Abstract
A versatile natural transformation protocol was established for and successfully applied to 18 of the 19 Streptococcus thermophilus strains tested. The efficiency of the protocol enables the use of in vitro-amplified mutagenesis fragments to perform deletion or insertion of large genetic fragments. Depending on the phenotype linked to the mutation, markerless mutants can be selected either in two steps, i.e., resistance marker insertion and excision using an adapted Cre-loxP system, or in one step using a powerful positive screening procedure as illustrated here for histidine prototrophy.
Streptococcus thermophilus is of major importance for the food market. The improvement of its industrially relevant properties is thus a main concern for dairy manufacturers. Since this step necessarily requires a deeper knowledge of the genetics of S. thermophilus in relation to its metabolism, there is a need to develop versatile and efficient tools for mutagenesis (genetic insertion/deletion and point mutation) for this species. Until now, they have classically included a DNA electrotransformation procedure (21, 24) and the use of thermosensitive pG+host-derivative plasmids that enable targeted mutagenesis by double homologous recombination (4, 9, 26). However, the low and variable electrotransformation frequencies obtained with existing protocols is a limiting factor in the manipulation of S. thermophilus (21, 24). This has been attributed to the existence of strain-specific host defense systems such as DNA restriction/modification (13, 22) and active CRISPR systems (2, 16). In addition, plasmid-based mutagenesis is time-consuming: it requires the cloning of recombination fragments, steps for double recombination with the target chromosome, and a tedious screening procedure to select the expected double recombination events (4). The latter steps can be quicker if a selectable marker is inserted between the recombination fragments, but this can lead to unwanted polar effects on downstream genes.
Recently, S. thermophilus was shown to be able to naturally acquire exogenous DNA and to stably maintain it in its genome by entering a physiological state known as “competence for transformation” (5, 8, 11). Natural transformation has been shown previously to facilitate the mutagenesis of streptococcal species since it allows the use of PCR products as recombinant DNA, avoiding the time-consuming steps of cloning into vectors (17, 27). The method used to select the mutant after natural transformation depends on the efficiency of competence development. For example, almost the entire S. pneumoniae population is transformable, which allows a simple PCR screening approach for the selection of transformants (17). For S. mutans, the lower competence rate (1%) led to the development of a two-step procedure based on the insertion and subsequent excision of a resistance marker using the Cre-loxP system (1). For S. thermophilus, some transformation and selection procedures have been proposed previously (6, 8). However, they were tested on a limited number of strains, i.e., strains LMD-9, LMG18311, and CNRZ1066, making them difficult to be retained as the most efficient and versatile techniques.
The objective of our study was to use natural transformation as a tool for the development of a versatile marker-free procedure for site-directed genetic modification in S. thermophilus. In a first step, we evaluated the efficiencies of different procedures to induce natural transformation with a large sample of strains. Nineteen strains were selected to represent the diversity existing within the S. thermophilus species: they exhibit unique combinations of industrially relevant properties (texturing and fast acidification) and can be divided into 11 clusters based on their CRISPR1 genetic contents (16). In a second step, we validated the use of PCR products as mutagenic DNA. Two-step and one-step strategies for genetic deletion and insertion, respectively, were tested with the model strains LMD-9 and LMG18311. The chosen genetic target was the histidine biosynthesis locus (his). The main reasons are (i) the relatively large size of the locus (10 genes; 7.348 kb), (ii) the strain-specific occurrence of the locus, and (iii) its associated phenotype, which allows the use of either positive (histidine prototrophy) or negative (histidine auxotrophy) screening procedures.
Plasmid-based systems for competence induction are restricted to a narrow range of strains.
For streptococcal species, the key step of competence development is the transcriptional induction of comX. It encodes the alternative sigma factor σX, which positively regulates genes required for DNA transformation and homologous recombination with the recipient genome (18). We compared the abilities of two comX expression plasmids to turn on competence in S. thermophilus. The pXL plasmid contains a fusion between the BlpCHR-regulated promoter PblpU and the comX gene from strain LMG18311 (comXLMG18311), which enables the inducible expression of comXLMG18311. This plasmid was shown previously to induce competence for natural transformation in strain LMG18311 grown in a complex culture medium (5). The constitutive expression system pMGX was constructed by cloning comXLMG18311 and its translation signals under the control of the constitutive P32 promoter of Lactococcus lactis into pMG36eT (10), yielding plasmid pMGX (Table 1).
TABLE 1.
Primers and plasmids used in this study
| Plasmid or primer | Relevant features or sequencea (5′ to 3′) | Reference(s) or target |
|---|---|---|
| Plasmids | ||
| pXL | Emr; pTRKH2 derivative containing a PblpU-comXLMG18311 fusion | 5 |
| pMGX | Emr; pMG36eT derivative containing a P32-comXLMG18311 fusion; comX was amplified with primers MX1/MX2 and cloned between the SmaI and SalI sites of pMG36eT | This study and reference 10 |
| pGIUD0855cat | Cmr Apr; pUC18 derivative containing P32-cat flanked by 1-kb fragments corresponding to the upstream and downstream regions of stu0855 | This study |
| pComECcat | Cmr Apr; pCR2.1-TOPO derivative allowing the insertion of P32-cat at the comEC locus | This study and reference 5 |
| pGhostcre | Emr RepA(Ts); pG+host9 derivative containing a P1144-cre fusion and the pepN transcriptional terminator from pNZ5348 between HindIII and KpnI sites | This study and reference 19 |
| Primers for construction of mutagenesis PCR products | ||
| Uplox66 | TAAGGAAGATAAATCCCATAAGG | lox66-P32-cat-lox71b |
| DNlox71 | TTCACGTTACTAAAGGGAATGTA | lox66-P32-cat-lox71b |
| UpHis1 | TTATGTCTTGGCCCTTGTCAAGGATTTGGG | his operon |
| UpHis2 | CCTTATGGGATTTATCTTCCTTATTCAATCTTTCGTAATCCTTT | his operon |
| DNHis1 | TACATTCCCTTTAGTAACGTGAAAAAGCAATGTTCATGACC | his operon |
| DNHis2 | TTTACTAGTCCCAGATGCACGCATACGACG | his operon |
| Primers for validation of recombinant strains | ||
| ChHis3 | TAAGTTGGAGTATGCTGTTGGTCGTGTGGACGC | his operon |
| ChHis4 | TTATCTTTAGCCTTGACCAATTCTTGTGAGGCC | his operon |
| IDHis1 | CTACCGAGTTAACAGCGGTTGGTTGGGC | his operon |
| IDHis2 | TTGGTCCTCAAAACCAGCCTCCAACTG | his operon |
| Chstu0855A | GGTATTGATCCCGAATTCAGATGTTTGTAG | stu0855 |
| Chstu0855B | GGCTGGATGGCATAACCGAGCTTTTGTTTC | stu0855 |
| Primers for sequence amplification for plasmid construction | ||
| Up0855KA | GGGGTACCATCCGTAAAACCATCAAATCTT | stu0855 |
| Up0855XB | CCGCTCGAGAACCCCAAGCAAAAAGTGGA | stu0855 |
| P32catXA | CCGCTCGAGGTACCATGCAGTTTAAATTCG | P32-catc |
| P32catXB | CCGCTCGAGTACAGTCGGCATTATCTCATA | P32-catc |
| DN0855XA | CCGCTCGAGTAATGTTTGGTGTAGGTAATA | stu0855 |
| DN0855SB | TCCCCCGGGTTAAACCAATCTGTCCTCGGG | stu0855 |
| MX1 | TATCCCGGGTATTATTGGAGGTTGAATGGAACAA | comX |
| MX2 | ACGCGTCGACTTCCTTGTATACTGGCTTCTA | comX |
Restriction sites introduced in the primers are underlined. Emr, Cmr, and Apr, erythromycin, chloramphenicol, and ampicillin resistance; Ts, temperature sensitive.
The PCR product obtained with primer pair Uplox66/DNlox71 was used to create all chimeric PCR fragments required for mutant construction. The Uplox66/DNlox71 primer set hybridized on pNZ5319 (19).
The P32catXA/P32catXB primers were used to amplify P32-cat from pGIZ850 (12).
The first step consisted of the introduction of both comX expression systems into the 17 selected strains and laboratory strains LMD-9 and LMG18311 by electroporation as described previously (5). We managed to transform 15 and 7 of 19 strains with pXL and pMGX, respectively (Table 2). Seven strains were permissive of electrotransformation with both pXL and pMGX, while four were recalcitrant. However, the pG+host9 vector was successfully introduced into the latter strains, indicating that electroporation failure was not due to poor quality of competent cells. The narrower host range of pMGX than of pXL could be explained by the different nature of its replication origin (pWV01 instead of pAMβ1), by the lack of target sequences for endogenous defense systems, or by a possible toxic effect due to strong and constitutive ComX production.
TABLE 2.
Electroporation test results and natural transformation rates for S. thermophilus strains
| Straina | Phenotypeb | Electroporation test result for plasmidc: |
Natural transformation rated for comX overexpression strain carrying plasmid: |
Natural transformation rated for strain in the presence of: |
||||
|---|---|---|---|---|---|---|---|---|
| pG+host9 | pXL | pMGX | pXL | pMGX | 0 μM ComS17-24 | 1 μM ComS17-24 | ||
| LMG18311 | F−, T+ | NT | + | + | 8.8 × 10−4 (−3.5 ± 0.8) | 7.7 × 10−3 (−2.7 ± 1.1) | 3.4 × 10−6 (−5.5 ± 0.3) | 1.3 × 10−1 (−0.9 ± 0.3) |
| LMD-9 | F+, T− | NT | + | + | 8.8 × 10−6 (−5.1 ± 0.2) | 5.9 × 10−5 (−4.4 ± 0.5) | 1.7 × 10−3 (−3.0 ± 0.5) | 5.4 × 10−3 (−2.5 ± 0.6) |
| DGCC7790 | F−, T+ | NT | + | + | 2.7 × 10−2 (−2.6 ± 1.3) | 5.9 × 10−5 (−5.0 ± 1.5) | ND | 4.3 × 10−2 (−2.1 ± 1.5) |
| DGCC7710 | F+, T+ | NT | + | + | NDe | 6.1 × 10−3 (−2.2 ± 0.2) | 6.7 × 10−7 (−6.3 ± 0.6) | 1.0 × 10−2 (−2.2 ± 0.5) |
| DGCC7853 | F+, T− | NT | + | + | ND | 8.4 × 10−4 (−3.6 ± 1.2) | 2.2 × 10−4 (−3.7 ± 0.3) | 4.9 × 10−4 (−3.3 ± 0.1) |
| DGCC7879 | F+, T− | NT | + | + | ND | 2.3 × 10−3 (−2.6 ± 0.4) | 8.1 × 10−7 (−6.3 ± 0.5) | 8.6 × 10−3 (−2.1 ± 0.2) |
| DGCC715 | F+, T− | NT | + | + | ND | 1.6 × 10−2 (−1.9 ± 0.3) | ND | 1.2 × 10−2 (−1.9 ± 0.2) |
| DGCC7773 | F−, T+ | NT | + | − | 1.5 × 10−2 (−1.9 ± 0.3) | ND | 1.5 × 10−1 (−1.0 ± 0.4) | |
| DGCC7785 | F−, T+ | NT | + | − | 2.5 × 10−4 (−3.7 ± 0.4) | ND | 4.1 × 10−5 (−4.5 ± 0.4) | |
| DGCC7796 | F+, T− | NT | + | − | 2.5 × 10−2 (−1.9 ± 0.7) | 3.1 × 10−6 (−5.5 ± 0.2) | 6.9 × 10−2 (−1.3 ± 0.4) | |
| DGCC7854 | F+, T− | NT | + | − | 6.9 × 10−5 (−4.5 ± 0.7) | 1.1 × 10−7 (−7.2 ± 0.6) | 5.3 × 10−3 (−2.5 ± 0.7) | |
| DGCC7891 | F−, T+ | NT | + | − | 1.4 × 10−4 (−4.1 ± 0.5) | ND | 1.4 × 10−4 (−4.2 ± 0.7) | |
| DGCC782 | F+, T− | NT | + | − | ND | ND | 2.0 × 10−3 (−2.8 ± 0.4) | |
| DGCC7666 | F−, T− | NT | + | − | ND | 1.8 × 10−6 (−5.9 ± 0.4) | 4.2 × 10−2 (−1.4 ± 0.2) | |
| DGCC7694 | F−, T− | NT | + | − | ND | ND | 3.9 × 10−2 (−1.5 ± 0.3) | |
| DGCC3367 | F+, T− | + | − | − | 4.7 × 10−7 (−6.5 ± 0.5) | 1.8 × 10−3 (−2.8 ± 0.3) | ||
| DGCC7809 | F+, T− | + | − | − | ND | 5.8 × 10−4 (−3.5 ± 0.6) | ||
| DGCC7909 | F−, T+ | + | − | − | ND | 4.5 × 10−3 (−2.4 ± 0.1) | ||
| DGCC7984 | F+, T+ | + | − | − | ND | ND | ||
All strains are from the Danisco collection, except strains LMD-9 (from the ATCC) and LMG18311 (from the LMG Collection).
F+ and F− refer to fast and slow acidification, respectively, by strains grown in pasteurized milk. T+ and T− refer to the presence and absence, respectively, of texturing properties of strains grown in pasteurized milk according to sensory analyses.
NT, not tested. + indicates that electrotransformants could be obtained under a range of conditions. − indicates that no electrotransformants could be obtained under a range of conditions.
Calculated as the ratio of transformants (chloramphenicol-resistant CFU) to the total CFU count per 1 μg of DNA (arithmetic mean of three independent experiments). Geometric means±standard deviations expressed in log10 are provided in parentheses.
ND, not detected (the transformation rate was below the detection limit of the test [<1×10−8]).
Natural transformation was then assessed with the following protocol. Each pXL- or pMGX-containing strain was grown for 6 h at 37°C in 300 μl of Todd-Hewitt broth (THB) medium supplemented with 1% glucose, 2.5 μg/ml erythromycin, and 1 μg plasmid (pGIUD0855cat) DNA. Mature BlpC from strain LMD-9 (BlpCLMD-9; 250 ng/ml of the D9C19 form) (9) was added to cultures of pXL-containing strains to induce comXLMG18311 expression. Plasmid pGIUD0855cat is a pUC18 derivative containing a chloramphenicol resistance cassette flanked by 1-kb fragments corresponding to the upstream and downstream regions of stu0855 (Table 1). This plasmid is unable to replicate in S. thermophilus, and upon natural transformation, it promotes the genetic replacement of stu0855 by P32-cat through homologous recombination. The presence of this locus in all selected strains was checked by PCR prior to further experiments. Transformation rates were calculated as the ratio of transformants (chloramphenicol-resistant CFU) to the total CFU count (Table 2). Constitutive and inducible comX expression systems yielded transformants for 7 of 7 and 8 of 15 strains, respectively, which indicates that pMGX is a more versatile system than pXL to induce competence. This was particularly clear when we compared efficiencies in the same genetic background: 4 of 7 of pXL-carrying strains were not transformable. We also found that transformation rates were highly strain dependent: between 10−2 and 10−5 with pMGX and between 10−2 and 10−6 with pXL. For pXL-bearing strains, poor or no transformability could be directly correlated with the poor level of comX expression. Indeed, we measured no or low luminescence driven from the PcomGA-luc reporter fusion carried by pXL, whose activity is dependent on the amount of ComX (comGA is a late competence gene) (5) (data not shown). This finding suggests that endogenous Blp regulatory systems that control comX expression are impaired in these strains.
Altogether, these results demonstrate that plasmid systems are not appropriate candidates to develop a universal and efficient competence induction procedure for S. thermophilus; their versatility is limited by (i) the electroporation efficiency (for the pMGX system) and (ii) the genetic background of the host strain (for the pXL system).
Addition of mature ComS induces competence in 18 of 19 S. thermophilus strains tested.
In S. thermophilus, induction of comX was shown recently to rely on the activation of a quorum-sensing system involving the transcriptional regulator ComR and its dedicated pheromone ComS. The current model proposes that ComS is secreted, matured, and reimported into the cells to interact with and activate ComR, leading to the binding of ComR to the comX promoter (8). Strain LMD-9 was shown previously to be naturally transformable in chemically defined medium (CDM) (11), while addition of synthetic heptapeptides corresponding to the C-terminal part of ComS (ComS18-24) was necessary to activate the ComRS systems of strains LMG18311 and CNRZ1066 (8). The same experiment was performed using the selected sample of strains with addition of the octapeptide ComS17-24 (8). This peptide has inducing properties equivalent to those of ComS18-24, but its activity is more stable over time (data not shown). Strains were grown at 37°C in 300 μl CDM, and after 1.5 h, 1 μM ComS17-24 was added to half of each culture. Plasmid pGIUD0855cat (1 μg) was used as donor DNA. Strains LMD-9 and LMG18311 were used as controls. The results presented in Table 2 show that in the absence of ComS17-24, most strains are either not competent (10 of 19 strains) or poorly competent (7 of 19 strains) (Table 2). Remarkably, supplementation with ComS17-24 renders 18 of the 19 strains tested highly transformable: rates between 10−2 and 10−4 were measured for most strains. Furthermore, strains LMG18311 and DGCC7773 developed rates of 10−1, which is comparable to that for Bacillus subtilis (14). These results show that production of a sufficient amount of ComS is critical to develop the competence state. Only one strain, DGCC7984, yielded no chloramphenicol-resistant transformants. The same result was obtained with plasmids pComECcat, which promotes the insertion of P32-cat at the comEC locus (data not shown) (Table 1). The sequences of ComR and ComS from strain DGCC7984 differ only at positions 239 (K239N) and 15 (A15P), respectively, from their counterparts from strain LMG18311. However, the substitutions do not account for the competence-negative phenotype of strain DGCC7984 since they were also found in the transformable strain DGCC7710. The presence of additional mutations that impaired natural transformation, at the level of comX induction and/or DNA processing, cannot be excluded. In conclusion, ComS supplementation can be regarded as an efficient procedure to transform S. thermophilus.
Two-step procedure for genetic modification without acquisition of a positively screenable phenotype.
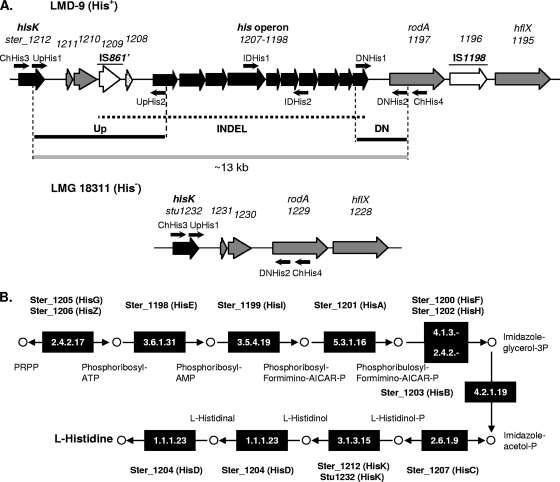
The choice of a markerless mutagenesis and screening procedure does not depend only on the natural transformation efficiency; it can also be influenced by the nature of the phenotype associated with the mutation. To create mutant phenotypes that cannot be positively selected, which is typical of genetic deletions, we developed a two-step mutagenesis procedure. For this purpose, we tested the possibility of performing allelic exchange of the his locus with a resistance marker by using chimeric PCR products as transforming DNA. The functionality of the improved Cre-loxP site-specific recombination system for marker excision (19) was then evaluated. For this purpose, a cre expression vector for S. thermophilus, pGhostcre, was constructed (Table 1). A HindIII-KpnI fragment from pNZ5348 (19) that encompasses the P1144-cre fusion and the pepN transcriptional terminator was cloned into the thermosensitive and erythromycin-selectable pG+host9 vector (20). Strain LMD-9 contains all histidine biosynthesis genes (Fig. 1) (15). They are organized into two loci: the putative histidinol phosphatase gene hisK (ster_1212) and the ster_1198-ster_1207 cluster (Fig. 1A). To create a His− strain, we decided to delete the ster_1198-ster_1207 cluster (10 genes; 7.348 kb).
FIG. 1.
Histidine biosynthesis in S. thermophilus. (A) Schematic representations of the hisK-hflX gene cluster of S. thermophilus LMD-9 (upper panel) and that of LMG18311 (lower panel). His+ and His− (in parentheses) indicate the histidine prototrophy and auxotrophy of strains LMD-9 and LMG18311, respectively. Genes encoding proteins are represented by thick arrows as follows: arrows for genes involved in histidine biosynthesis (the his operon and the putative histidinol phosphatase gene ster_1212 or stu1232) are black, arrows for insertion sequences are white, arrows for genes conserved between LMD-9 and LMG18311 are gray, and the arrow for a gene of unknown function unique to LMD-9 (ster_1208) is dotted. The dotted line in the upper panel delimits a potential insertion/deletion event (INDEL). The following elements are indicated: the names of the insertion sequences (underlined), the region amplified by PCR to transfer the histidine prototrophy from LMD-9 to LMG18311 (thick gray line), the Up and DN recombination fragments used to delete the his operon of LMD-9 (thick black lines), and the primers used to amplify the Up/DN regions and to validate the identities of His+ and His− recombinant strains (thin black arrows). (B) l-Histidine biosynthesis pathway and respective contributions of S. thermophilus his genes. The EC numbers of the enzymes catalyzing the reactions from phosphoribosyl pyrophosphate (PRPP) to l-histidine are black boxed. The corresponding gene products from strains LMD-9 and LMG18311 are indicated in bold at the top or bottom of each box. The His nomenclature of the enzymes is indicated in parentheses. The intermediate metabolites are indicated and represented by open circles. AICAR, aminoimidazole carboxamide ribonucleotide.
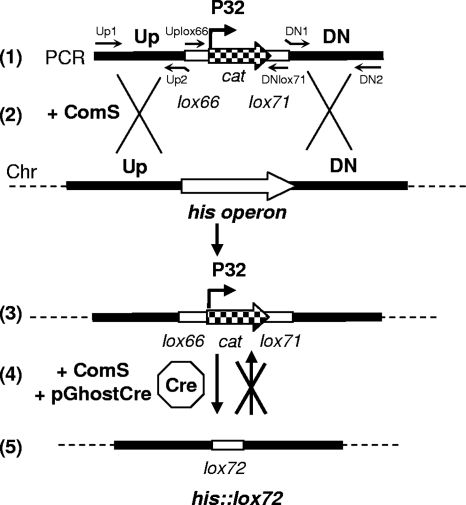
The cassette allowing in-frame exchange of the his locus with the chloramphenicol marker was assembled in vitro by overlapping PCR as described previously (8). This cassette consists of a central lox66-P32-cat-lox71 fragment flanked by sequences corresponding to the upstream (Up) and downstream (DN) regions of the his locus (Fig. 1A and Fig. 2). Primers UpHis1/UpHis2 and DNHis1/DNHis2 (Fig. 1A) used to amplify the recombination regions are listed in Table 1. lox66 and lox71 are mutated loxP sites that create the doubly mutated lox72 site, which has a reduced affinity for the Cre recombinase, after site-specific recombination (Fig. 2 illustrates the general strategy) (1, 19). To obtain the marker-free ster_1198-ster_1207::lox72 strain, LMD-9 underwent two steps of natural transformation in CDM supplemented with ComS17-24. First, 25 ng of the chimeric PCR products was used as donor DNA, which yielded the Cmr ster_1198-ster_1207::lox66-P32-cat-lox71 strain (transformation rate, 2.6 × 10−5). The allelic substitution was confirmed by PCR analysis (using primers ChHis3/ChHis4 [Table 1]) and NcoI restriction of PCR products. Second, the strain was transformed with 1 μg of pGhostcre. Emr colonies were recovered after 48 h of incubation at 29°C (mean transformation rate, 1 × 10−4) and transferred onto solid medium in the presence or absence of chloramphenicol. Cre recombinase was expressed during incubation at 29°C since 100% of pGhostcre-containing His− colonies were Cms (50 colonies were tested). Recombination and loss of P32-cat was also confirmed by PCR (using primers Uplox66 and DNlox71). As a final step, Emr Cms colonies were cured of the pGhostcre plasmid by growth at 37°C (nonpermissive for pGhostcre replication) in the absence of antibiotic as described previously (1). After 16-h culture, 100% of ster_1198-ster_1207::lox72 colonies were Ems (50 colonies were tested). Finally, phenotypic tests were performed to validate the biological effect of the mutagenesis performed. To check histidine auxotrophy, we compared the growth kinetics of wild-type (LMD-9) and ster_1198-ster_1207::lox72 (LMD-9 His−) strains in the presence and absence of histidine. Results showed that they grow similarly in complete CDM (Fig. 3A) and that the ster_1198-ster_1207::lox72 (LMD-9 His−) strain, solely, has a clear growth defect in CDM lacking histidine (Fig. 3B).
FIG. 2.
Schematic representation of the markerless deletion procedure developed for S. thermophilus. (1) In vitro construction of mutagenesis PCR products. They consist of 3 individual PCR fragments joined together by overlapping extension: the upstream region (obtained using primers generically named Up1/Up2) and the downstream region (obtained using primers generically named DN1/DN2) of the target gene/locus, separated by a lox66-P32-cat-lox71 fragment (obtained using primers Uplox66/DNlox71). (2) S. thermophilus cells growing in ComS-supplemented CDM are naturally transformed with chimeric PCR fragments. (3) The target gene/locus is replaced by the lox66-P32-cat-lox71 cassette through double homologous recombination. The transformants are selected on chloramphenicol-containing M17 medium. (4) Mutants from step 3 are naturally transformed with plasmid pGhostcre by growing cells in ComS-supplemented CDM. Site-specific recombination between lox66 and lox71 by Cre recombinase (represented by a polygon) promotes P32-cat excision. This step is performed by growing cells at 29°C in the absence of chloramphenicol. (5) The lox72 site created has reduced affinity for Cre, which prevents the reintroduction of the cassette. The following elements are represented: primer pairs used to separately amplify the Up, DN, and lox66-P32-cat-lox71 fragments (thin black arrows); the target gene/locus (white arrow); cat (checkered arrows); the chromosome (Chr; dotted lines); lox sites (thick white lines); recombination fragments (thick black lines); and recombination events (crossed lines).
FIG. 3.
Growth (expressed as the optical density at 600 nm [OD600]) of wild-type (WT) strains LMD-9 and LMG18311 and their respective His− and His+ derivatives. (A and B) Growth in CDM (A) and CDM lacking histidine (B) of the LMD-9 WT, LMD-9 His− (ster_1198-ster_1207::lox72) (3 clones), the LMG18311 WT, and LMG18311 His+ PCR (3 clones). (C and D) Growth in CDM (C) and CDM lacking histidine (D) of the LMD-9 WT, the LMG18311 WT, and LMG18311 His+ Ch (3 clones). Growth was monitored at 10-min intervals with the Varioskan Flash multimode reader (Thermo Fisher Scientific, Zellic, Belgium) as described previously (8). Mean values calculated from triplicates are presented for WT strains.
In conclusion, the efficiency of our natural transformation protocol allows the combined use of chimeric PCR products and the Cre-loxP system to produce large markerless deletions (7.278 kb in the case of the his locus). This strategy was successfully applied to other loci, such as lacZ and comRS (data not shown). The efficiency of the first step, i.e., allelic exchange, depends mostly on the yield of the overlapping PCR extension rather than on the sizes of the target loci (data not shown).
One-step procedure for genetic modification that leads to the acquisition of a positively screenable phenotype.
Compared to strain LMD-9, strain LMG18311 lacks homologues of ster_1198-ster_1207 (Fig. 1A), and it was shown previously to be a histidine auxotroph (25). Since insertion of the ster_1198-ster_1207 genes into strain LMG18311 should lead to the acquisition of a positively selectable phenotype, i.e., histidine prototrophy, a one-step mutagenesis protocol was attempted.
Two types of DNA were used as transforming DNA: a 13-kb PCR fragment amplified from strain LMD-9 (by using primer pair UpHis1/DNHis2) (Fig. 1A) and the whole LMD-9 chromosome. The PCR fragment contains the ster_1198-ster_1207 genes flanked by the Up and DN sequences, used previously to create strain LMD-9 His− (Fig. 1A). The 5′ end of the Up sequence and the 3′ end of the DN sequence will mediate double homologous recombination events between the PCR fragment and the LMG18311 chromosome since these sequences contain an ∼1-kb region that is homologous (with 99% identity) to stu1229 and stu1230-stu1231, respectively (Fig. 1A). Regarding the LMD-9 chromosome, the potentially homologous sequences (with 99% identity to LMG18311) allowing insertion of the his locus into the LMG18311 chromosome are longer than 10 kb (data not shown). The competence experiments were performed with ComS17-24-supplemented CDM in the presence of 200 ng unpurified PCR product or 45 μg total chromosomal DNA prepared as described previously (7). For both experiments, transformants having acquired the histidine prototrophy were selected on CDM lacking histidine. After 48 h of incubation at 37°C, we obtained 30 CFU/ml with PCR fragments and 3,840 CFU/ml with chromosomal DNA. The higher number of transformants obtained with the chromosomal DNA may be related to the longer regions available for homologous recombination (see above). The respective strains were designated LMG18311 His+ PCR and LMG18311 His+ Ch. The insertion of the ster_1198-ster_1207 locus between stu1299 and stu1230 in three His+ colonies from both strains was validated by PCR (using primer pairs IDHis1/IDHis2, ChHis3/ChHis4, ChHis3/IDHis2, and ChHis4/IDHis1) (Fig. 1A and Table 1). In addition, the identities of the transformants were also checked with primers that are specific to strain LMG18311. To study the functionality of his transfer, we measured the growth kinetics of strains LMG18311 His+ PCR and LMG18311 His+ Ch in complete CDM and in CDM lacking histidine. The results are presented in Fig. 3. In the presence of histidine, we measured no significant growth difference between the wild-type strain and the three LMG18311 His+ PCR clones (Fig. 3A). However, in the absence of histidine, the latter displayed growth similar to that in the presence of histidine while the wild-type strain was clearly growth deficient, which confirmed the histidine prototrophy of the LMG18311 His+ PCR clones (Fig. 3B). Similar results were obtained for strain LMG18311 His+ Ch, except that some growth disparity among clones of strain LMG18311 His+ Ch was observed (Fig. 3C and D and data not shown). This could indicate that their genetic contents are different. Indeed, we cannot exclude the possibility that, besides the his locus, LMG18311 His+ Ch isolates have acquired other LMD-9-specific loci. The corollary is that they could also have lost LMG18311-specific clusters. Genetic cotransfer during natural transformation has already been observed in S. mutans (3).
With the example illustrated above, we showed that an efficient natural transformation procedure combined with a powerful positive screen allows the construction of recombinant strains in one step without using any antibiotic resistance marker.
Concluding remarks.
The transformation protocol developed in this work is applicable to all S. thermophilus strains tested except one recalcitrant strain, and its efficiency for most strains is close to that for S. mutans (transformation rate, 1%). Consequently, quick and effective selection of transformants depends on the use of a powerful phenotypic screen. We showed that it can be based on either a two-step procedure consisting of the insertion and subsequent deletion of an antibiotic marker, similar to the procedure for S. mutans (1), or the acquisition/loss of metabolic properties. Blomqvist and coworkers recently proposed a markerless strategy based on colony hybridization with digoxigenin-labeled probes to select small genetic changes in S. thermophilus (6). However, it could turn out to be labor-intensive in the case of strains displaying a transformation rate of ≤10−4, which applies to 4 of 18 strains in our sample of competent strains. The reason for their poor transformability remains to be elucidated. We found no correlation with some metabolic properties (fast acidification and texturing). We cannot exclude that these strains lack some important competence determinants located either upstream or downstream of comX induction. Demonstration of competence in S. thermophilus is recent, and a better study of the factors involved and their regulation is thus a prerequisite to improve transformation protocols. In addition, it is interesting that our procedure for genetic modification is potentially applicable to S. salivarius, since this species also uses the ComRS system to control competence (8).
Natural transformation in a microorganism of importance for the food sector, like S. thermophilus, represents a unique opportunity for the industrial market. Indeed, natural transformation could be considered a self-cloning strategy as long as the DNA used, such as a total chromosome or a PCR product containing no intentionally introduced mutations, includes no recombinant DNA. This approach is excluded from the European legislation on genetically modified microbes (GMM) (23). The transfer of the histidine prototrophy between strains showed for the first time that it is possible to use natural transformation to insert large genetic fragments without altering the sequence of the donor DNA and without using any foreign non-S. thermophilus genes. This opens interesting perspectives to engineer novel non-GMM starter strains by combining industrially relevant traits such as fast acidification (corresponding to the prtS locus), texture production (corresponding to the eps locus), or phage resistance (corresponding to CRISPR spacers).
Acknowledgments
This research was carried out with financial support from Danisco and FNRS. C. Boutry holds a doctoral fellowship from FRIA. L. Fontaine is a postdoctoral researcher at FNRS. P. Hols is a research associate at FNRS.
We are grateful to E. Maguin for providing the pG+host9 vector, J. Kok for providing plasmid pMG36e, L. S. Håvarstein for providing plasmid pXL, and M. Kleerebezem for providing plasmids pNZ5319 and pNZ5348. We warmly thank P. Goffin for critically reading the manuscript.
Footnotes
Published ahead of print on 8 October 2010.
REFERENCES
- 1.Banerjee, A., and I. Biswas. 2008. Markerless multiple-gene-deletion system for Streptococcus mutans. Appl. Environ. Microbiol. 74:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrangou, R., C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, D. A. Romero, and P. Horvath. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709-1712. [DOI] [PubMed] [Google Scholar]
- 3.Biswas, I., L. Drake, S. Johnson, and D. Thielen. 2007. Unmarked gene modification in Streptococcus mutans by a cotransformation strategy with a thermosensitive plasmid. Biotechniques 42:487-490. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomqvist, T., H. Steinmoen, and L. S. Håvarstein. 2006. Natural genetic transformation: a novel tool for efficient genetic engineering of the dairy bacterium Streptococcus thermophilus. Appl. Environ. Microbiol. 72:6751-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomqvist, T., H. Steinmoen, and L. S. Håvarstein. 2010. A food-grade site-directed mutagenesis system for Streptococcus thermophilus LMG 18311. Lett. Appl. Microbiol. 50:314-319. [DOI] [PubMed] [Google Scholar]
- 7.Ferain, T., J. N. Hobbs, Jr., J. Richardson, N. Bernard, D. Garmyn, P. Hols, N. E. Allen, and J. Delcour. 1996. Knockout of the two ldh genes has a major impact on peptidoglycan precursor synthesis in Lactobacillus plantarum. J. Bacteriol. 178:5431-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontaine, L., C. Boutry, M. H. de Frahan, B. Delplace, C. Fremaux, P. Horvath, P. Boyaval, and P. Hols. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontaine, L., C. Boutry, E. Guedon, A. Guillot, M. Ibrahim, B. Grossiord, and P. Hols. 2007. Quorum-sensing regulation of the production of Blp bacteriocins in Streptococcus thermophilus. J. Bacteriol. 189:7195-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine, L., and P. Hols. 2008. The inhibitory spectrum of thermophilin 9 from Streptococcus thermophilus LMD-9 depends on the production of multiple peptides and the activity of BlpGSt, a thiol-disulfide oxidase. Appl. Environ. Microbiol. 74:1102-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardan, R., C. Besset, A. Guillot, C. Gitton, and V. Monnet. 2009. The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 191:4647-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffin, P., F. Lorquet, M. Kleerebezem, and P. Hols. 2004. Major role of NAD-dependent lactate dehydrogenases in aerobic lactate utilization in Lactobacillus plantarum during early stationary phase. J. Bacteriol. 186:6661-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guimont, C., P. Henry, and G. Linden. 1993. Restriction/modification in Streptococcus thermophilus: isolation and characterization of a type II restriction endonuclease Sth455I. Appl. Microbiol. Biotechnol. 39:216-220. [DOI] [PubMed] [Google Scholar]
- 14.Hamoen, L. W., G. Venema, and O. P. Kuipers. 2003. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology 149:9-17. [DOI] [PubMed] [Google Scholar]
- 15.Hols, P., F. Hancy, L. Fontaine, B. Grossiord, D. Prozzi, N. Leblond-Bourget, B. Decaris, A. Bolotin, C. Delorme, E. S. Dusko, E. Guedon, V. Monnet, P. Renault, and M. Kleerebezem. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 29:435-463. [DOI] [PubMed] [Google Scholar]
- 16.Horvath, P., D. A. Romero, A. C. Coute-Monvoisin, M. Richards, H. Deveau, S. Moineau, P. Boyaval, C. Fremaux, and R. Barrangou. 2008. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 190:1401-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannelli, F., and G. Pozzi. 2004. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Mol. Biotechnol. 26:81-86. [DOI] [PubMed] [Google Scholar]
- 18.Johnsborg, O., and L. S. Håvarstein. 2009. Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol. Rev. 33:627-642. [DOI] [PubMed] [Google Scholar]
- 19.Lambert, J. M., R. S. Bongers, and M. Kleerebezem. 2007. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl. Environ. Microbiol. 73:1126-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marciset, O., and B. Mollet. 1994. Multifactorial experimental design for optimizing transformation: electroporation of Streptococcus thermophilus. Biotechnol. Bioeng. 43:490-496. [DOI] [PubMed] [Google Scholar]
- 22.Mercenier, A. 1990. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol. Rev. 7:61-77. [DOI] [PubMed] [Google Scholar]
- 23.Official Journal of the European Communities. 2001. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Off. J. Eur. Communities L 106:1-38. [Google Scholar]
- 24.O'Sullivan, T. F., and G. F. Fitzgerald. 1999. Electrotransformation of industrial strains of Streptococcus thermophilus. J. Appl. Microbiol. 86:275-283. [DOI] [PubMed] [Google Scholar]
- 25.Pastink, M. I., B. Teusink, P. Hols, S. Visser, W. M. de Vos, and J. Hugenholtz. 2009. Genome-scale model of Streptococcus thermophilus LMG18311 for metabolic comparison of lactic acid bacteria. Appl. Environ. Microbiol. 75:3627-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renye, J. A., Jr., and G. A. Somkuti. 2009. Insertion of a heterologous gene construct into a non-functional ORF of the Streptococcus thermophilus chromosome. Biotechnol. Lett. 31:759-764. [DOI] [PubMed] [Google Scholar]
- 27.Weng, L., I. Biswas, and D. A. Morrison. 2009. A self-deleting Cre-lox-ermAM cassette, Cheshire, for marker-less gene deletion in Streptococcus pneumoniae. J. Microbiol. Methods 79:353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]