Abstract
Grx3 and Grx4 are two monothiol glutaredoxins of Saccharomyces cerevisiae that have previously been characterized as regulators of Aft1 localization and therefore of iron homeostasis. In this study, we present data showing that both Grx3 and Grx4 have new roles in actin cytoskeleton remodeling and in cellular defenses against oxidative stress caused by reactive oxygen species (ROS) accumulation. The Grx4 protein plays a unique role in the maintenance of actin cable integrity, which is independent of its role in the transcriptional regulation of Aft1. Grx3 plays an additive and redundant role, in combination with Grx4, in the organization of the actin cytoskeleton, both under normal conditions and in response to external oxidative stress. Each Grx3 and Grx4 protein contains a thioredoxin domain sequence (Trx), followed by a glutaredoxin domain (Grx). We performed functional analyses of each of the two domains and characterized different functions for them. Each of the two Grx domains plays a role in ROS detoxification and cell viability. However, the Trx domain of each Grx4 and Grx3 protein acts independently of its respective Grx domain in a novel function that involves the polarization of the actin cytoskeleton, which also determines cell resistance against oxidative conditions. Finally, we present experimental evidence demonstrating that Grx4 behaves as an antioxidant protein increasing cell survival under conditions of oxidative stress.
Living cells contain regulatory proteins that are involved in maintaining the redox status of their respective molecules. Two groups, glutaredoxins and thioredoxins, play an important role in the reduction and reparation of oxidized proteins (19). Two dicysteinic glutaredoxins with antioxidant functions have been identified in Saccharomyces cerevisiae (16, 33) and five monocysteinic glutaredoxins: Grx3, Grx4, Grx5, Grx6, and Grx7 with different functions (24, 32, 36, 39, 41). Grx3 and Grx4 play roles in the regulation of the transcription factor Aft1 and also, as a consequence, in the regulation of iron homeostasis (36, 39). Grx3 and Grx4 interact with Aft1 in the nucleus, regulating Aft1 nuclear localization (39). Grx3 and Grx4 form heterodimeric complexes with Fra2 to assemble a [2Fe-2S] cluster that could act as a signal for controlling iron regulon (29). Grx3 physically interacts with Grx4 in the nucleus (39), and both glutaredoxins localize to the nucleus (30, 34). However, Kumánovics et al. (28) also localized them in the cytoplasm. Grx3 and Grx4 are highly homologous, and each possesses both an N-terminal Trx-like domain and a C-terminal Grx-like domain (22, 34). The Trx-like domain of Grx3 has recently been crystallized by Gibson et al. (17), in what was the first structural report relating this domain to any monothiol glutaredoxin. In Schizosaccharomyces pombe, one gene: grx4+, has been identified as being highly homologous to Grx3 (42% identity) and Grx4 (41% identity) (51). The gene grx4+ mainly localizes in the nucleus (8) and has been shown to be involved in nitrosative, osmotic, oxidative, and metabolic stress (26, 27). There is a human homologue for Grx3/Grx4: the PICOT protein (PKC-interacting cousin of Trx), which has also been proposed for involvement in the oxidative stress response (4, 50).
The yeast actin cytoskeleton is organized into actin cables and cortical patches (1, 35). Actin cables, which extend throughout the growth axis of the mother cell, represent the highest level of polymerization and structural organization. Actin cables are required to deliver organelle and work as tracks for movement (13a, 38, 42). Cortical actin patches normally cluster inside the expanding bud. Oxidative stress is a consequence of aerobic life. Reactive oxygen species (ROS), which are generated through the activation of several enzymatic molecules, cause oxidative damage to many macromolecules (3, 14), with actin being a known target for oxidation (15). This is probably because actin contains an elevated number of cysteine residues. One of the cellular responses to oxidative stress characterized in S. cerevisiae consists of the transient disassembly of actin cables; this leads to a depolarized actin cytoskeleton. As a result, actin is organized in patches that are randomly distributed throughout the cell (46). Under oxidative stress conditions, actin depolarization is associated with actin depolymerization, which is dosage dependent (15). After a time, which may vary, cell growth resumes. This occurs once actin polymerization activity and the polarity of the actin cytoskeleton have both been restored. All of these processes imply the potentially oxidized actin cytoskeleton having previously been repaired.
Actin plays a relevant role in ageing and apoptosis (18). Actin oxidative repair would therefore seem an important marker for cell survival under oxidative stress.
We present evidence here demonstrating that the monothiol glutaredoxins Grx3 and Grx4 play a role in the defense against oxidative stress through the regulation of iron homeostasis. The novelty of this study is that it describes how the Grx3 and Grx4 proteins each play their own specific roles in the organization of the actin cytoskeleton in a way that is independent of their previously described function in maintaining iron homeostasis. Grx4 also maintains the integrity of the actin cables under nonstress conditions, whereas both Grx3 and Grx4 contribute to the repolarization of the actin cytoskeleton in response to oxidizing agents. We have also characterized two different protein regions involved in the regulation of two different functions. Each of the Trx domains of Grx3 and Grx4 regulates the remodeling of the actin cytoskeleton, whereas the Grx domains regulate the correct redox status of the cells. Both functions are essential for cell survival under oxidative conditions.
MATERIALS AND METHODS
Media, growth conditions, and chemicals.
Yeasts were grown in SD synthetic medium (2% glucose, 0.67% yeast nitrogen base, and the required amino acids) (25). Unless otherwise stated, the temperature was 30°C. Hydrogen peroxide was purchased from Sigma and diluted in sterilized Milli-Q water when required. Dihydroethidium was purchased from Sigma and a stock solution of 5 mg/ml was prepared in sterilized Milli-Q water. Manganese sulfate monohydrate was also acquired from Sigma.
Yeast strains and plasmids.
The yeast strains and plasmids used in the present study have been previously described (see reference 39). The wild-type, grx4, grx3, aft1, grx3 grx4, and grx3 grx4 aft1 genotypes and constructions have been were described previously (see Table S1 in reference 39). Plasmid tetO7GRX3 corresponded to plasmid pMM367 (39), whereas plasmid tetO7GRX4 corresponded to plasmid pMM518 (39). The plasmids bearing each of the Trx and Grx domains of the Grx3 and Grx4 used in the present study corresponded to plasmids pMM491, pMM486, pMM506, and pMM502 (39).
Point mutations in the putative catalytic cysteines of the Trx domains of Grx3 and Grx4 were constructed by the ExSite method (48), and the former plasmids pMM486 and pMM502 were used as templates. We designed different oligonucleotides in order to create a restriction site near the desired point mutation. This did not affect the protein product, and it was used as a marker of a correct substitution. We constructed two amino acid replacements. In the Trx domain of Grx3, Cys72 was replaced by Arg, giving rise to the pTP150 plasmid. In the Trx domains of Grx4, Cys37 was replaced by Arg, which was contained in the pTP117 plasmid.
Detection of ROS through the quantification of superoxide anion.
We used an adaptation of a previously described method (see references 2 and 7). Cells were grown to logarithmic phase in SD medium plus the required amino acids at 30°C. We collected 1 ml of culture for each time point. After centrifugation at 6,000 rpm for 4 min at room temperature, cells were resuspended in 1 ml of phosphate buffer containing 0.1% glucose. This suspension was then transferred to quartz cuvettes, to which DHE probe was added to a final concentration of 5 μg/ml. We then used a RF-5,000 spectrophotometer to register the fluorescence released by ethidine upon the oxidation of the dihydroethidium. Readings were registered at 590 nm at 5-min intervals for 30 min. The wavelengths used for this assay were 520 nm for excitation and 590 nm for emission.
Actin staining.
Cells growing exponentially at 30°C were stained with rhodamine-phalloidin as previously described (see reference 44). For quantification, we counted 1,000 budded cells in which the bud was smaller than the mother cell. We considered that cells were depolarized when no actin cables were microscopically detected. We repeated this experiment three times to obtain average values for the experiments with their corresponding error bars plotted in the histograms shown in the figures.
Yeast extracts and immunoblot analyses.
Both methods were performed as described previously (37). As primary antibodies, we used anti-hemagglutinin (anti-HA) and anti-α-tubulin (Sigma). Anti-HA was used at 1:1,000 dilution in TBST buffer containing 0.25% nonfat milk; as secondary antibody, we used horseradish peroxidase-linked anti-mouse (NA931V; Amersham Biosciences) at a 1:10,000 dilution in TBST buffer containing 0.25% nonfat milk. Anti-α-tubulin was used at a 1:5,000 dilution in TBST buffer containing 5% nonfat milk, as secondary antibody we also used anti-mouse in TBST buffer containing 5% nonfat milk.
In all cases, chemiluminescent detection was performed using the Supersignal substrate (Pierce) in a Lumi-Imager (Roche Molecular Biochemicals).
RESULTS
Grx4 and Grx3 play a role in cellular protection against oxidative stress by assuring correct iron homeostasis.
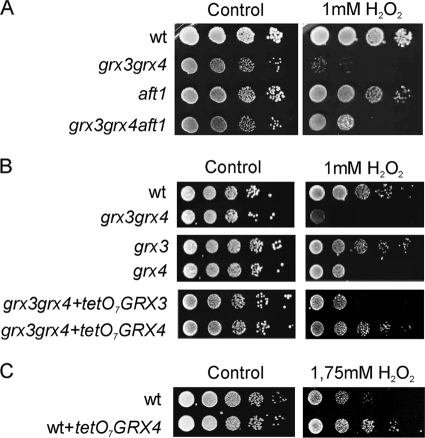
In a previous study, we had already demonstrated that the grx3 grx4 double mutant was sensitive to oxidizing agents. However, we observed that the sensitivity to oxidizing agents determined in the double mutant was not totally due to the high intracellular iron accumulation that occurred as a consequence of Aft1 nuclear localization. This conclusion was based on the observation that an Aft1 deletion in the grx3 grx4 double mutant did not efficiently rescue sensitivity to the oxidizing agent hydrogen peroxide. As shown in Fig. 1A, the grx3 grx4 aft1 triple mutant was still sensitive to the oxidative treatment, although to a lesser degree than the grx3 grx4 double mutant. We also detected that the single grx4 mutant was sensitive to hydrogen peroxide (Fig. 1B). In order to ascertain whether both the Grx3 and the Grx4 proteins were capable of increasing cell resistance to oxidizing agents, we increased the concentration of hydrogen peroxide from 1 to 1.75 mM. We had previously observed (data not shown) that H2O2 concentrations above 1 mM impaired wild-type cell survival. As a consequence, these conditions should allow us to detect a possible rescue in cell viability when either Grx3 or Grx4 was overexpressed. Similarly, when we overexpressed Grx4 in a wild-type strain, we observed that this protein enhanced cellular resistance to oxidative stress (Fig. 1C). However, we did not observe this effect in the case of Grx3 overexpression (not shown). This suggested that Grx4 plays a direct role in the oxidative stress response.
FIG. 1.
(A and B) The absence of Grx4 increases cell sensibility to hydrogen peroxide and its overexpression induces cell resistance against the oxidizing agent. All of these effects occur independently of Aft1. The following strains were exponentially grown in SD medium plus amino acids before being serial diluted and spotted onto plates either containing, or not containing, 1 mM H2O2. The plates were incubated at 30°C for 3 days. (C) Same as in panels A and B, except that the plates contained 1.75 mM hydrogen peroxide. wt, wild type.
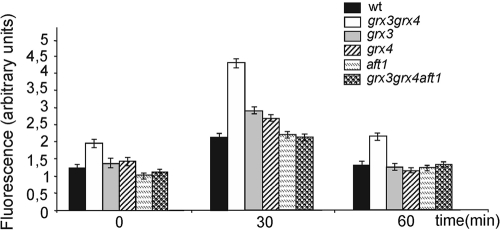
In order to study this phenomenon in greater depth, we decided to investigate the levels of intracellular oxidation found in the wild type, the grx3 mutant, the grx4 mutant, and the grx3 grx4 double mutant, both in exponentially growing cultures and in response to treatment with the exogenous oxidizing agent, hydroxide peroxide. To do this, we measured the intracellular levels of ROS by quantifying the accumulation of the superoxide ion (as described in Materials and Methods). We were then able to determine that the absence of either Grx3 or Grx4 did not affect normal ROS levels in the cells with respect to wild-type cells (Fig. 2). Each of the single mutants, i.e., the grx3 and grx4 mutants, presented a slightly higher accumulation of ROS than those determined in wild-type cells after 30 min of treatment with hydrogen peroxide. However, at 60 min, the oxidative levels of grx3 mutant, grx4 mutant, and wild-type cells were indistinguishable (Fig. 2). We had previously demonstrated that Grx3 and Grx4 formed a complex in the nucleus and that their functions were redundant and additive with respect to Aft1 regulation (39). When we analyzed the intracellular oxidative status in the grx3 grx4 double mutant, we detected that the absence of both proteins induced endogenous oxidative stress at levels that were significantly higher than those determined in grx4 mutant, grx3 mutant, and wild-type cells and that this stress increased considerably in response to oxidative treatment with hydrogen peroxide (Fig. 2).
FIG. 2.
The grx3 grx4 double mutant exhibits endogenous constitutive oxidative stress caused by ROS accumulation as a consequence of Aft1 misregulation. Cells were exponentially grown in SD plus amino acids. Aliquots (1 ml) were removed from each culture to quantify ROS levels using the dihydroethidine method described in Materials and Methods. Time points represent 30 and 60 min after the addition of 1 mM hydrogen peroxide to each of the growing exponentially cell cultures. Histograms represent the average of three independent experiments. wt, wild type.
Our results demonstrate that both Grx3 and Grx4 are required to maintain the correct redox equilibrium in the cell and also to protect cells against exogenous oxidation. We next reasoned that one explanation for the elevated oxidative levels observed in the grx3 grx4 double mutant could have been the high accumulation of iron as a consequence of the Aft1 constitutive nuclear localization (39). Elevated levels of intracellular iron could have provoked an accumulation of ROS throughout the Fenton reaction (49). In order to confirm this hypothesis, we next determined the intracellular levels of ROS in the grx3 grx4 aft1 mutant and compared these values to those observed in wild-type and grx3, grx4, and grx3 grx4 mutant strains. We observed that both grx3 grx4 aft1 mutant and wild-type cells presented equivalent levels of ROS in cells, both under logarithmic conditions and upon hydrogen peroxide treatment. Our data demonstrate that it was the accumulation of intracellular iron that caused both the constitutive and the induced oxidative stress observed in the absence of both GRX3 and GRX4 genes (Fig. 2).
Grx3 and, above all, Grx4 play a role in remodeling the actin cytoskeleton under both normal conditions and in response to oxidative stress.
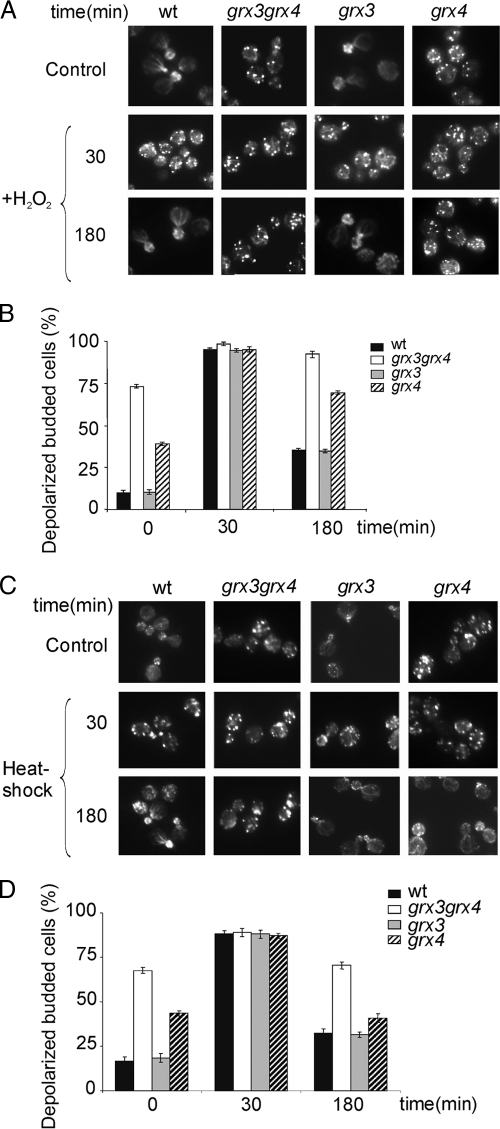
One of the known cellular consequences deriving from oxidative stress is the depolarization and depolymerization of the actin cytoskeleton (18, 46). In view of earlier results, we sought to determine the organization of the actin cytoskeleton in wild-type, grx3 grx4, grx3, or grx4 cells either treated with hydrogen peroxide or untreated as a control. In grx4 cells growing exponentially, we had already observed a substantial defect in the integrity of the actin cytoskeleton (Fig. 3A). Actin filaments were almost absent, and actin patches were clearly scattered throughout the grx4 cells. Interestingly, in the grx3 grx4 double mutant, the actin cytoskeleton presented an even greater degree of disorganization; this can be seen in Fig. 3A. This became even more evident when the depolarized cells were quantified (Fig. 3B). In this figure, we can observe that in cells growing exponentially, nearly 75% of the grx3 grx4 cells had actin cytoskeletons that were totally depolarized and with actin distributed in patches that were scattered throughout the mother and daughter cells; however, no cables were detected (Fig. 3A and B). The grx4 mutant showed that 40% of cells contained depolarized actin cytoskeleton. In contrast, the actin cytoskeleton in the grx3 cells was morphologically identical to that observed in wild-type cells (Fig. 3A), with fewer than 10% of the cells being depolarized. When cells were treated with the oxidizing agent hydrogen peroxide, we detected a total and transient depolarization of the actin cytoskeleton in all of the backgrounds tested. This was in accordance with our previous studies (46). After 1 h of peroxide treatment, wild-type and grx3 cells recovered most of their wild-type actin polarity. However, more than 50% of the grx4 cells remained depolarized and, more significantly, more than 90% of the cells recorded in the grx3 grx4 mutant were totally depolarized (Fig. 3B). We decided to determine whether grx4 and grx3 grx4 mutants exhibited similar responses to other types of stress with respect to actin dynamics. To do this, we treated wild-type and grx3, grx4, and grx3 grx4 mutant strains with another known type of stress, heat shock, in order to induce transient actin cytoskeleton depolarization. In Fig. 3C, it can be observed that the wild type, grx3, and grx4 strains had similar responses to this stress: their cells presented an actin cytoskeleton transiently depolarized (30 min of treatment), but by 180 min, the majority of their cells had perfectly repolarized. In the case of the grx3 grx4 double mutant, although most of the cells were depolarized, it was possible to observe a transient increase in depolarization and subsequent recovery, with values reaching levels similar to those recorded at time zero and after 60 min. These results demonstrate that mainly Grx4, but also Grx3, were involved in the repolarization of the actin cytoskeleton in a specific response to oxidative stress.
FIG. 3.
Grx3 and Grx4 perform an additive function in remodeling actin cables both under normal conditions and upon treatment with hydrogen peroxide. (A) Actin staining of samples from wild-type and grx3, grx4, and grx3 grx4 mutant cultures treated with 1 mM hydrogen peroxide. Samples were collected for actin staining at the times indicated in the figure. (B) Histograms representing the quantification of panel A. (C) Actin staining of the same samples as in panel A with temperatures shifted from 30 to 38°C for the times indicated. (D) Quantification of the experiment represented in panel C. wt, wild type.
In a previous study, we showed that iron accumulation was identical in each of the grx3 and grx4 backgrounds. We also demonstrated that in both single mutants, the intracellular accumulation of iron was only slightly higher than that found in wild-type cells (39). Moreover, neither grx3 nor grx4 cultures presented a substantial accumulation of ROS in comparison to wild-type values (Fig. 2). All of these results prompted us to conclude that the actin cytoskeleton depolarization observed in grx4 and grx3 grx4 mutant cells could not be fully attributed to either the iron or the ROS accumulation inside the cells. Grx4 must therefore play a novel role in the organization of the actin cytoskeleton. Another conclusion from these experiments was that Grx4 and Grx3 perform an additive and redundant function in actin cable maintenance both under normal conditions and in the adaptive response to oxidative treatment.
The function that both Grx3 and Grx4 play in actin cable remodeling is independent of their role in the regulation of iron homeostasis.
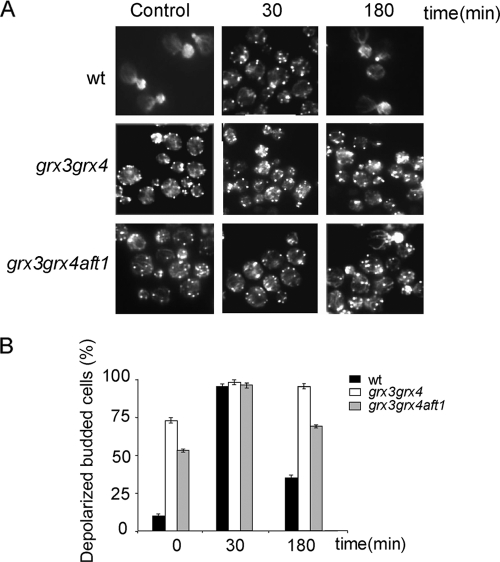
To investigate whether the total actin depolarization observed in the grx3 grx4 double mutant was again a consequence of the endogenous oxidative stress caused by iron accumulation, we decided to analyze the organization of the actin cytoskeleton in the grx3 grx4 aft1 triple mutant. In Fig. 4, we show that the deletion of AFT1 only caused a partial rescue of actin cytoskeleton organization in the grx3 grx4 double mutant with respect to wild-type levels. In order to deplete the cells of iron, we also treated grx3 grx4 cells with ferrozine, which is a very well-known iron chelator. As expected, and supporting our model, iron depletion caused a marked reduction in the concentration of ROS in the grx3 grx4 double mutant, which fell to levels equivalent to those typically detected in wild-type cells under standard experimental conditions (Fig. 5A). Furthermore, iron depletion did not restore actin cytoskeleton polarity in the grx3 grx4 double mutant, as can be observed in Fig. 5B. We therefore concluded that Aft1 misregulation and the consequent intracellular iron accumulation caused ROS accumulation but was not the main cause of the actin cytoskeleton depolarization that occurred when both Grx4 and Grx3 were absent.
FIG. 4.
Neither iron nor ROS accumulation provoked by Aft1 misregulation causes the actin cytoskeleton disorganization observed in the grx3 grx4 double mutant. (A) Actin staining of samples from the wild-type and grx3 grx4 and grx3 grx4 aft1 mutant cultures either treated, or not treated, with 1 mM hydrogen peroxide. (B) Quantification of the experiment shown in panel A. wt, wild type.
FIG. 5.
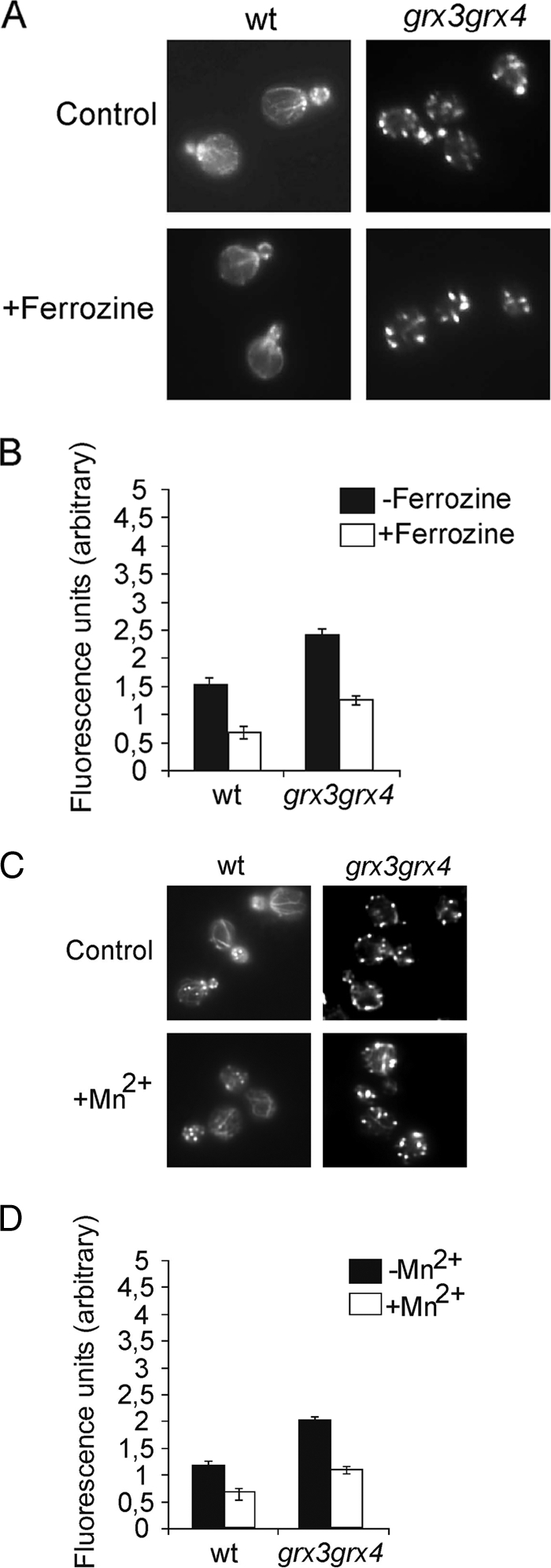
Elevated concentration of intracellular iron and hydroxyl radicals are not sufficient to explain actin disorganization in the grx3 grx4 mutant. Wild-type and grx3 grx4 mutant strains were logarithmically grown and treated with 2 mM ferrozine for 6 h. Samples were collected at time zero and after 6 h of treatment for actin staining (A) and to quantify the levels of superoxide (B). (C) Exponentially growing wild-type and grx3 grx4 mutant cells were grown in the presence of the ROS scavenger Mn2+. Samples were treated with 2 mM Mn2SO4 for 2 h. Samples were collected for actin staining (C) and to quantify the levels of superoxide (D). Histograms represent the averages of three independent experiments. wt, wild type.
We also speculated whether a reduction in ROS accumulation, perhaps caused by mechanisms other than the regulation of iron homeostasis, could be sufficient to restore the organization of the actin cytoskeleton in the grx3 grx4 mutant. In order to investigate this hypothesis, we treated wild-type and grx3 grx4 cells with manganese sulfate (Mn2+), which is a superoxide radical scavenger (9). We observed that scavenging superoxide radicals did not restore the wild-type actin cytoskeleton in the grx3 grx4 double mutant (Fig. 5C and D). We therefore concluded that the actin polarization defect observed in the grx3 grx4 mutant was not an indirect consequence of iron accumulation or of the generation of oxidative stress, but rather of a specific role played by Grx4 and Grx3 in the polymerization of the actin cytoskeleton.
Grx3 and Grx4 play a redundant role in promoting actin cable formation and cell viability.
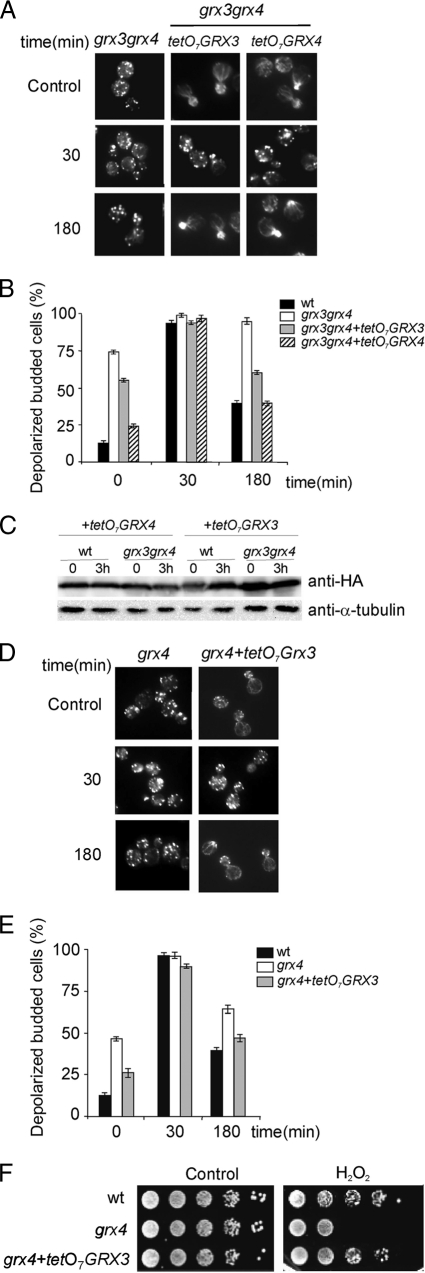
In order to demonstrate a specific role for Grx3 and Grx4 in remodeling the actin cytoskeleton, we overexpressed each protein in the grx3 grx4 double mutant to study the organization of the actin cytoskeleton. Under these conditions, we detected a clear and significant restoration of the integrity of actin cables (Fig. 6A); this was reflected in histograms showing the percentage of depolarized cells (Fig. 6B). It is interesting that Grx4 overexpression was significantly more efficient in the formation of actin cables, and therefore in the restoration of cell polarity, than Grx3. This was observed under both normal conditions and hydrogen peroxide treatment (Fig. 6B). As already shown, in Fig. 1B, Grx4 overexpression was also more efficient than Grx3 at rescuing low grx3 grx4 cell viability in response to oxidative stress. We were able to determine that these observed differences did not result from different steady-state levels of the overexpressed proteins. This is reflected in Fig. 6C, in which a Western blot reflects an equivalent level of expression of either Grx3 or Grx4 under the conditions used in the present study. These results demonstrate that Grx4 plays an important, novel role in actin cable remodeling in the cellular response to oxidative stress. In order to ascertain whether Grx3 could compensate for the absence of the Grx4 function in actin cable remodeling, we overexpressed Grx3 in the grx4 mutant. In Fig. 6D and E, it can be observed that high levels of Grx3 rescued the defect in actin cable integrity that was observed in the grx4 mutant. Moreover, high levels of Grx3 also rescued the lack of viability that the single mutant grx4 exhibited when it was treated with hydrogen peroxide (Fig. 6F). These results demonstrate that both Grx3 and Grx4 play a role in the organization of the actin cytoskeleton under normal conditions and in the adaptive response to oxidative stress and that both proteins also work in cooperation and share a redundant role in this mechanism.
FIG. 6.
Overexpression of Grx3 compensates for the lack of Grx4 function in the restoration of actin cytoskeleton polarity and in cell viability, both under normal and oxidative conditions. (A) Actin staining of exponentially growing cells of grx3 grx4, grx3 grx4+pGrx3, and grx3 grx4+pGrx4. Cultures were either treated, or not treated, with hydrogen peroxide, and samples were collected for actin staining at the times indicated. (B) Quantification of the experiment depicted in panel A. The histograms represent the number of cells without visible cables. (C) Western blot analysis performed with anti-HA polyclonal antibody to detect the desired protein in total protein extracts. We used anti-α-tubulin as a loading control (see Materials and Methods). Cultures from the wild-type and grx3 grx4 mutant strains, transformed with each of the plasmids containing either tetO7GRX3 or tetO7GRX4 were grown in SD media to exponential phase (0) and subsequently treated with 1 mM H2 O2 for 3 h (3 h). (D) Actin staining of grx4 and grx4+pGrx3 cultures, as in panel A. (E) Quantification of the experiment depicted in panel C, as in panel B. (F) Serial dilutions from exponentially growing cultures of the different strains stated in the figure. SD plates plus the required amino acids either containing or not containing 1 mM hydrogen peroxide were used. Plates were grown at 30°C for 3 days.
Trx domains from either Grx3 or Grx4 are sufficient to complement actin defects and cell viability in Grx3- and Grx4-deficient cells.
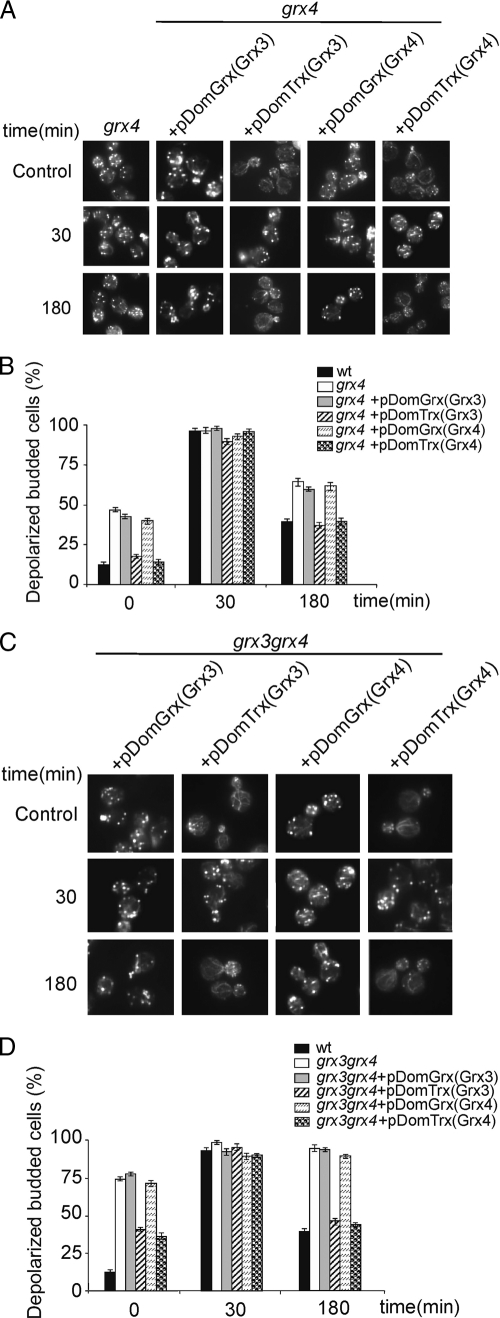
In order to look more deeply into the role that either Grx3 or Grx4 could play in cell morphogenesis through the regulation of actin dynamics, we separately analyzed each of the two Grx and Trx domains from Grx3 and Grx4. We first overexpressed Grx and Trx domains from each Grx3 and Grx4 protein in the single grx4 mutant. We could determine that the levels of protein expression, corresponding to each of the four domains cloned in plasmids, were essentially similar in all of the samples used (data not shown). In Fig. 7A and B, it can be seen that both Trx domains efficiently restored grx4 actin cytoskeleton polarization and cable formation in logarithmically growing cells and also in response to the hydrogen peroxide treatment. We then decided to investigate whether the single domains were also able to restore actin cable morphology and cell growth in the grx3 grx4 double mutant. Again, only the two Trx domains, but not the Grx domains, were able to efficiently repolarize the actin cytoskeleton in the grx3 grx4 double mutant. This induced substantial and efficient cable formation in both cells growing exponentially and in response to treatment with the oxidant hydrogen peroxide, as is clearly shown and quantified in Fig. 7C and D.
FIG. 7.
The Trx domains of Grx3 and Grx4 are sufficient to induce actin cable formation either in grx4 or in grx3 grx4 mutants. (A) Actin staining of the grx4 mutant transformed, or not transformed, with each of the Grx or Trx domains of proteins Grx3 and Grx4, both in logarithmic growth and in response to hydrogen peroxide treatment at the times indicated. (B) Histograms representing statistical values for the experiment performed in panel A. (C) Same as in panel A, but with the grx3 grx4 double mutant. (D) Quantification of the experiment performed in panel C.
Both Trx domains contain a WAD/EPCK sequence that is reminiscent of the authentic active site motif of thioredoxins WCGPCK (45). We therefore raised the question as to whether the function that both Trx domains demonstrate in actin cytoskeleton polarization could have been exerted through the cysteine residues contained in their putative active site motives. In order to answer to this question, we constructed the following point mutations: we substituted the Cys72 of Grx3 for Arg and replaced the Cys37 of Grx4 with Arg (see Materials and Methods). We then transformed the pMM486 and pMM502 plasmids containing each of the point mutations in each of the wild-type, grx4, and grx3 grx4 strains and analyzed cell viability, actin cytoskeleton organization, and protein expression under normal conditions and after 3 h of treatment with 1 mM hydrogen peroxide. We obtained identical results to those shown in Fig. 7A to D with the nonmutated Trx domains (data not shown). Our results clearly demonstrated that the cysteine in the putative catalytic thioredoxin motives in each of the Trx domains of Grx3 and Grx4 was not responsible for the restoration of the organization of the actin cytoskeleton. We therefore concluded that the Trx domains perform a function in actin dynamics by some other means than the expected thioredoxin active motif.
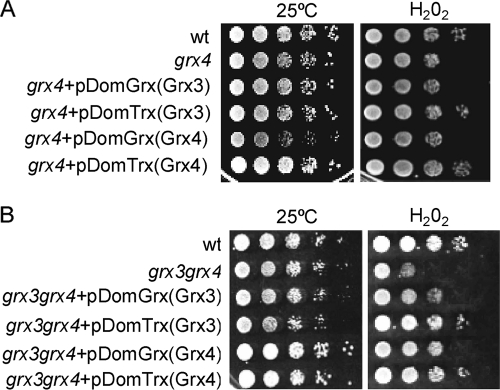
We then studied cell survival in response to oxidation in each of the two grx4 and grx3 grx4 mutants transformed with either Grx or Trx domains from Grx3 or Grx4. Only the Trx domains from Grx3 or Grx4 suppressed the sensitivity of grx4 mutants to hydrogen peroxide (Fig. 8A). Interestingly, each of the four domains—Trx or Grx from either Grx3 or Grx4 proteins—was able to individually rescue the growth deficiency observed in the grx3 grx4 double mutant in response to oxidative stress, although the Trx domains did this more efficiently (Fig. 8B).
FIG. 8.
The Trx domains of Grx3 and Grx4 are involved in cell survival under conditions of oxidative stress. grx4 (A) and grx3 grx4 (B) mutants were transformed with the Grx and Trx domains of Grx3 and Grx4. Exponentially growing cells of the different cultures from this figure were serial diluted and plated onto plates either containing, or not containing, 1 mM hydrogen peroxide. Plates were grown at 30°C for 3 days.
This result demonstrates that the lack of viability observed in the grx4 single mutant in response to oxidative stress was due to its inability to efficiently remodel the actin cytoskeleton.
Although both the Trx and the Grx domains of Grx3 and Grx4 are sufficient to promote cell survival in response to oxidative stress, only the Grx domains participate in ROS detoxification.
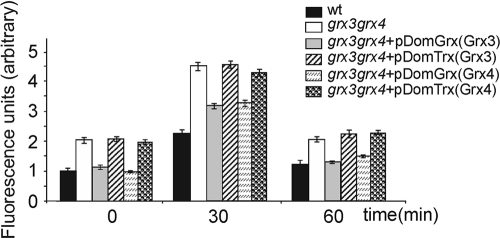
We also decided to investigate whether the previously described function for both Grx3 and Grx4, as ROS scavengers, was specifically exerted through any of the domains analyzed here. For this function, we also observed separate roles for each of the domains. Both of the Grx domains from the Grx3 and Grx4 proteins were individually able to reduce intracellular levels of ROS, and therefore oxidative stress, in the grx3 grx4 double mutant (Fig. 9). Overexpression of these domains did not have any effect on the levels of superoxide accumulated in either wild-type or grx4 mutant cells (data not shown).
FIG. 9.
Each of the Grx domains of either Grx3 or Grx4 favor ROS detoxification in grx3 grx4 mutant cells. The grx3 grx4 mutant and wild-type strains were either transformed, or not transformed, with each of the plasmids, which alternatively contained the Trx or Grx domains of either Grx3 or Grx4 proteins. Exponentially growing cultures were either treated, or not treated, with hydrogen peroxide to determine the anion superoxide concentration in the cells, which was represented in units of fluorescence. Histograms represent averages of three independent experiments. wt, wild type.
However, neither of the two Trx domains of Grx3 or Grx4 participated in the detoxification of grx3 grx4 mutant cells of ROS (Fig. 9).
We concluded that both Grx domains play a role in detoxifying ROS cells by inducing reductions in the levels of intracellular iron. This is due to the previously published mechanism by which the Grx domains of Grx3 and Grx4 promote Aft1 translocation from the nucleus to the cytoplasm (39). On the other hand, both of the Trx domains play direct and independent roles in promoting the organization of the actin cytoskeleton. Both functions lead to higher resistance to oxidative stress and consequently contribute to improved cell survival.
DISCUSSION
In this study, we present evidence demonstrating a role for Grx3, and especially for Grx4, in actin cable remodeling and in the general defense against oxidative stress. The absence of Grx4 causes a defect in actin polarization and also in the process of actin repolarization; the latter occurs in response to external oxidative stress caused by hydrogen peroxide. The observation that the grx4 mutant is sensitive to peroxide (but that the grx3 mutant is not) and, on the contrary, that Grx4 overexpression (but not Grx3 overexpression) confers cells with greater resistance to oxidative stress suggests a direct role for Grx4 in the oxidative stress response. This differs from the role that both proteins play in iron homeostasis regulation (39). We previously reported that the two mutants, the grx3 and grx4 mutants, were not significantly sensitive to hydrogen peroxide (39), but in the current study, a deeper characterization revealed that the grx4 mutant exhibited a slight, but significant, degree of sensitivity to hydrogen peroxide that had not been previously detected.
Our results also suggest that the defect in cell survival observed in the grx4 mutant was due to its inability to efficiently remodel the actin cytoskeleton under oxidative conditions. This hypothesis is supported by the fact that both the Trx domains of either Grx3 or Grx4 suppressed the lethality of the grx4 mutant in the presence of hydrogen peroxide, and this was concomitant with the restoration of the actin cytoskeleton structure. However, neither of the two Grx domains was able to compensate for the lack of viability of the grx4 mutant or the disorganization of the actin cytoskeleton. This demonstrated that the deregulation of iron homeostasis was not responsible for the grx4 phenotypes.
We have presented data that demonstrate that the simultaneous absence of the two glutaredoxins provokes constitutive oxidative stress. The explanation for this is as follows. The nuclear Aft1 localization that occurs in the grx3 grx4 double mutant induces a constitutive expression of the genes involved in iron uptake (39, 52). The intracellular accumulation of iron that then follows as a consequence of Aft1 misregulation induces ROS accumulation due to the Fenton reaction (49).
We had previously demonstrated that the cellular consequences of oxidative stress include the depolarization of the actin cytoskeleton, the disappearance of large actin filaments, and the transient organization of the cytoskeleton into patches scattered throughout the cell (46). Moreover, and also in response to oxidizing agents such as hydrogen peroxide, cells significantly lose their capacity to polymerize F-actin in a way that is dependent on the dosage of the oxidizing agent (40). The actin cytoskeleton is a known redox target. Examples of this have been widely demonstrated in such different eukaryotic systems as yeast (15, 46), Mytilus edulis (31) and human cells (6, 12). Actin contains many cysteine residues that are known to be particularly sensitive to oxidation. Oxidative damage can induce the formation of intermolecular disulfides in actin molecules. These then induce the formation of amorphous aggregates (43) and cause the absence of functional filaments. The oxidation of methionine residues is another known consequence of actin oxidation and dramatically impairs the function of the actin cytoskeleton (6, 10, 11, 15). It has also been demonstrated that the physiological consequence of actin oxidation is the acceleration of cell death in yeast.
In the present study, we demonstrate that mainly Grx4, but also Grx3, plays a direct role in actin cable formation, which is important for cell viability, and that protein oxidation may be reversible through the action of thioredoxins, glutathione, and glutaredoxins. Finding a function for Grx4 and Grx3 as glutaredoxins involved in the processes required to repair oxidized actin would therefore not be unexpected. We have also demonstrated that the role that both Grx3 and Grx4 play in actin cable formation is not the consequence of the intracellular accumulation of iron described in the grx3 grx4 double mutant (39), or of ROS accumulation, which was determined in the absence of Grx3 and Grx4 in this study. Each of the two Grx3 and Grx4 proteins has one thioredoxin domain (Trx), followed by one glutaredoxin domain (Grx) (5, 39, 45). In a previous study, we demonstrated that each Grx domain of both Grx3 and Grx4 played a role in Aft1 translocation from the nucleus to the cytoplasm. Consequently, for both Grx3 and Grx4, Grx overexpression caused a reduction in the intracellular concentration of iron in the grx3 grx4 mutant (39). We have now characterized an independent role for the Trx domains of both Grx4 and Grx3 in actin cable remodeling and in promoting cell survival under conditions of oxidative stress. Grx3 and Grx4 therefore have a function in actin cytoskeleton dynamics and organization, both under normal conditions and in response to specific types of stress, such as oxidative stress. In a recent study (13), it was demonstrated that the glutaredoxin domain of TXNRD1_v3, a human thioredoxin reductase 1, regulates the actin cytoskeleton by inducing cytoplasm filaments and cell membrane filopodia. In our system, the thioredoxin domains of two glutaredoxins participate in the regulation of the actin cytoskeleton organization. Many proteins are readily susceptible to oxidization in cysteine residues, giving rise to disulfide bonds. This -S-S- formation can, however, be reversibly reduced by several proteins, including glutaredoxins and thioredoxins (5, 19, 23, 32, 47). Haarer et al. (21) recently described a genetic interaction between both Grx3 and Grx4 and actin. It should not therefore be surprising to find that Grx3 and Grx4, which play an important role in the response to oxidative stress, may also be involved in the protection of the redox integrity of the actin cytoskeleton.
We have presented results that demonstrate that the role that each of the two Trx domains plays in actin dynamics is independent from its catalytic cysteines. This suggests that the mechanism used by Grx3 and Grx4 to regulate and repair the cytoskeleton is different from that attributed to a putative thioredoxin function. The study of this mechanism merits future investigation. Our results were consistent with the idea that Grx3 and Grx4 form a functional complex that works additively, not only for iron homeostasis regulation, through the regulation of Aft1 nuclear localization, but also to maintain actin cytoskeleton integrity. However, once again, both proteins presented functional redundancy for the second of these functions. That said, Grx4 is the main protein that regulates actin cytoskeleton dynamics, although by a different and currently unknown mechanism that differs from the classical activities of thioredoxin and glutaredoxin. Since Grx3 and Grx4 present homologies with other eukaryotic systems, both their function in cytoskeleton remodeling and their role in cell survival in response to oxidative stress merit further investigation.
We therefore conclude that both the regulation of iron homeostasis by Grx domains and the regulation of actin cytoskeleton organization by Trx domains are important for cell viability and the cellular responses to oxidative stress.
Acknowledgments
We thank Mireia Aresté and Silvia Martínez for invaluable technical assistance.
This research was supported by grant BFU2009-11215 from the MEC (Spanish Government). N.P.-C. was supported by a fellowship from the Generalitat de Catalunya (Spain) and a contract associated with grant BFU2009-11215.
Footnotes
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Adams, A. E., and J. R. Pringle. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic mutant Saccharomyces cerevisiae. J. Cell Biol. 98:934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilaniu, H., L. Gustafsson, M. Rigoulet, and T. Nyström. 2003. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299:1751-1753. [DOI] [PubMed] [Google Scholar]
- 3.Ames, B., M. Shigenaga, and T. Hagen. 1993. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. U. S. A. 90:7915-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babichev, Y., and N. Isakov. 2001. Tyrosine phosphorylation of PICOT and its translocation to the nucleus in response of human T cells to oxidative stress. Adv. Exp. Med. Biol. 495:41-45. [DOI] [PubMed] [Google Scholar]
- 5.Bellí, G., J. Polaina, J. Tamarit, M. A. de la Torre, M. T. Rodríguez-Manzaneque, J. Ros, and E. Herrero. 2002. Structure function analysis of yeast Grx5 monothiol glutaredoxins defines essential amino acids for the function of the protein. J. Biol. Chem. 277:37590-37596. [DOI] [PubMed] [Google Scholar]
- 6.Bencsath, F. A., A. Shartava-Monteiro, and S. R. Goodman. 1996. Identification of the disulfide-linked peptide in irreversibly sickle cell beta-actin. Biochem. 35:4403-4408. [DOI] [PubMed] [Google Scholar]
- 7.Binkodas, V. P., J. Jordan, C. C. Lee, and R. J. Millar. 1996. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 16:1324-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, W. H., K. D. Kim, and J. H. Roe. 2005. Localization and function of three monothiol glutaredoxins in Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 330:604-610. [DOI] [PubMed] [Google Scholar]
- 9.Corson, L. B., J. Folmer, J. J. Strain, V. C. Culotta, and D. W. Cleveland. 1999. Oxidative stress and iron are implicated in fragmenting vacuoles of Saccharomyces cerevisiae lacking Cu,Zn-superoxide dismutase. J. Biol. Chem. 274:27590-27596. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne, I., R. Rossi, A. Milzani, P. Di Simplicio, and R. Colombo. 2001. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 31:1624-1632. [DOI] [PubMed] [Google Scholar]
- 11.Dalle-Donne, I., R. Rossi, D. Giustarini, N. P. Gagliano, L. Lusini, P. Di Simplicio, R. Colombo, and A. Milzani. 2001. Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic. Biol. Med. 31:1075-1083. [DOI] [PubMed] [Google Scholar]
- 12.Dalle-Donne, I., R. Rossi, D. Giustarini, N. Gagliano, P. Di Simplicio, R. Colombo, and A. Milzani. 2002. Methionine oxidation as a major cause of the functional impairment of oxidized actin. 2002. Free Radic. Biol. Med. 32:927-937. [DOI] [PubMed] [Google Scholar]
- 13.Damdimopoulou, P. E., A. Miranda-Vizuete, E. S. Arnér, J. A. Gustafsson, and A. E. Damdimopoulos. 2009. The human thioredoxin reductase-1 splice variant TXNRD1_v3 is an atypical inducer of cytoplasmic filaments and cell membrane filopodia. Biochim. Biophys. Acta 1793:1588-1596. [DOI] [PubMed] [Google Scholar]
- 13a.Drubin, D. G. 1991. Development of cell polarity in budding yeast. Cell 65:1093-1096. [DOI] [PubMed] [Google Scholar]
- 14.Finkel, T., and N. J. Holbrook. 2000. Oxidants, oxidative stress, and the biology of ageing. Nature 408:239-247. [DOI] [PubMed] [Google Scholar]
- 15.Farah, M. E., and D. C. Amberg. 2007. Conserved actin cysteine residues are oxidative stress sensors that can regulate cell death in yeast. Mol. Biol. Cell 18:1359-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan, Z. R. 1992. Cloning and sequencing of a gene encoding yeast thioltransferase. Biochem. Biophys. Res. Commun. 187:949-955. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, L. M., N. N. Dingra, C. E. Outten, and L. Lebioda. 2008. Structure of the thioredoxin-like domain of yeast glutaredoxin 3. Acta Crystallogr. 64:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gourlay, C. W., and K. R. Ayscough. 2005. A role for actin in aging and apoptosis. Biochem. Soc. Trans. 33:1260-1264. [DOI] [PubMed] [Google Scholar]
- 19.Grant, C. 2001. Role of the glutathione/glutaredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 39:533-541. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Haarer, B., S. Viggiano, M. A. Hibbs, O. G. Troyanskaya, and D. C. Amberg. 2007. Modeling complex genetic interactions in a simple eukaryotic genome: actin displays a rich spectrum of complex haploinsufficiencies. Genes Dev. 21:148-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero, E., and M. A. de la Torre-Ruiz. 2007. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol. Life Sci. 64:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmgren, A., C. Johansson, C. Berndt, M. E. Lönn, C. Hudemann, and C. H. Lilling. Thiol redox control via thioredoxin and glutaredoxin systems. 2005. Biochem. Soc. Trans. 33:1375-1377. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo, A., C. Casas, U. Mühlenhoff, C. H. Lilling, and E. Herrero. 2008. Saccharomyces cerevisiae Grx6 and Grx7 are monothiol glutaredoxins associated with the early secretory pathway. Eukaryot. Cell 7:1415-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Kim, H. G., B. C. Kim, E. H. Park, and C. J. Lim. 2005. Stress-dependent regulation of a monothiol glutaredoxin gene from Schizosaccharomyces pombe. Can. J. Microbiol. 51:613-620. [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. G., J. H. Kim, B. C. Kim, E. H. Park, and C. J. Lim. 2005. Carbon source dependent-regulation of a second gene encoding glutaredoxin from the fission yeast Schizosaccharomyces pombe. Can. J. Microbiol. 32:15-24. [DOI] [PubMed] [Google Scholar]
- 28.Kumánovics, A., O. Chen, L. Li, D. Bagley, E. Adkins, H. Lin, N. N. Dingra, C. E. Outten, G. Keller, D. Winge, D. Ward, and J. Kaplan. 2008. Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron-sulfur cluster synthesis. J. Biol. Chem. 283:10276-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, H., T. D. T. Mapolelo, N. N. Dingra, S. G. Naik, N. S. Lees, B. M. Hoffman, P. J. Iggs-Gelasco, B. H. Huynh, M. K. Johnson, and C. E. Outten. 2009. The yeast iron regulatory proteins Grx3/Grx4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 48:9569-9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopreiato, R., S. Facchin, G. Sartori, G. Arrigoni, S. Casonato, M. Ruzzene, L. A. Pinna, and G. Carignani. 2004. Analysis of the interactions between piD261/Bud32, an evolutionarily conserved protein kinase of Saccharomyces cerevisiae, and the Grx4 glutaredoxin. Biochem. J. 377:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonagh, B., and D. Sheehan. 2008. Effects of oxidatived stress on protein thiols and disulphides in Mytilys edulis revealed by proteomics: actin and protein disulphide isomerase are redox targets. Mar. Environ. Res. 66:193-195. [DOI] [PubMed] [Google Scholar]
- 32.Mesecke, N., A. Spang, M. Deponte, and J. M. Herrmann. 2008. A novel group of glutaredoxins in the cis-Golgi critical for oxidative stress resistance. Mol. Biol. Cell 19:2673-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer, Y., B. B. Buchanan, F. Vignols, and J. P. Reichheld. 2009. Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43:335-367. [DOI] [PubMed] [Google Scholar]
- 34.Molina, M. M., G. Bellí, M. A. de la Torre, M.-A. Rodríguez-Manzaneque, and E. Herrero. 2004. Nuclear monothiol glutaredoxins of Saccharomyces cerevisiae can function as mitochondrial glutaredoxins. J. Biol. Chem. 279:51923-51930. [DOI] [PubMed] [Google Scholar]
- 35.Moseley, B., and B. L. Goode. 2006. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70:605-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojeda, L., G. Keller, U. Mühlenhoff, J. C. Rutherford, R. Lill, and D. R. Winge. 2006. Role of glutaredoxin-3 and glutaredoxin-4 in the iron-regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae J. Biol. Chem. 281:17661-17669. [DOI] [PubMed] [Google Scholar]
- 37.Petkova, M. I., N. Pujol-Carrion, J. Arroyo, J. García-Cantalejo, and M. A. de la Torre-Ruiz. 2010. Mtl1 is required to activate general stress response through Tor1 and Ras2 inhibition under conditions of glucose starvation and oxidative stress. J. Biol. Chem. 285:19521-19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruyne, D., M. A. Legesse, L. Gao, Y. Dong, and A. Bretscher. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20:559-591. [DOI] [PubMed] [Google Scholar]
- 39.Pujol-Carrion, N., G. Bellí, E. Herrero, A. Nogues, and M. A. de la Torre-Ruiz. 2006. Glutaredoxins Grx3 and Grx4 regulate nuclear localization of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J. Cell Sci. 119:4554-4564. [DOI] [PubMed] [Google Scholar]
- 40.Pujol, N. C. Bonet, F. Vilella, M. I. Petkova, A. Mozo-Villarías, and M. A. de la Torre-Ruiz. 2009. Two proteins from Saccharomyces cerevisiae: Pfy1 and Pkc1, play a dual role in activating actin polymerization and in increasing cell viability in the adaptive response to oxidative stress. FEMS Yeast Res. 9:1196-1207. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Manzaneque, M. T., J. Ros, E. Cabiscol, A. Sorribas, and E. Herrero. 1999. Grx5 plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:8180-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schott, D., T. Huffaker, and A. Bretscher. 2002. Microfilaments and microtubules: the news from yeast. Curr. Opin. Microbiol. 5:564-574. [DOI] [PubMed] [Google Scholar]
- 43.Shvetson, A., R. Musib, M. Phillips, P. A. Rubenstein, and E. Reisler. 2002. Locking the hydrophobic loop 262-274 to G-actin surface by a disulfide bridge prevents filament formation. Biochemistry 41:10787-10793. [DOI] [PubMed] [Google Scholar]
- 44.Torres, J., C. J. Di Como, E. Herrero, and M. A. de la Torre-Ruiz. 2002. Regulation of the cell integrity pathway by rapamycin sensitive TOR function in budding yeast. J. Biol. Chem. 277:42495-42504. [DOI] [PubMed] [Google Scholar]
- 45.Vilella, F., R. Alves, M. T. Rodriguez-Manzaneque, G. Bellí, S. Swaminathan, S. Sunnerhagen, and E. Herrero. 2004. Evolution and cellular function of monothiol glutaredoxins: involvement in iron-sulphur cluster assembly. Comp. Funct. Genom. 5:328-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilella, F., E. Herrero, J. Torres, and M. A. de la Torre-Ruiz. 2005. Pkc1 and the elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280:9149-9159. [DOI] [PubMed] [Google Scholar]
- 47.Wang, J., E. S. Boja, W. Tan, E. Tekle, H. M. Fales, S. English, J. J. Mieyal, and P. B. Chock. 2001. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 276:47763-47766. [DOI] [PubMed] [Google Scholar]
- 48.Weiner, M. P., and G. L. Costa. 1995. Rapid PCR-site directed mutagenesis. PCR Methods Appl. 4:S131-S136. [DOI] [PubMed] [Google Scholar]
- 49.Wink, D. A., C. B. Wink, R. W. Nims, and P. C. Ford. 1994. Oxidizing intermediates generated in the Fenton reagent: kinetic arguments against the intermediacy of the hydroxyl radical. Environ. Health Perspect. 112:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witte, S., M. Villalba, K. Bi, Y. Liu, N. Isakov, and A. Altman. 2000. Inhibition of the c-Jun N-terminal kinase/AP-1 and NF-κB pathways by PICOT, a novel protein kinase C-interacting protein with a thioredoxin homology domain. J. Biol. Chem. 275:1902-1909. [DOI] [PubMed] [Google Scholar]
- 51.Wood, V. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi-Iwai, Y., R. Ueta, A. Fukunaka, and R. Sasaki. 2002. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 277:18914-18918. [DOI] [PubMed] [Google Scholar]