine-induced increase in responsiveness to coordinated sensory inputs, and decreases off-task behavior, likely owing to the dopamine-induced decrease in pyramidal neuron responsiveness to weaker background inputs. In contrast, D1R antagonism by SCH attenuates cue-reward learning and the associated plasticity, which may reflect increases in the inhibition of lateral amygdala neurons by intercalated GABA neurons, which densely express D1Rs27,43. In comparison, D2R inhibition selectively increases task-irrelevant behavior, in agreement with the notion that D2R antagonism facilitates the ability of weak excitation from task-irrelevant stimuli to drive neuronal excitation.
In conclusion, our findings suggest that MPH enhances learning performance through a dopamine-dependent mechanism by gating cortico-amygdala potentiation, facilitating cue-reward learning through a D1R-dependent mechanism and enhancing the ability to suppress task-irrelevant behavior through a D2R-dependent mechanism. Although NET inhibition did not acutely facilitate performance of our cue-reward learning task within the first exposure, the improvement in memory retention would likely contribute to behavioral enhancements observed across sessions. Furthermore, the NET may also act to clear dopamine from the extracellular space in brain regions with low DAT expression45.
Although DAT inhibition enhances task performance during the initial training session, it is still unclear whether DAT inhibition enhances task acquisition, consolidation, expression or a combination of these processes. Future studies may determine whether these mechanisms of MPH action generalize to other brain regions involved in learning and attention or to other behavioral assays of cognitive performance.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Acknowledgments
We thank H.L. Fields, G.D. Stuber, J.A. Rosenkranz and E.E. Steinberg for helpful comments; and A.C. Hollowell, S.L. Cho, S.J. Chang, L. Wang and F.W. Hopf for technical assistance. This research was supported by the State of California for Medical Research on Alcohol and Substance Abuse through the University of California at San Francisco (A.B. and P.H.J.), NIDA DA15096-01 (A.B.) and a Massachusetts Institute of Technology Peter J. Eloranta Summer Undergraduate Research Fellowship (L.D.T.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
K.M.T. supervised experiments and performed all whole-cell recordings. K.M.T., A.B. and P.H.J. contributed to study design, results analysis, interpretation and manuscript writing. K.M.T., L.D.T., J.J.C. and E.F.H. surgically implanted guide cannulae, performed intra-LA drug infusions, conducted behavioral experiments, sectioned acute slice preparations and performed data entry and analyses. A.B. and P.H.J. provided mentorship and resources.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprintsandpermissions/.
References
- 1.Swanson JM, Lerner M, Williams L. More frequent diagnosis of attention deficit-hyperactivity disorder. N Engl J Med. 1995;333:944. doi: 10.1056/NEJM199510053331419. [DOI] [PubMed] [Google Scholar]
- 2.Aman MG, Vamos M, Werry JS. Effects of methylphenidate in normal adults with reference to drug action in hyperactivity. Aust N Z J Psychiatry. 1984;18:86–88. doi: 10.3109/00048678409161040. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing; Arlington, Virginia, USA: 1994. [Google Scholar]
- 4.Rodriguez A, et al. Do inattention and hyperactivity symptoms equal scholastic impairment? Evidence from three European cohorts. BMC Public Health. 2007;7:327. doi: 10.1186/1471-2458-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhill LL, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Chung LC, Chen CS, Chen CC. Rapid improvement in academic grades following methylphenidate treatment in attention-deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58:37–41. doi: 10.1111/j.1440-1819.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 11.Davis M. The role of the amygdala in emotional learning. Int Rev Neurobiol. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- 12.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 13.Plessen KJ, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doron NN, Ledoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. J Comp Neurol. 1999;412:383–409. [PubMed] [Google Scholar]
- 15.Nakashima M, et al. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res. 2000;36:297–309. doi: 10.1016/s0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 16.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 18.Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–3945. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng X, Liu F, Wu X, Li B. Infusion of methylphenidate into the basolateral nucleus of amygdala or anterior cingulate cortex enhances fear memory consolidation in rats. Sci China C Life Sci. 2008;51:808–813. doi: 10.1007/s11427-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of D-, L-, and DL-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- 21.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty DD, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 23.Waldman ID, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkow ND, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 26.Andersen PH. The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 27.Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 28.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 29.Hess G, Kuhnt U, Voronin LL. Quantal analysis of paired-pulse facilitation in guinea pig hippocampal slices. Neurosci Lett. 1987;77:187–192. doi: 10.1016/0304-3940(87)90584-2. [DOI] [PubMed] [Google Scholar]
- 30.Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala d-amphetamine. Psychopharmacology (Berl) 1997;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- 31.Bernal S, et al. Role of amygdala dopamine D1 and D2 receptors in the acquisition and expression of fructose-conditioned flavor preferences in rats. Behav Brain Res. 2009;205:183–190. doi: 10.1016/j.bbr.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hitchcott PK, Bonardi CM, Phillips GD. Enhanced stimulus-reward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Psychopharmacology (Berl) 1997;133:240–248. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- 33.Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2009;30:289–298. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrzejewski ME, Spencer RC, Kelley AE. Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience. 2005;135:335–345. doi: 10.1016/j.neuroscience.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 36.Guarraci FA, Frohardt RJ, Young SL, Kapp BS. A functional role for dopamine transmission in the amygdala during conditioned fear. Ann NY Acad Sci. 1999;877:732–736. doi: 10.1111/j.1749-6632.1999.tb09312.x. [DOI] [PubMed] [Google Scholar]
- 37.Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- 38.Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- 39.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 40.Kienast T, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- 41.Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- 42.Loretan K, Bissiere S, Luthi A. Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology. 2004;47:631–639. doi: 10.1016/j.neuropharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.