Abstract
Enzymatic lignocellulose hydrolysis plays a key role in microbially driven carbon cycling and energy conversion and holds promise for bio-based energy and chemical industries. Cellulases (key lignocellulose-active enzymes) are prone to interference from various noncellulosic substances (e.g., metal ions). During natural cellulolysis, these substances may arise from other microbial activities or abiotic events, and during industrial cellulolysis, they may be derived from biomass feedstocks or upstream treatments. Knowledge about cellulolysis-inhibiting reactions is of importance for the microbiology of natural biomass degradation and the development of biomass conversion technology. Different metal ions, including those native to microbial activity or employed for biomass pretreatments, are often tested for enzymatic cellulolysis. Only a few metal ions act as inhibitors of cellulases, which include ferrous and ferric ions as well as cupric ion. In this study, we showed inhibition by ferrous/ferric ions as part of a more general effect from oxidative (or redox-active) metal ions and their complexes. The correlation between inhibition and oxidation potential indicated the oxidative nature of the inhibition, and the dependence on air established the catalytic role that iron ions played in mediating the dioxygen inhibition of cellulolysis. Individual cellulases showed different susceptibilities to inhibition. It is likely that the inhibition exerted its effect more on cellulose than on cellulase. Strong iron ion chelators and polyethylene glycols could mitigate the inhibition. Potential microbiological and industrial implications of the observed effect of redox-active metal ions on enzymatic cellulolysis, as well as the prevention and mitigation of this effect in industrial biomass conversion, are discussed.
The microbial degradation of lignocellulosic biomass (derived mostly from plant cell walls) is important for carbon cycling, energy conversion, microbiota vitality, animal-microbe symbiosis, and other microbiological or ecological processes. In general, the biodegradation of lignocellulose takes place in highly heterogeneous environments (e.g., soil, sediment, swamp, marsh, or animal digestive tract), in which different microbes employ different biological ways (of a lytic, redox, synthetic, or other nature) to seek nutrients, compete with others, or fight off inactivation. Lignocellulolytic microbes use an array of specific hydrolases, lyases, oxidoreductases, and accessory enzymes to constitute enzymatic machineries able to effectively degrade or convert highly complex lignocellulosic substances (1, 23). In nature, these enzymes have to have not only high specificity and reactivity toward their substrates (targeted lignocellulosic substances) but also resilience toward inhibitors or inactivators derived from other biomass-targeting enzymatic reactions as well as other coexisting microbial or abiotic processes. The potential impact from redox chemistry, one of the important ecological or environmental properties of many microbiotas, might become significant because of two sets of microbial activities.
First, many microbes employ oxidoreductase-driven redox agents to degrade or modify lignin, a major plant cell wall substance that not only coexists with but also protects cellulose (from degradation by foreign agents). For instance, white rots secrete diffusible heme-containing peroxidases (lignin peroxidase, Mn peroxidase, and versatile peroxidase) to attack lignin, supported by H2O2 generated by secreted carbohydrate or alcohol oxidases and assisted by radicalized surface amino acid residues, dissociated Mn(III), or separate redox mediators (for a recent review, see reference 33). Brown rots secrete redox-active quinoids or Fe-chelating peptides, H2O2-generating carbohydrate or alcohol oxidase, and other oxidoreductases (including laccase) to modify lignin and degrade cellulose by OH radicals from Fenton-type chemistry (for recent reports, see references 1 and 41). These oxidoreductases and their redox metabolites, products, or mediators [e.g., Mn(III) and H2O2] might act on or affect cellulases (secreted by the same or different lignocellulolytic microbes) or their lignocellulose hydrolysis.
Second, all microbial life cycles depend on redox chemistry, and many general microbial processes are of a redox nature. Based on their final electron acceptors (or cellular respiration modes), microbes can be grouped as aerobes (able to reduce O2 to H2O), nitrate reducers or denitrifiers (able to reduce NO3− to N2), Mn(IV) reducers [able to reduce MnO2 to Mn(II)], Fe(III) reducers [able to reduce Fe2O3 or FeO(OH) to Fe(II)], sulfate reducers (able to reduce SO42− or S to HS−), or methanogens (able to reduce CO2 to CH4). Among them, the Mn(IV) and Fe(III) reducers, as well as general anoxygenic photosynthesis, can generate diffusible redox-active mediators and Mn or Fe species. The spatial and temporal distribution of different microbes or respiration modes, and, consequently, redox environments or an abundance of redox-active agents, in microbiota is subjected to air O2 availability and geochemical conditions (for recent reviews, see references 4 and 34). When exposed to air, aerobic cellulolytic microbes (mostly fungi) degrade cellulose by coupling the process to their respiration. Local anoxicity/anaerobicity may be formed due to fast metabolisms of these and other aerobes, thus allowing anaerobic cellulolytic microbes (mostly bacteria) to degrade cellulose. Aerobic cellulolytic microbes may border with, and anaerobic cellulolytic microbes may live within, noncellulolytic anaerobes (facultative or obligate and saccharolytic or syntrophic) relying on non-O2 respirations. Consequently, metabolites or products (including redox-active agents) from the noncellulolytic anaerobes might contact and affect cellulolytic microbes, their cellulases, or targeted lignocelluloses as part of a more general cohabitation, collaboration, or competition among different cellulolytic and noncellulolytic species (for a recent review, see reference 23).
Biomass-active microbes and enzymes, as well as natural microbial biomass degradation processes, are highly attractive for the emerging biomass conversion/utilization industry (biorefinery). Three of the major challenges faced by the biomass conversion research and development efforts are the general recalcitrance of biomass, heterogeneity of common feedstocks, and inhibition of specific transformation reactions by coexisting nonsubstrate substances. In general, the structural and compositional complexity of biomass makes the separation of its components and subsequent transformation into valuable chemicals difficult. For effectively and efficiently converting cellulose (one of the main targets of biomass conversion), one needs to not only optimize the efficacy of cellulases (cellulolytic biocatalysts) but also minimize the detrimental effects of various cellulose derivatives and noncellulosic substances originating from biomass feedstock or upstream treatments (for recent reviews, see references 11, 13, 22, 41, and 42).
Biomass (as either a direct substrate for natural microbial biomass degradation or feedstock for industrial bioalcohol and other chemical productions) is in general highly heterogeneous and complex, comprising cellulose, hemicellulose, lignin, protein, extractive, mineral, and other substances. Although carbohydrates are the main targets for the development of biomass-active enzymes, other minor biomass components are of interest as well, because they may affect the activity of biomass-active enzymes. Metal ions could be such effectors, since they exist universally in microbiota habitats [e.g., Ca(II), Al(III), and other metal ions adsorbed in soil or dissolved in water; Fe(III), Fe(II), Mn(IV), and Mn(II) from anaerobes' respiration and anoxygenic photosynthesis] and often serve as structural/functional cofactors [e.g., Ca(II), Zn(II), Fe(II), Mn(II), and Cu(II)] or inhibitors [e.g., Ag(I), Hg(II), and Pb(II)] of many enzymes, including cellulases. In corn stover, a major feedstock, iron oxide may account for 0.5% of the ash, and Mn may be present at 23 mg/kg of body weight (25). In nature, biomass-degrading microbial enzymes (e.g., cellulases and hemicellulases) are most likely in contact with various metal ions (including redox-active ones) related to other enzymatic, microbial, or abiotic processes. Some metal ions may also be present at significant levels in industrial enzymatic biomass-converting systems, after being added as reagents, dissolved from a reactor/pipeline, or brought from water or other raw material sources.
As part of cellulase characterization, common divalent metal ions [e.g., Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), and Hg(II)] as well as mono- or trivalent metal ions [e.g., Na(I), K(I), and Fe(III)] are often tested for their effect on cellulase activity (24). In general, Hg(II) behaves as a fatal cellulase inhibitor, likely because of its interaction with key sulfur-containing amino acid residues (as it does for other proteins). Among the divalent metal ions, most often only Cu(II) and Fe(II) are found to exert strong inhibition on cellulose-hydrolyzing reactions of cellobiohydrolase (CBH), endo-β-glucanase (EG), or β-glucosidase (BG) (for examples, see references 12, 15, and 30). The cellulase-inhibiting actions of Fe(II) and Cu(II), but not Mn(II), Co(II), Ni(II), and Zn(II) (all have the same 2+ charge and similar size), have not been well understood from a mechanistic aspect. In general, the effect of redox-active metal ions or other compounds on cellulases during natural microbial biomass degradation has not been extensively investigated.
To further probe the effects of Fe(II) and Cu(II), we carried out a study to examine the hypothesis that the inhibition of the reaction of cellulases by metal ions is of a redox nature. Various redox-active metal ions and complexes were tested comparatively, and a possible correlation between the redox and inhibitory properties of these substances was sought. The potential ecological or environmental significance of the redox effect on the action of cellulase is discussed.
MATERIALS AND METHODS
Materials and instruments.
Chemicals used as reagents or buffers were commercial products of reagent or purer grade unless specified otherwise. A diluted acid-pretreated corn stover (PCS) preparation (∼59% glycan and 28% lignin), kindly provided by the U.S. National Renewable Energy Laboratory, was first ground and sieved, extensively washed by water, and then adjusted to pH ∼5. Carboxymethyl cellulose (CMC) was obtained from Hercules (7L2 type; 70% substitution). Phosphoric acid-swollen cellulose (PASC) was prepared from Avicel (FMC; PH101) according to a previously reported method (35). An Fe-(2,2′-bipyridyl)2 complex was prepared by mixing 0.1 M FeCl2 or FeCl3 with 0.2 M 2,2′-bipyridyl (2,2′BP) in water at 23°C. An Fe-(1,10-phenanthroline)2 complex was prepared by mixing 0.1 M FeCl2 or FeCl3 with 0.2 M 1,10-phenanthroline in water. An Fe-EDTA complex was prepared by mixing 0.1 M FeCl2 or FeCl3 with 0.1 M EDTA in water.
An experimental cellulase preparation from Novozymes (“cellulase mix”) was used as cellulase mixtures for cellulose hydrolysis (cellulolysis). The mix contained extracellular Hypocrea jecorina (Trichoderma reesei) cellulases and Aspergillus oryzae CEL3A BG. Wild-type H. jecorina CEL7A CBH-I; recombinant H. jecorina CEL6A CBH-II, CEL7B EG-I, and CEL5A EG-II; as well as recombinant CEL3A were prepared as previously reported (14, 44, 45).
Spectrophotometric measurements were carried out with a Molecular Devices SpectraMax 340PC or Gemini XPS reader with Costar 96-well microplates. Sugar analysis was carried out with an Agilent 1100 high-performance liquid chromatography (HPLC) instrument equipped with a Bio-Rad Aminex HPX-87H column and a refractive index detector under a 5 mM H2SO4 elution. Temperature-controlled incubations were made with New Brunswick Scientific Innova 4080 incubation shakers. Polyacrylamide gel electrophoresis (PAGE) was carried out with a Bio-Rad Criterion cell with Criterion precast gels stained with Bio-Rad's Bio-Safe Coomassie kit.
Enzymatic cellulose hydrolysis.
PCS hydrolysis was carried out in either 1.7-ml Eppendorf microcentrifuge tubes or a 2-ml 96-well VWR Deep Well plate, typically with 43.4 g/liter PCS (dry weight), 0.25 g/liter (∼4 μM, based on an average mass of 60 kDa) cellulase mix, and 50 mM Na-acetate (pH 5), in 1-ml suspensions and at 50°C for up to 4 days under shaking at 150 rpm. Heat sealing of the plate was done by use of an ABgene ALPS-300 device (160°C for 2 s). Aliquots (80 μl) of the suspension were sampled and then centrifuged or filtered (on Millipore Multiscreen-HV 96-well plate filters), and the supernatants were analyzed for soluble reducing sugars by HPLC. The extent of hydrolysis was estimated from the observed glucose and cellobiose (longer cellodextrins were negligible) and, based on the glycan content of the PCS, taking into consideration water incorporation after glucosidic bond cleavage. The initial rate was obtained from hydrolysis during the first day. The hydrolysis of PASC, Avicel, and cellobiose was carried out under conditions similar to those for the hydrolysis of PCS, and their typical doses were 2, 23, and 2 g/liter level, respectively. Typical cellulase dosing was 0.25 g/liter cellulase mix or 0.04 g/liter (or ∼0.7 μM) individual cellulase. For 50-ml-scale hydrolysis, the enzyme/substrate levels were scaled up accordingly, and hydrolysis was carried out with rubber-stopped 125-ml Erlenmeyer flasks. CMC hydrolysis was performed with 1-ml solutions with 10 or 20 g/liter CMC, 1 to 20 mg/liter cellulase, and 50 mM Na-acetate at pH 5 and at 50°C. Aliquots of the suspension were sampled, and their supernatants were analyzed for soluble reducing sugars by p-hydroxybenzoic acid hydrazide (PHBAH) according to a previously reported method (16). Background absorption was corrected by control reactions.
When anaerobicity was needed, 125-ml Erlenmeyer flasks with a glass stopper (Chemglass CG-8506) and an inflatable/sealed/gas-controlled chamber (Aldrich AtmosBag) were used. The chamber was filled with N2 (Airgas; 99.9%), and the premade cellulose suspension was deaerated by gentle bubbling with N2 (for 2 min). The suspension and enzyme stock were mixed, and the flasks were sealed inside the chamber. Temporal sampling of the hydrolysis mixture was made inside a deaerated chamber. The glass stoppers provided lasting anaerobicity, although they needed to be held tightly against increased (∼10% higher) internal gas pressure (when the sealed flasks were warmed up from 23°C to 50°C) by copper wires anchored around the flask necks. Hydrolysis suspensions assembled/sampled in open air served as controls.
At least duplicates were run in each experiment.
Inhibition from metal ions or complexes.
The evaluation of selected metal ions for their effects on enzymatic cellulolysis was exemplified by that of Fe(II). Stock solutions of Fe(II) were prepared at 0.25 M as FeSO4 in water, unless specified otherwise. FeSO4 was added to the cellulose hydrolysis suspension, and their inhibitions were evaluated by the decrease of hydrolysis. For PASC hydrolysis, 0.6 to 4 g/liter PASC, 0.01 g/liter cellulase mix, and 0.1 to 10 mM FeSO4 were reacted in 50 mM Na-acetate at pH 5. As a control, FeSO4 was incubated with PASC, and no hydrolysis was seen. No effect of FeSO4 on the HPLC profiles of glucose and cellobiose was seen. For Avicel hydrolysis, 0.6 to 4 g/liter Avicel, cellulase mix, and 0.5 to 4 mM FeSO4 were reacted in 50 mM Na-acetate at pH 5. For Avicel hydrolysis, 0.6 to 4 g/liter Avicel, 0.25 g/liter cellulase mix, and 0.5 to 4 mM FeSO4 (or 0.2 to 2 mM other selected redox-active ions/complexes) were reacted in 50 mM Na-acetate at pH 5. For PCS hydrolysis, 43.4 g/liter PCS, 0.25 g/liter cellulase mix, and 0.1 to 10 mM FeSO4 were reacted in 50 mM Na-acetate at pH 5. For individual cellulases, Fe(II) inhibition was tested similarly, with 0.6 to 4 g/liter PASC, 0.04 g/liter CEL7A or other cellulases, and 3 to 15 mM FeSO4, for up to 4 h of incubation. For CEL3A, cellobiose was used instead of PASC.
The initial hydrolysis rate was obtained from the first two hydrolysis time points [with a <20% hydrolysis extent in general, rate = (hydrolysis difference)/(time difference)]. Double-reciprocal plots {1/(initial rate) versus 1/[cellulose] as a function of [inhibitor]} (square brackets indicate concentrations) were employed to study inhibition types and derive the inhibition constant, Ki, when possible. Initial rate-versus-[inhibitor] plots (linear interpolation), as a function of [cellulose], were used to derive the I50 (the inhibitor concentration resulting in a 50% loss of the initial rate).
Mitigation of iron ion inhibition on enzymatic cellulolysis.
Potential mitigators of iron ion inhibition were tested by PCS hydrolysis. In one experiment, the hydrolysis of 43 g/liter PCS by 0.25 g/liter cellulase mix, in 50 mM Na-acetate at pH 5 and at 50°C, with 2.5 mM FeSO4, 10 mM H2O2, and 10 mM desferrioxamine (siderophore), added either alone or in combination after 30 min of preincubation prior to the addition of cellulases, was monitored. In another PCS hydrolysis experiment, 10 mM desferrioxamine, 1,10-phenanthroline, or 2,2′-bipyridyl was added so that the effect of the Fe(II) chelators on hydrolysis could be evaluated.
Potential effect from polyethylene glycol (PEG) on the Fe(II)-caused inhibition of enzymatic cellulolysis was studied with PEG 4000. In one experiment, 10 mM FeSO4 and 0.625 to 5 g/liter PEG 4000 were added either alone or in combination to PCS hydrolysis and cellulase mix. In another experiment, 10 mM FeSO4 and 5 g/liter PEG 4000 were added to Avicel hydrolysis and cellulase mix. The effects of 50 g/liter PEG 4000 on the inhibition by 10 mM FeSO4 of the hydrolysis of either 50 g/liter PCS or 25 g/liter Avicel by 0.25 g/liter cellulase mix were also compared.
Preincubation of cellulases or cellulose with inhibitory iron species.
To preincubate Avicel or PASC with inhibitory iron species, prior to hydrolysis with cellulase mix, 25 g/liter Avicel or 2 g/liter PASC was mixed with 10 mM FeSO4, FeCl2, or FeCl3 in water at 23°C for 2 h. Pretreated cellulose was then extensively washed with 50 mM Na-acetate at pH 5 (until the final concentration of Fe species in supernatant reached <0.05 mM) and hydrolyzed with cellulase mix. To preincubate cellulases with inhibitory iron species, prior to enzyme cellulolysis, 2.5 g/liter (for Avicel hydrolysis) or 0.01 g/liter (for PASC hydrolysis) cellulase mix was mixed with 10 mM FeSO4, FeCl2, or FeCl3 in buffer at 23°C for 2 h or 3 days. Pretreated cellulases were then desalted on Bio-Rad Bio-Spin 6 columns (to remove iron species) and applied to hydrolyze Avicel or PASC. Hydrolyses of untreated or buffer-only-preincubated Avicel, PASC, and cellulase mix, with or without iron inhibitors, served as controls.
Other assays.
Protein determinations for cellulase samples were made with a Pierce BCA kit after subjecting samples to gel filtration.
Fe ion quantification by 2,2′-bipyridyl was carried out by mixing Fe(II)- or Fe(III)-containing samples with 5 mM 2,2′-bipyridyl and measuring electronic absorption at 520 nm. Solutions of 0.005 to 0.1 mM FeSO4 or FeCl3 were used for calibration. At the same concentration, the absorption of Fe(III)(2,2′BP)3 was negligible compared to that of Fe(II)(2,2′BP)3.
Possible Fe ion adsorption onto cellulose was detected by incubating 5 to 100 μM FeSO4 with 14 to 25 g/liter Avicel in 50 mM Na-acetate at pH 5 (1-ml suspension) at 50°C. After 1 to 24 h, the supernatants were subjected to 2,2′-bipyridyl quantification.
The possible effect of Fe(II) on cellulases was examined by nondenaturing PAGE. Cellulase mix (2.5 g/liter) or individual cellulases were first incubated with 10 mM FeSO4 in 50 mM Na-acetate at pH 5 at 50°C for 4 days. The samples were then subjected to nondenaturing (SDS-free, native PAGE sample buffer and Tris-glycine running buffer) PAGE on 8 to 16% gels. Fresh cellulase mix and individual cellulases were also run as controls.
To strip away the potential accessible Fe ion in PCS, washed PCS was incubated with 10 mM 2,2′-bipyridyl, 1,10-phenanthroline, or 1,7-phenanthroline for 2 days and then washed extensively to remove the chelator or Fe-chelator complex. The treated PCS was then subjected to hydrolysis with selected cellulases.
RESULTS
Inhibitory effect of the addition of Fe(II) on enzymatic cellulolysis.
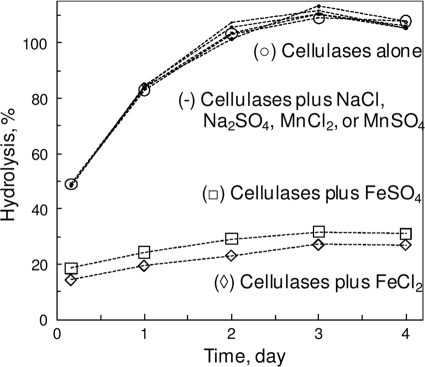
When several metal cations, as either chloride or sulfate salt, were added (individually) to the enzymatic hydrolysis mixtures of PCS, only FeSO4 and FeCl2 caused a significant inhibition of the hydrolysis (Fig. 1). Since other tested sulfates and chlorides were benign, the inhibition was most likely due to Fe(II).
FIG. 1.
Effect of Fe(II) and other ions on enzymatic PCS hydrolysis. Added salt concentrations were 10 mM. Hydrolysis mixtures included 43 g/liter PCS, 0.25 g/liter cellulase mix, and 50 mM Na-acetate (pH 5) at 50°C.
The concentration dependence of the inhibition of Fe(II) on enzymatic cellulolysis was probed with the hydrolysis of Avicel or PASC by the cellulase mix. For the hydrolysis PASC by a cellulase mix, the addition of FeSO4 resulted in plots of 1/(initial rate) versus 1/[PASC] (as a function of [FeSO4]), indicative of a mix-type inhibition, whose complexity prevented the extraction of simple Ki. For the hydrolysis of Avicel by the cellulase mix, the addition of FeSO4 also resulted in double-reciprocal plots indicative of a mixed-type inhibition, but no simple Ki could be extracted due to plot complexity. Table 1 shows the I50 obtained from plots of the initial rate versus [FeSO4]. Apparently, Fe(II)'s inhibition of Avicel hydrolysis was more pronounced than that of PASC. For the hydrolysis of PCS, an I50 of ∼7 mM was observed for FeSO4.
TABLE 1.
Inhibition parameters of Fe(II) on enzymatic cellulolysis
| Mixture | Mean I50 (mM) ± SDa |
|||||
|---|---|---|---|---|---|---|
| Cellulase mix | CEL7A | CEL6A | CEL7B | CEL5A | CEL3A | |
| Avicel | 2 ± 1 | ND | ND | ND | ND | ND |
| PASC | 14 ± 1 | 7 ± 2 | ∼7 | 1.8 ± 0.2 | 12 ± 4 | 13 ± 2b |
ND, not determined.
On cellobiose hydrolysis.
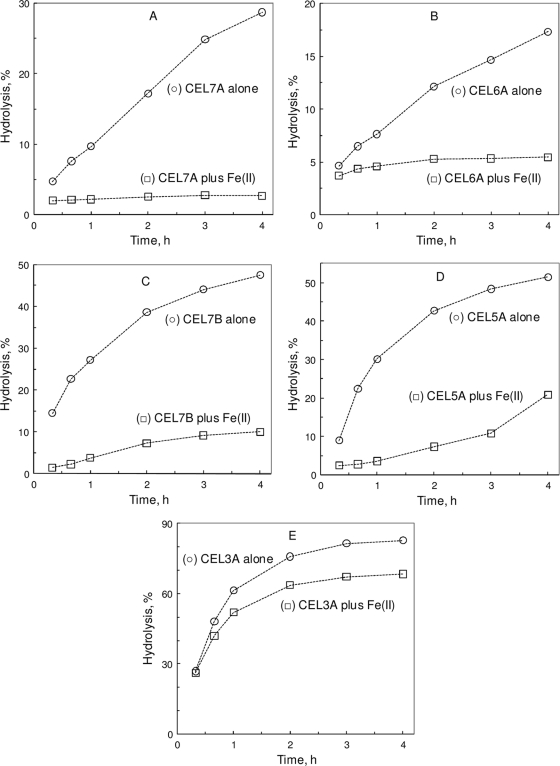
Fe(II) also exerted an inhibition of PASC hydrolysis by the major components of the cellulase mix (Fig. 2). For CEL7A, CEL7B, CEL5A, and CEL3A, the plots of the initial rate versus [Fe(II)] appeared as a mixed type whose complexity also prevented the extraction of simple Ki. Table 1 shows the I50 obtained from plots of the initial rate versus [FeSO4]. Apparently, Fe(II) exerted a more pronounced inhibition on CEL7B's reaction than those on CEL7A and CEL5A. For CEL3A's reaction, Fe(II)'s inhibition seemed minor. CMC hydrolysis was also tested for Fe(II)'s inhibition on CEL7B and CEL5A (both EGs), but Fe(II) significantly interfered with the PHBAH assay, making the reducing sugar measurement highly variable.
FIG. 2.
Effect of Fe(II) on individual cellulases. (A) CEL7A CBH-I. (B) CEL6A CBH-II. (C) CEL7B EG-I. (D) CEL5A EG-II. (E) CEL3A BG. The inhibitor was 10 mM FeSO4. The hydrolysis mixture included 2 g/liter PASC (A to D) or cellobiose (E), 40 mg/liter (A to D) or 1 mg/liter (E) cellulase, and 50 mM Na-acetate (pH 5) at 50°C.
Differential inhibition of enzymatic cellulolysis by redox-active metal ions.
To probe whether or not the inhibitory effect of Fe(II) is related to its ionic properties (size and charge), various (biologically common) divalent metal ions with variable ionic radii were tested with Avicel hydrolysis by the cellulase mix. At the 10 mM level, MgCl2 and CaCl2 showed a slight enhancement, while CoSO4, MnSO4, NiCl2, and ZnSO4 showed slight or moderate inhibition upon hydrolysis. In contrast, FeSO4 (as well as FeCl2) and CuSO4 resulted in a ∼70% or greater loss of both the initial hydrolysis rate and hydrolysis extent after 4 days (Table 2). MnSO4 was also tested at the 0.1 to 10 mM range, and no effect on hydrolysis was observed, although FeSO4 at the same range caused a concentration-dependent inhibition.
TABLE 2.
Properties and effects of tested metal ions
| Iona | Charge | Radius (Å)b | Eo (V)b,c | Effect on enzymatic cellulolysisd |
|---|---|---|---|---|
| Mg(II) | 2+ | 0.72 | ∼20% gain in initial rate, ∼20% gain in extended hydrolysis extent | |
| Ca(II) | 2+ | 1.00 | ∼20% gain in initial rate, ∼20% gain in extended hydrolysis extent | |
| Cr(III) | 3+ | 0.62 | −0.407 | ∼40% loss in initial rate, ∼40% loss in extended hydrolysis extent |
| Mn(II) | 2+ | 0.67 | ∼10% loss in initial rate, no change on extended hydrolysis extent | |
| Fe(II) | 2+ | 0.61 | ∼70% loss in initial rate, ∼70% loss in extended hydrolysis extent | |
| Fe(III) | 3+ | 0.55 | 0.771 | ∼90% loss in initial rate, ∼90% loss in extended hydrolysis extent |
| Co(II) | 2+ | 0.65 | ∼10% loss in initial rate, no change on extended hydrolysis extent | |
| Ni(II) | 2+ | 0.69 | ∼20% loss in initial rate, ∼10% loss in extended hydrolysis extent | |
| Cu(II) | 2+ | 0.73 | 0.153 | ∼90% loss in initial rate, ∼80% loss in extended hydrolysis extent |
| Ru(III) | 3+ | 0.68 | 0.249 | ∼90% loss in initial rate, ∼80% loss in extended hydrolysis extent |
| Zn(II) | 2+ | 0.74 | ∼20% loss in initial rate, ∼20% loss in extended hydrolysis extent |
The counteranions of these metal ion compounds, SO42− and Cl−, were inert under the hydrolysis conditions of this study.
Ionic radii in crystals for six-ligand coordination (19).
Single-electron oxidation potential versus a normal hydrogen electrode. Metal ions without Eo entry are stable against either reduction or oxidation under the conditions of this study.
One-milliliter-scale hydrolysis of 23 g/liter Avicel by 0.25 g/liter cellulase mix in 50 mM Na-acetate at pH 5 and at 50°C for 4 days.
Because of its susceptibility to O2 oxidation, the initial added Fe(II) was steadily transformed to Fe(III), as indicated by the appearance of a brown color of the hydrolysis suspension. To test whether Fe(II) exerted its inhibition via air-oxidized Fe(III), a series of 50-ml-scale hydrolyses of 25 g/liter Avicel by 0.25 g/liter cellulase mix with or without 1 mM FeSO4, in 50 mM Na-acetate (pH 5) at 50°C, was carried out in “normal” (aerated) or N2-deaerated solutions. After 1 day, FeSO4 caused a ∼30% decrease in “aerobic” cellulolysis but no effect on “anaerobic” cellulolysis, indicating the involvement of O2 in the apparent inhibition of cellulolysis by Fe(II).
The effect of adding H2O2 to Fe(II)-inhibited PCS hydrolysis mixtures was studied. For the hydrolysis of 43.5 g/liter PCS with 0.25 g/liter cellulase mix with or without 10 mM FeSO4, 0, 1.25, 2.5, 5, and 10 mM H2O2 were added. No change of the inhibition was seen, indicating that it was mainly the Fe species, rather than oxygen-based species derivable from Fenton chemistry, that caused the inhibition.
To directly study the effect of Fe(III) on enzymatic cellulolysis, FeCl3 was added into Avicel hydrolysis mixtures with the cellulase mix. At the 10 mM level, FeCl3 resulted in a ∼90% loss of both the initial hydrolysis rate and extent of hydrolysis after 4 days (Table 2). For the hydrolysis of 2 g/liter PASC by 0.01 g/liter cellulase mix, 10 mM FeCl3 resulted in an ∼80% loss of both the initial hydrolysis rate and extent of hydrolysis after 4 days, compared to the ∼30% loss brought about by FeCl2. Thus, Fe(III) exerted more potent inhibition than did Fe(II).
In addition to Fe(III), two other trivalent and oxidative metal ions, Cr(III) and Ru(III), were also tested. At the 10 mM level, CrCl3 and RuCl3 exerted moderate and pronounced inhibitions of enzymatic Avicel hydrolysis, respectively (Table 2).
Differential inhibition of enzymatic cellulolysis by iron-chelator complexes.
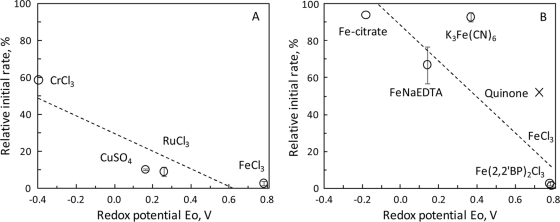
To further probe how iron ions inhibited enzymatic cellulolysis, iron-chelator complexes with variable sizes or redox properties were tested. For Avicel hydrolysis by cellulase mix, 10 mM Fe(2,2′-BP)3Cl3, FeCl3, FeCl2, Fe(2,2′-BP)3Cl2, FeNa(EDTA), K4Fe(CN)6, K3Fe(CN)6, or Fe-citrate exerted various degrees of inhibition (Table 3). Iron complexes with a higher oxidation potential, Eo, tended to cause more hydrolysis inhibition (Fig. 3).
TABLE 3.
Oxidation potential and inhibitory effect of iron-chelator complexes
| Complex | Eo (V)a | Effect on enzymatic cellulolysisc |
|---|---|---|
| Fe(III)(2,2′BP)2Cl3 | 0.78 | ∼100% loss in initial rate, ∼100% loss in extended hydrolysis extent |
| Fe(II)(2,2′BP)2Cl2 | ∼40% loss in initial rate, ∼40% loss in extended hydrolysis extent | |
| K3Fe(III)(CN)6 | 0.358 | No significant change in initial rate, no change in extended hydrolysis extent |
| K4Fe(III)(CN)6 | ∼10% loss in initial rate, ∼20% loss in extended hydrolysis extent | |
| Fe(III)NaEDTA | 0.13b | ∼10% loss in initial rate, ∼20% loss in extended hydrolysis extent |
| Fe(II)Na2EDTA | ∼80% loss in initial rate, ∼80% loss in extended hydrolysis extent | |
| Fe(III)-citrate | −0.191b | No significant change in initial rate, no change in extended hydrolysis extent |
Single-electron oxidation potential (19).
Data from references 3 and 6. Iron-chelator complexes without Eo entry are reduced counterparts of the corresponding oxidized complexes (Eo given).
Hydrolysis of 23 g/liter Avicel by 0.25 g/liter cellulase mix in 50 mM Na-acetate at pH 5 and at 50°C for 4 days.
FIG. 3.
Correlation between redox potential and cellulolysis-inhibiting effect of selected oxidative metal ions (A) and Fe-ligand complexes (also including p-quinone) (B). Data for the Eo and initial rate of cellulase mix-catalyzed Avicel hydrolysis are described in Tables 2 and 3 and the text. (A) Relative rate = −48Eo + 29; r2 = 0.795. (B) Relative rate = −97Eo + 88; r2 = 0.766.
The concentration dependence of the effect of inhibitory metal ions [in addition to Fe(II)] and complexes on Avicel hydrolysis by cellulase mix was also studied. Oxidative Fe(III), Ru(III), and Cu(II) species had I50 values much lower than those of Fe(II) species, with I50 values of 0.5 ± 0.4 mM for FeCl3, 0.6 ± 0.1 mM for Fe(2,2′BP)3Cl3, ∼20 mM for Fe(2,2′BP)3Cl2, ∼10 mM for FeNaEDTA, <10 mM for FeNa2EDTA, ∼0.2 mM for CuSO4, ∼0.15 mM for RuCl3, >10 mM for CrCl3, and ∼5 mM for quinone (tested with 23 g/liter Avicel hydrolysis by 0.25 g/liter cellulase mix).
To further probe the connection between Eo and cellulolysis inhibition of oxidative substances, p-benzoquinone and p-hydroquinone (reduced quinone), with an Eo of 0.715 V (10), were tested for Avicel hydrolysis by cellulase mix. At the 5 mM level, quinone resulted in a ∼50% loss in both the initial hydrolysis rate and extended hydrolysis extent, while p-hydroquinone exerted no effect.
Differential targeting of inhibition of the iron ion on cellulase and cellulose.
To test whether Fe(II) affects cellulose or cellulase, FeCl2 was preincubated with either Avicel or cellulase mix for 3 days. “Fe-pretreated” Avicel and the cellulase mix were washed and gel filtered, respectively, to remove residual Fe ions. Fe-pretreated Avicel was hydrolyzed with fresh cellulase mix or cellulase mix subjected to “FeCl2-less” preincubation and gel filtration. Fe-pretreated cellulase mix was applied to hydrolyze fresh Avicel or Avicel subjected to “FeCl2-less” preincubation and washing, in comparison with the hydrolysis of fresh Avicel (2 g/liter) by fresh cellulase mix (0.16 g/liter), with or without 10 mM FeCl2. The preincubation of FeCl2 with Avicel or cellulase mix led to a ∼10% or 20% loss, respectively, in both the initial rate and extended extent of hydrolysis. Fresh FeCl2 led to a ∼50% loss in both the initial rate and extended extent of hydrolysis of fresh Avicel by fresh cellulase mix. The preincubation of cellulase mix or Avicel with buffer resulted in no effect on hydrolysis.
To test whether Fe(III) affects cellulose or cellulase, FeCl3 was also preincubated with either Avicel or cellulase mix for 3 days. The preincubation of FeCl3 with Avicel or cellulase mix led to an ∼80% or 20% loss, respectively, in both the initial rate and extended extent of hydrolysis. Fresh FeCl3 led to a ∼90% loss in both the initial rate and extended extent of hydrolysis of fresh Avicel by fresh cellulase mix.
The “preincubation” study was also done for the hydrolysis of PASC. The preincubation of FeCl2 with PASC led to a ∼60% loss and no loss in the initial rate and extended hydrolysis extent, respectively. The preincubation of FeCl2 with cellulase mix led to a ∼70% loss and no loss in the initial rate and extended hydrolysis extent, respectively. Fresh FeCl2 led to a ∼30% loss and no loss in the initial rate and extended hydrolysis extent, respectively, for the hydrolysis of PASC by cellulase mix (fresh or buffer preincubated). The replacement of FeCl2 with FeSO4 led to a similar result. The preincubation of FeCl3 with PASC led to ∼50% and 10% losses in the initial rate and extended hydrolysis extent, respectively. The preincubation of FeCl3 with cellulase mix led to a ∼50% loss and no loss in the initial rate and extended hydrolysis extent, respectively. Fresh FeCl3 led to ∼70% and 30% losses in the initial rate and extended hydrolysis extent, respectively, for the hydrolysis of PASC by cellulase mix (fresh or buffer preincubated).
To probe whether Fe(II) could be adsorbed onto cellulose, a partition study was performed. After incubating Avicel and FeSO4, no detectable Fe(II) adsorption onto Avicel was seen, indicating that the inhibition of enzymatic cellulolysis by Fe(II) was likely not related to cellulose adsorption. The presence of Avicel did not significantly affect the auto-oxidation rate of Fe(II) into Fe(III) in aerated solution.
Preincubation with FeCl2 resulted in a PASC whose initial reactivity to cellulases was inferior to those of fresh or buffer-preincubated PASC, indicating a detrimental modification of PASC by Fe(II). The washing of FeCl2-preincubated PASC with 2,2′-bipyridyl, to strip off adsorbed iron ions, did not improve the initial reactivity of PASC to cellulases, indicating that the Fe-caused PASC modification was not simple Fe adsorption.
The possible interaction between iron ion and cellulase proteins was probed by electrophoresis. On nondenaturing PAGE gels, in comparison with fresh individual cellulases, the FeSO4 preincubation led to a decrease (or diffusion) of the intensity of the major protein bands in cellulase mix, particularly that of BG. Higher concentrations of [FeSO4] in the preincubation mixture led to a more pronounced decrease in the PAGE band intensity. Thus, the interaction with the inhibitors seemed to affect the folding or stability of the major cellulases.
Mitigation of iron ion-caused cellulolysis inhibition.
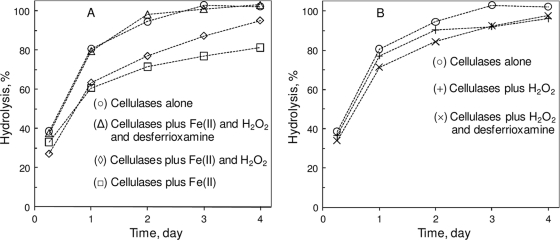
In one experiment, Fe(II) was subjected to oxidation by H2O2, and the oxidation effect on the cellulolysis inhibitor was studied. As shown in Fig. 4A, the preincubation of FeSO4, H2O2, and PCS, prior to the addition of cellulases, led to a relief of the inhibition by Fe(II). The presence of desferrioxamine, a strong Fe(III) chelator, resulted in complete mitigation against the iron ion-caused inhibition of enzymatic cellulolysis. In the absence of Fe(II), H2O2 with or without desferrioxamine affected hydrolysis only slightly (Fig. 4B). At 10 mM, desferrioxamine itself did not affect the hydrolysis of PCS.
FIG. 4.
Effect of Fe(II) oxidation and Fe(III) chelation on enzymatic cellulolysis. The hydrolysis mixture included 43 g/liter PCS, 0.25 g/liter cellulase mix, and 50 mM Na-acetate (pH 5) at 50°C. Concentrations of inhibitor, oxidant, and chelator added to the hydrolysis suspension were 2.5 mM FeSO4, 10 mM H2O2, and 10 mM desferrioxamine.
At 1 and 10 mM, Fe(III)-citrate did not affect the hydrolysis of PCS by cellulase mix 2, likely due to the Fe(III) chelation by citrate, similar to the case with cellulase mix (Table 3).
Two widely used Fe(II) chelators, 1,10-phenanthroline and 2,2′-bipyridyl, were tested as potential mitigators for the Fe(II) inhibition of enzymatic cellulolysis. Unexpectedly, the chelators themselves exhibited significant inhibitions of PCS hydrolysis by the cellulase mix: 10 mM 1,10-phenanthroline or 2,2′-bipyridyl resulted in a ∼40% or 60% decrease, respectively, in the hydrolysis (in terms of both the initial rate and extended hydrolysis extent) of 43 g/liter PCS by 0.25 g/liter cellulases. In the presence of the chelators, the cellobiose level in the hydrolysis products was about twice that in the absence of the compounds. Such an inhibition by the chelators prevented their use as mitigators against the inhibition of enzymatic cellulolysis by Fe(II) under our conditions.
PEG 4000 was tested for a potential mitigation of the Fe(II)-caused inhibition of enzymatic cellulolysis. Only an insignificant mitigation effect was seen with 5 or 50 g/liter PEG 4000 for the inhibition of 10 mM FeSO4 imposed on the hydrolysis of 25 g/liter Avicel by 0.25 g/liter the cellulase mix. For PCS hydrolysis, however, a detectable mitigation effect from PEG 4000 was seen. For example, 5 g/liter PEG 4000 apparently fully mitigated the ∼20% loss of hydrolysis of 43 g/liter PCS from 10 mM FeSO4 by 0.25 g/liter cellulase mix 2. However, in the absence of FeSO4, PEG 4000 also enhanced PCS (but not Avicel) hydrolysis by 5%, an effect reported previously (E. Vlasenko, E., J. Cherry, and F. Xu, 28 July 2005, PCT international patent application WO/2005/067531). With [PEG 4000] at various levels of 0, 0.625, 1.25, 2.5,and 5 g/liter, PEG 4000 showed a dose-dependent mitigation of Fe(II) inhibition.
The ability of PEG 4000 to mitigate Fe(II)'s inhibition could be reduced when H2O2 was present. In a hydrolysis experiment with PCS and cellulase mix, the presence of 10 mM H2O2 led to a lesser effect from 0.31 g/liter PEG on the inhibition of 10 mM FeSO4, probably indicating a lower efficacy of PEG 4000 against Fe(III) [produced from Fe(II) by H2O2].
To test whether there was any significant amount of (accessible) Fe(II) in PCS and whether removing it with a chelator might help PCS hydrolysis, washed PCS was “stripped” with 10 mM 2,2′-bipyridyl or 1,10-phenanthroline prior to hydrolysis by the cellulase mix. No detectable effect of the treatment on hydrolysis was seen, a result that might be attributable to the lack of a significant amount of Fe(II) in (washed) PCS.
DISCUSSION
The apparent inhibition of various cellulases (more precisely, their reactions) by Fe(II) or Cu(II) ion complexes was reported previously (5, 7-9, 12, 15, 17, 18, 26-28, 30, 32, 38). In this study, we also observed a significant inhibition by Fe(II) of the major cellulases (CEL7A CBH-I, CEL6A CBH-II, CEL7B EG-I, and CEL5A EG-II) in H. jecorina and, to a lesser extent, of A. oryzae CEL3A BG. A mechanistic understanding of the Fe(II) or Cu(II) effect has not been addressed in previous reports, although it might be ascribed to a detrimental binding to cellulases that causes a conformational change or replacement of native metal cofactors or to cellulose that blocks the accessibility for cellulase. Our study suggested that Fe(II) and Cu(II) exert their inhibitory effect on enzymatic cellulolysis via a redox mechanism.
It is known that cellulose can be oxidized, at its “reducing ends,” by Cu(II) and that oxidized cellulose is less reactive to cellulase action than unoxidized cellulose, including that of CEL6 CBH-II, which in general has specificity for cellulose's nonreducing end (45). Although it has been postulated that cellulose oxidation might decrease cellulase accessibility, the molecular mechanism of the effect remains to be fully elucidated. Such a detrimental oxidative effect is anticipated for oxidants other than Cu(II). In this study we showed that various oxidants [including Fe(II) and Cu(II)], whether as metal ions, organics, or metallo-organics, could inflict a significant inhibition of enzymatic cellulolysis (Tables 2 and 3). The inhibition of cellulase reactions by Fe(III), Cr(III), and KMnO4 (a strong oxidant) was also reported previously (5, 30, 32, 39). The inhibitory effect depended on the Eo, the thermodynamic measure of oxidation potency, of the oxidants. Among various ionic compounds, the inhibition effect seemed independent of anions or the charge/size of cations (metal ions) under the conditions of this study (∼10 mM level).
In this study, Fe(III) demonstrated a great inhibitory effect (among the tested iron species) on enzymatic cellulolysis. Having an Eo of 0.77 V, free Fe(III) [or, more likely, Fe(III)-acetate (stability constant, K, of 101.4 M−1) in 50 mM Na-acetate buffer] is an oxidant much more potent than Fe(III)-citrate and Fe(III)-desferrioxamine, in which strong chelations by citrate and desferrioxamine greatly stabilize Fe(III) over Fe(II) (K ratios of 1010.9/103.2 for citrate and 1030.6/107.2 for desferrioxamine [37, 40]) and, consequently, a lower Eo for Fe(III). Chelating the iron ion with EDTA (K ratio of 1025.1/1014.3), 2,2′-bipyridyl (cumulative stability constant, β2, ratio of 1016.3/1017.2), 1,10-phenanthroline (β3 ratio of 1014.1/1021), and CN− (β6 ratio of 1043.9/1036.9) should also affect the oxidation potency of Fe(III) according to the relative stability of chelated Fe(III) and Fe(II) (2, 21, 36).
Because Fe(II) is readily oxidized by air to Fe(III) in aqueous solution, the observed inhibitory effect of Fe(II) on cellulolysis likely involved Fe(III). Being an oxidant, Fe(III) is known to be able to oxidize the reducing ends (hemiacetals) of carbohydrates (Eo of −0.4 V [10]), while it is reduced to Fe(II) (31). The formed Fe(II) can then be reoxidized by O2, starting an Fe-catalyzed cycle of cellulose oxidation by O2 in which Fe shuttles electrons from the reducing ends of cellulose to O2 (Eo of ∼0.9 V for O2/H2O [2]). The oxidation can lead to less cellulase-active cellulose and inhibited cellulolysis. Such a mechanism was strongly supported by the effect of anaerobicity on inhibition by Fe(II) and consistent with the effect of Cu(II) on cellulose oxidation (45).
The preincubation of cellulases or cellulose with iron ions led to slightly or significantly inactivated cellulases or cellulose, respectively. Likely, iron targeted mainly cellulose for its inhibition of enzymatic cellulolysis. No significant iron ion adsorption onto cellulose seemed to rule out any major role of iron-cellulose complexing in the inhibitory effect of iron.
Fe(II) showed a stronger inhibition of the hydrolysis of Avicel than of PASC (Table 1), attributable to the fewer accessible reducing ends in Avicel (most of them were within crystalline microfibrils) than those in PASC (swollen and amorphous). Fe(II)'s inhibition of PASC hydrolysis by the cellulase mix seemed to be determined by Fe(II)'s inhibition of the actions of both CBH and EG, based on the I50 values (Table 1).
Because of their dependence on the Eo, the inhibitory effect of oxidative (or redox-active) metal ions may be alleviated by lowering the Eo via differentially complexing their oxidized and/or reduced forms. Chelators strongly favoring (and, thus, binding more tightly) the oxidized metal ion form may stabilize it more against the reduced form, leading to lower Eo values and consequently lower inhibition of enzymatic cellulolysis, as exemplified by Fe(III)-desferrioxamine and Fe(III)-citrate. For Fe(II) and other O2-labile, redox-active, enzymatic cellulolysis inhibitors, the elimination of O2 (or oxidative conditions) and/or maintenance of a reductive hydrolysis environment may be applied to curtail the substances' inhibition and regeneration. For practical biomass conversion, citric acid, oxalic acid, and EDTA, etc., may serve as low-cost, effective Fe(III) chelators, and CO2 and sulfite, etc., may be used for nonoxidative or reductive cellulolysis.
Cooccurring Fe(III)/Fe(II) reactions or other redox events could impact natural lignocellulose hydrolysis by microbial cellulases and hemicellulases, taking place in highly heterogeneous environments in which lignocellulolytic microbes cohabitate with other microbes under diverse aeration, moisture, sunlight, and soil composition conditions and other abiotic or biotic conditions. As a part of local microbiota, the growth and action of lignocellulolytic microbes are subjected to the presence and temporal/spatial variations of not only nutrients or substrates but also inhibitors or regulators, which may include redox-active agents. In addition to (hemi)cellulases, many lignocellulolytic microbes cosecrete oxidoreductases (e.g., peroxidase and laccase) or small redox molecules (e.g., quinone) and generate or utilize environmental redox agents [e.g., H2O2 and Fe(III)/Fe(II)] to degrade or modify lignin (1, 33, 41). It remains unclear how a lignocellulolytic microbe regulates its oxidative delignifying or cellulolyzing enzymes or agents to control their potential inhibition of the concomitant hydrolytic cellulase activity or whether a lignocellulolytic microbe might use its oxidative enzymes/agents to compete with a (ligno)cellulolytic microbe in degrading biomasses. Conventionally, microbial enzymatic delignifying systems and cellulolytic systems are studied separately, even when they are part of the whole lignocellulolytic enzyme machinery of the same microbes. Our finding of the oxidative effect on enzymatic cellulolysis calls for a more extensive investigation of the interaction between the two systems and its effect on lignocellulose biodegradation.
Redox chemistry not directly related to microbial lignocellulolysis might also impact biodegradation. Redox-active Fe and Mn species and other diffusible redox mediators are involved in Fe(III) and Mn(IV) reducers' anaerobic respirations and anoxygenic photosynthesis (4, 34). Such redox agents from nonlignocellulolytic cohabitants of local microbiota could affect anaerobic or even aerobic (depending on the local O2 distribution) lignocellulolytic microbes and their (hemi)cellulases, either “unintentionally” or competitively. Historically, microbial biodegradations of biomass and lignocellulolytic microbes are studied mostly under simplified laboratory conditions with isolated strains. Our observation of oxidatively impacted enzymatic cellulolysis suggests that other ecological or environmental factors (including those of a redox nature) should be taken into account so that various “outdoor” lignocellulose biodegradations, as part of either natural carbon cycling, native biota preservation, or artificial soil/water bioremediation, can be further understood.
Variable amounts of oxidative or redox-active iron species or other chemicals may exist in industrial biomass hydrolysis reactions, originating from either feedstock, a reaction vessel/pipeline/water, or upstream steps. For instance, a laccase mediator system has been applied for biomass pretreatment, and the tested N-hydroxy mediators became inhibitory to cellulases after being oxidized by laccase (29). Recently, an FeCl3 system was also tested for biomass pretreatment (20). Our study shows the importance of taking into account the potential detrimental effect of oxidants or redox-active substances on the enzymatic hydrolysis step for biomass conversion as well as including such considerations in the overall development/optimization of biomass conversion technologies. A further mechanistic understanding of the interaction between cellulase and oxidized cellulose and biophysical characterization of oxidized cellulose (quantification and localization of oxidized sites) could assist us in finding and engineering cellulases that are more tolerant of this oxidative inhibition effect.
Acknowledgments
We thank Robert L. Starnes and Claus C. Fuglsang from Novozymes for critical reading of the manuscript.
This material is based upon work supported by the Department of Energy under award number DE-FC36-08GO18080. This report was prepared as an account of work sponsored by an agency of the U.S. Government.
Neither the U.S. Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the U.S. Government or any agency thereof. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the U.S. Government or any agency thereof.
Footnotes
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Baldrian, P., and V. Valaskova. 2008. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 32:501-521. [DOI] [PubMed] [Google Scholar]
- 2.Dawson, R. M. C., D. C. Elliott, W. H. Elliott, and K. M. Jones. 1991. Data for biochemical research, 3rd ed. Oxford University Press, New York, NY.
- 3.Dhungana, S., C. H. Taboy, D. S. Anderson, K. G. Vaughan, P. Aisen, T. A. Mietzner, and A. L. Crumbliss. 2003. The influence of the synergistic anion on iron chelation by ferric binding protein, a bacterial transferrin. Proc. Natl. Acad. Sci. U. S. A. 100:3659-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkowski, P. G., T. Fenchel, and E. F. Delong. 2008. The microbial engines that drive earth's biogeochemical cycles. Science 320:1034-1039. [DOI] [PubMed] [Google Scholar]
- 5.Ferchak, J. D., and E. K. Pye. 1983. Effect of cellobiose, glucose, ethanol, and metal ions on the cellulase enzyme complex of Thermomonospora fusca. Biotechnol. Bioeng. 25:2865-2872. [DOI] [PubMed] [Google Scholar]
- 6.Florence, T. M. 1984. The production of hydroxyl radical from hydrogen peroxide. J. Inorg. Biochem. 22:221-230. [DOI] [PubMed] [Google Scholar]
- 7.Gardner, R. M., K. C. Doerner, and B. A. White. 1987. White purification and characterization of an exo-beta-1,4-glucanase from Ruminococcus flavefaciens FD-1. J. Bacteriol. 169:4581-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada, K. M., K. Tanaka, Y. Fukuda, W. Hashimoto, and K. Murata. 2005. Degradation of rice bran hemicellulose by Paenibacillus sp. strain HC1: gene cloning, characterization and function of beta-D-glucosidase as an enzyme involved in degradation. Arch. Microbiol. 184:215-224. [DOI] [PubMed] [Google Scholar]
- 9.Heredia, A., J. Fernandez-Bolafios, and R. Guillen. 1989. Inhibitors of cellulolytic activity in olive fruits (Olea europaea, Hojiblanca var.). Z. Lebensm. Unters. Forsch. 189:216-218. (In German.) [Google Scholar]
- 10.Hewitt, L. F. 1950. Oxidation-reduction potentials in bacteriology and biochemistry, 6th ed. E&S Livingstone, Edinburgh, United Kingdom.
- 11.Himmel, M. E., S. Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady, and T. D. Foust. 2007. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804-807. [DOI] [PubMed] [Google Scholar]
- 12.Kim, D. W., Y. H. Jang, C. S. Kim, and N.-S. Lee. 2001. Effect of metal ions on the degradation and adsorption of two cellobiohydrolases on microcrystalline cellulose. Bull. Korean Chem. Soc. 22:716-720. [Google Scholar]
- 13.Kumar, R., S. Singh, and O. V. Singh. 2008. Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 35:377-391. [DOI] [PubMed] [Google Scholar]
- 14.Langston, J., N. Sheehy, and F. Xu. 2006. Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochim. Biophys. Acta 1764:972-978. [DOI] [PubMed] [Google Scholar]
- 15.Lee, T.-K., and C.-H. Kim. 1999. Molecular cloning and expression of an endo-β-1,4-D-glucanase I (Avicelase I) gene from Bacillus cellulyticus K-12 and characterization of the recombinant enzyme. Appl. Biochem. Biotechnol. 80:121-140. [DOI] [PubMed] [Google Scholar]
- 16.Lever, M. 1972. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47:273-279. [DOI] [PubMed] [Google Scholar]
- 17.Li, D.-C., M. Lu, Y.-L. Li, and J. Lu. 2003. Purification and characterization of an endocellulase from the thermophilic fungus Chaetomium thermophilum CT2. Enzyme Microb. Technol. 33:932-937. [Google Scholar]
- 18.Li, Y.-H., M. Ding, J. Wang, G.-J. Xu, and F. Zhao. 2006. A novel thermoacidophilic endoglucanase, Ba-EGA, from a new cellulose-degrading bacterium, Bacillus sp. AC-1. Appl. Microbiol. Biotechnol. 70:430-436. [DOI] [PubMed] [Google Scholar]
- 19.Lide, D. R. 1993. CRC handbook of chemistry and physics, 73rd ed. CRC Press, Boca Raton, FL.
- 20.Liu, L., J. Sun, M. Li, S. Wang, H. Pei, and J. Zhang. 2009. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 100:5853-5858. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Z. D., and R. C. Hider. 2001. Design of clinically useful iron(III)-selective chelators. Med. Res. Rev. 22:26-64. [DOI] [PubMed] [Google Scholar]
- 22.Lynd, L. R., M. S. Laser, D. Bransby, B. E. Dale, B. Davison, R. Hamilton, M. Himmel, M. Keller, J. D. McMillan, J. Sheehan, and C. E. Wyman. 2008. How biotech can transform biofuels. Nat. Biotechnol. 26:169-172. [DOI] [PubMed] [Google Scholar]
- 23.Lynd, L. R., P. J. Weimer, W. H. van Zyl, and I. S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandels, M., and E. T. Reese. 1965. Inhibition of cellulases. Annu. Rev. Phytopathol. 3:85-102. [Google Scholar]
- 25.Morey, R. V., D. L. Hatfield, R. Sears, and D. G. Tiffany. 2006. Characterization of feed streams and emissions from biomass gasification/combustion at fuel ethanol plants, paper 064180. Abstr. 2006 Am. Soc. Agr. Biol. Eng. Annu. Int. Meet. http://www.biomasschpethanol.umn.edu/papers/ASABEPaper064180.pdf.
- 26.Ohmiya, Y., T. Takeda, S. Nakamura, F. Sakai, and T. Hayashi. 1995. Purification and properties of a wall-bound endo-1,4-beta-glucanase from suspension-cultured poplar cells. Plant Cell Physiol. 36:607-614. [PubMed] [Google Scholar]
- 27.Okada, G. 1988. Cellulase of Aspergillus niger. Methods Enzymol. 160:259-264. [Google Scholar]
- 28.Oyekola, O. O., N. Ngesi, and C. G. Whiteley. 2007. Isolation, purification and characterisation of an endoglucanase and β-glucosidase from an anaerobic sulphidogenic bioreactor. Enzyme Microb. Technol. 40:637-644. [Google Scholar]
- 29.Palonen, H., and L. Viikari. 2004. Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 86:551-557. [DOI] [PubMed] [Google Scholar]
- 30.Pitson, S. M., R. J. Seviour, and B. M. McDougall. 1997. Purification and characterization of an extracellular β-glucosidase from the filamentous fungus Acremonium persicinum and its probable role in β-glucan degradation. Enzyme Microb. Technol. 21:182-190. [DOI] [PubMed] [Google Scholar]
- 31.Reddy, K. N., M. K. R. Reddy, and K. C. Rajanna. 1996. A kinetic study of the 1,10-phenanthroline-catalysed iron(III) oxidation of epimeric aldo-, and D-, and L-ketohexoses. Transit. Met. Chem. 21:112-116. [Google Scholar]
- 32.Riou, C., J. M. Salmon, M. J. Vallier, Z. Günata, and P. Barre. 1998. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 64:3607-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Dueñas, F. J., and Á. T. Martínez. 2009. Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2:164-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schink, B. 2006. Microbially driven redox reactions in anoxic environments: pathways, energetics, and biochemical consequences. Eng. Life Sci. 6:228-233. [Google Scholar]
- 35.Schülein, M. 1997. Enzymic properties of cellulases from Humicola insolens. J. Biotechnol. 57:71-81. [DOI] [PubMed] [Google Scholar]
- 36.Shifrin, N. S., B. D. Becka, T. D. Gauthierb, S. D. Chapnicka, and G. Goodman. 1996. Chemistry, toxicology, and human health risk of cyanide compounds in soils at former manufactured gas plant sites. Regul. Toxicol. Pharmacol. 23:106-116. [DOI] [PubMed] [Google Scholar]
- 37.Shvartsman, M., R. Kikkeri, A. Shanzer, and Z. I. Cabantchik. 2007. Non-transferrin-bound iron reaches mitochondria by a chelator-inaccessible mechanism: biological and clinical implications. Am. J. Physiol. Cell Physiol. 293:1383-1394. [DOI] [PubMed] [Google Scholar]
- 38.Singh, J., N. Batra, and R. C. Sobti. 2004. Purification and characterisation of alkaline cellulase produced by a novel isolate, Bacillus sphaericus JS1. J. Ind. Microbiol. Biotechnol. 31:51-56. [DOI] [PubMed] [Google Scholar]
- 39.Singh, R. N., and V. K. Akimenko. 1993. Isolation of a cellobiohydrolase of Clostridium thermocellum capable of degrading natural crystalline substrates. Biochem. Biophys. Res. Commun. 192:1123-1130. [DOI] [PubMed] [Google Scholar]
- 40.Verma, P. S., R. C. Saxena, and A. Jayaraman. 1997. Cyclic voltammetric studies of certain industrially potential iron chelate catalysts. Fresenius J. Anal. Chem. 357:56-60. [Google Scholar]
- 41.Wei, D., C. J. Houtman, A. N. Kapich, C. G. Hunt, D. Cullen, and K. E. Hammel. 2010. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol. 76:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei, H., Q. Xu, L. E. Taylor, J. O. Baker, M. P. Tucker, and S. Y. Ding. 2009. Natural paradigms of plant cell wall degradation. Curr. Opin. Biotechnol. 20:330-338. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, D. B. 2009. Cellulases and biofuels. Curr. Opin. Biotechnol. 20:295-299. [DOI] [PubMed] [Google Scholar]
- 44.Xu, F., and H. Ding. 2007. A new kinetic model for heterogeneous (or spatially confined) enzymatic catalysis: contributions from the fractal and jamming (overcrowding) effects. Appl. Catal. A 317:70-81. [Google Scholar]
- 45.Xu, F., H. Ding, and A. Tejirian. 2009. Detrimental effect of cellulose oxidation on cellulose hydrolysis by cellulase. Enzyme Microb. Technol. 45:203-209. [Google Scholar]
- 46.Xu, F., H. Ding, D. Osborn, A. Tejirian, K. Brown, W. Albano, N. Sheehy, and J. Langston. 2007. Partition of enzymes between the solvent and insoluble substrate during the hydrolysis of lignocellulose by cellulases. J. Mol. Catal. B. Enzym. 51:42-48. [Google Scholar]