Abstract
Different fermentation strategies were employed for the cultivation of a new poly(3-hydroxybutyrate)-accumulating thermophilic bacterium, Chelatococcus sp. strain MW10, with the aim of achieving high-cell-density (HCD) growth and high poly(3-hydroxybutyrate) [poly(3HB)] productivity. Enhanced cultivation was achieved by a cyclic fed-batch fermentation (CFBF) technique (42-liter scale). Maximal poly(3HB) productivity was obtained during the second cycle [16.8 ± 4.2 g poly(3HB)/liter]. At the end of CFBF (265 h), an HCD of up to 115.0 ± 4.3 g cell dry weight/liter was achieved.
Fermentations, which are performed with thermophilic microorganisms, are energy-efficient processes because few cooling efforts are necessary. Moreover, thermophilic fermentations are self-heated systems because the heat generated by the cell's metabolism during high-cell-density (HCD) growth (28, 49) and also by stirring can be used for providing heat to the fermentation process itself. Thus, both heating and cooling costs are lowered (14, 46). Additionally, unlike when mesophilic bacteria are used in fermentations, sterile conditions may not be essential during a process involving thermophilic bacteria (41, 42).
Unfortunately, thermophiles usually grow to only low cell densities. Recently, with the use of advanced fermentation technology, a few thermophilic bacteria were cultivated successfully, yielding HCD growth and enhanced product formation (15, 25). In industry, HCD cultivation is often a prerequisite for high productivity during fermentations, in particular if intracellular compounds are being produced. HCD cultivations are enhanced cultivations of the microbial strain achieving cell dry weight (CDW) concentrations exceeding 100 g/liter. However, a lower cell density can be regarded as an HCD as well, depending on the microorganism and its cultivation conditions (39). In general, HCD cultivations represent a 10- to 20-fold increase in growth in comparison to normal cell density growth. Problems encountered by HCD cultivation are numerous, such as partial O2 pressure (pO2) deficiency, by-product formation, and/or metabolic heat production. As a result of the growing industrial interest in HCD, many attempts have been made to develop HCD fermentations, for example, by improving potent strains and/or using different types of bioreactors and cultivation strategies (39).
The most industrially applicable technique is fed-batch fermentation (FBF). An FBF is a batch culture which is fed frequently with the limiting substrates without the removal of the culture fluid; therefore, the volume of an FBF culture discontinuously increases (32). A new, attractive alternative strategy of semicontinuous cultivation, cyclic fed-batch fermentation (CFBF), was investigated for HCD cultivation of microorganisms (31, 32). During CFBF, a partial withdrawal of the culture broth and subsequent refilling of the reactor with fresh medium can be utilized to prevent increases in concentrations of toxic by-products as well as increases in culture volume usually experienced during FBF (5, 6, 8, 24, 32, 33). In this context and because of chemical changes expected in growth medium during thermophilic cultivations, CFBF is considered to enhance the growth of thermophiles to achieve higher concentrations of final biomass and higher product yields.
Biodegradable biopolymers are often the preferred materials not only for environmental considerations but also in medical applications, such as developing therapeutic devices for tissue engineering and for slow-release drug delivery systems (20, 35). An attractive group of biopolymers are polyhydroxyalkanoates (PHAs), which are intracellularly accumulated as a source for carbon and energy (2). Numerous studies have been conducted to produce PHAs at reduced costs to replace conventional petroleum-based plastic, which would reduce the dependency on exhaustible fossil resources and contribute to a sustainable economy (48), advantages over and above the environmental advantages of polymer biodegradability (12).
Several fermentation strategies using different potent strains for HCD growth and high PHA contents in cheap cultivation media and enabling easy recovery have been described and used in numerous investigations and patented processes (4, 7, 22, 26, 29, 44). Not only should the costs of fermentation substrates be considered with regard to cost efficiency, but also every step of the production process. In this context, all costs of bioreactor operation are important, especially the cost of cooling during the exothermic HCD fermentation process (28), where thermophilic fermentations are recommended (49). Since most PHA-producing strains are mesophiles growing optimally at temperatures between 30 and 37°C, alternative PHA-producing thermophilic strains should be used (19).
To date, there have been only a few reports on the biosynthesis of PHAs in thermophilic microorganisms (13, 34, 36, 37, 40). However, none of them was used for the fermentative production of PHAs. Very recently, new thermophilic strains were isolated which accumulate poly(3-hydroxybutyrate) [poly(3HB)] up to 73% (wt/wt) of CDW at 50°C in cheap medium and which utilize renewable resources such as glucose or glycerol as carbon sources for growth and poly(3HB) synthesis (19). From these strains, Chelatococcus sp. strain MW10 was selected for this study. Several cultivation techniques were used for HCD thermophilic fermentation at the 2- and 42-liter scales.
Strain Chelatococcus sp. MW10 was grown in mineral salts medium (MSM) as described previously (19). FBF was performed in a 2-liter glass bioreactor (Biostat B plus; Sartorius BBI Systems GmbH, Melsungen, Germany). A well-grown second MSM preculture (36 h at 50°C) of the cells was used to inoculate 1.5 liters MSM (4% [vol/vol] inoculum size). Cultivations were carried out at 50°C. NaOH (2 N) was used for automatic pH control at 7.3. Glucose was supplied by occasional feeding using a 30% (wt/vol) glucose solution. Aeration and stirring were increased gradually up to 1.25 volumes per volume per minute (vvm) and to 700 rpm, respectively, according to growth requirements. The chemical antifoam agent Silikon Antischaum Emulsion SLE (Wacker, Darwin Vertriebs GmbH, Ottobrunn, Germany) was used automatically to combat foam formation in the culture. Samples of 40 ml were withdrawn at different intervals for analyses.
A Biostat UD-30 stainless steel reactor (B. Braun Biotech International, Melsungen, Germany) with a total volume of 42 liters was used for cultivations at the 42-liter scale as previously described (17). Cultivations were performed at 50°C. Unless stated otherwise, the pH in the medium was held at 6.7 by the controlled addition of NaOH (5 N). Foam was removed by a mechanical foam destroyer; if this was not sufficient, a chemical antifoam agent was added. Samples of 100 ml were withdrawn from the culture fluid for analytical purposes. Cells were harvested by centrifugation in a CEPA type Z41 or type Z61 continuous centrifuge (Carl Padberg Zentrifugenbau GmbH, Lahr, Germany). Harvested cells were frozen at −30°C and then lyophilized (Beta 1-16; Christ, Osterode, Germany).
Analyses of ammonium, residual carbon sources, CDW, and poly(3HB) contents of the cells were performed as previously described (18). Quick information about residual glucose concentration during fermentation was obtained using glucose strips (Diabur-Test 5000; Roche Diagnostics GmbH, Mannheim, Germany). The substrate conversion factors [g poly(3HB)/g glucose or g CDW/g glucose] were calculated as grams of poly(3HB) produced or grams of cells produced per gram of glucose used, respectively. All results are from duplicate measurements, and mean values and standard deviations are presented.
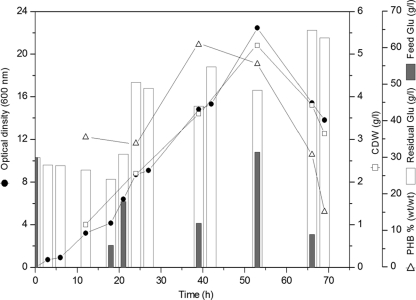
Chelatococcus sp. MW10 was cultivated in FBF at the 2-liter scale to provide excess carbon during growth, with the aims of achieving HCD growth and high poly(3HB) productivity by the strain and overcoming the problem of poly(3HB) degradation, which has been observed before in flask batch cultures (19). For this purpose, glucose was fed occasionally to keep its concentration above 20 g/liter. Figure 1 shows that maximum growth and poly(3HB) productivity were reached at 53 h [5.2 ± 0.6 g CDW/liter and 2.9 ± 0.7 g poly(3HB)/liter, respectively]. However, considerable decreases in both CDW (3.2 ± 0.2 g CDW/liter) and in poly(3HB) content (15.1% ± 3.7%, wt/wt) were recorded, in spite of the excess of carbon (>20 g glucose/liter) during the entire fermentation course. A corresponding decrease in optical density (OD) was also recorded (from 22.5 ± 0.2 to 13.8 ± 0.3 OD at 600 nm) during the polymer degradation phase.
FIG. 1.
Fed-batch fermentation for cultivation of the poly(3HB)-accumulating thermophile Chelatococcus sp. MW10. Cells were cultivated in a 2-liter glass bioreactor (Biostat B plus) containing 1.5 liters MSM with glucose as the sole carbon source. The bioreactor was inoculated with a 24-h-grown MSM preculture (4% [vol/vol] inoculum size). Aeration and agitation rates were increased gradually up to 1.25 vvm and 700 rpm, respectively. Cells were grown for 69 h at 50°C and pH 7.3. During the time course of cultivation, samples were withdrawn, and the concentrations of glucose as well as the cell dry weight (CDW) and poly(3HB) content of the cells were determined as described in the text.
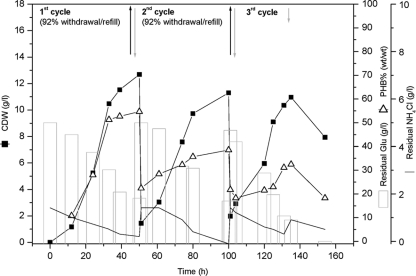
Cyclic batch fermentation (CBF) was conducted at a larger scale in a 42-liter bioreactor at 50°C (pH 6.7, 1.5 g NH4Cl/liter, and 50 g glucose/liter), utilizing the information obtained from the 2-liter FBF (Fig. 1) and from flask experiments (data not shown). Cycling time was designed (50-h cultivation batches) according to the results obtained with the 2-liter-scale FBF (Fig. 1). Cultivation was started with 25 liters MSM (4% [vol/vol] inoculum size). The pO2 in the medium was controlled automatically at 20% saturation by increasing the airflow and stirrer speed to 1.67 vvm and 400 rpm, respectively. During the first cycle, a high growth rate (μmax = 0.125/h) was achieved, as shown in Fig. 2. A significant increase in cell density up to 12.7 ± 0.9 g CDW/liter had occurred by the end of cycle 1 (50 h), which represents an increase of the cell density by a factor of 2.4 (in comparison to the cell density achieved by the 2-liter-scale FBF). The poly(3HB) content after 50 h of cultivation (55.0% ± 5.7%, wt/wt) (Fig. 2) was similar to that obtained after the same cultivation time during the FBF (Fig. 1; Table 1). Withdrawal of 23 liters of MSM and refilling with an equal volume of fresh MSM (without sterilization), representing 92% of the original culture volume, were performed at the end of each cycle. The culture broth remaining in the bioreactor (2 liters) was used as an inoculum for the next cycle (8% [vol/vol] inoculum size). Similar cell densities (11.3 ± 0.3 and 11.0 ± 0.8 g CDW/liter) but lower poly(3HB) contents (38.5% ± 6.4% and 32.5% ± 3.0%, wt/wt) were obtained during the second and third cycles, respectively. Enough residual glucose (between 15 and 20 g/liter) was detected at the end of each cycle. It was exhausted only when additional nitrogen was fed after the third cycle (at 139 h).
FIG. 2.
Cyclic batch fermentation (CBF) for cultivation of Chelatococcus sp. MW10 under thermophilic conditions. Cultivation was conducted in a Biostat UD-30 stirred-tank reactor containing 25 liters MSM with an initial glucose concentration of 50 g/liter as the sole carbon source. The fermentor was inoculated with a 24-h-grown MSM preculture (4% [vol/vol] inoculum size). Culture temperature and pH were controlled at 50°C and at pH 6.7, respectively, during the entire course of the fermentation. Aeration and agitation rates were controlled automatically by adjusting the pO2 at 20% saturation. Cycling of cultures was operated on a 50-h cycle by withdrawal of 92% of the cultivation medium (black arrows) and refilling with an equal volume of fresh MSM (gray arrows) with glucose (50 g/liter). During the time course of cultivation, samples were withdrawn, and the concentrations of glucose and ammonium as well as the cell dry weight (CDW) and poly(3HB) content of the cells were determined as described in the text.
TABLE 1.
Comparison of different techniques for cultivation of the poly(3HB)-accumulating thermophile Chelatococcus sp. MW10a
| Fermentation technique | Cultivation scale | OD at 600 nm | CDW (g/liter) | Poly(3HB) content (%, wt/wt) | Poly(3HB) produced (g/liter) | Cell yield (g/g)b | Poly(3HB) yield (g/g)c |
|---|---|---|---|---|---|---|---|
| Batch culture | 250-ml flaskd | 18.5 ± 1.4 | 4.4 ± 0.3 | 69.0 ± 4.8 | 3.0 ± 0.4 | 0.15 | 0.10 |
| Fed-batch fermentation | 2-liter fermentor | 22.5 ± 0.2 | 5.2 ± 0.6 | 55.5 ± 7.1 | 2.9 ± 0.7 | 0.08 | 0.05 |
| Cyclic batch fermentation | 42-liter fermentor | ||||||
| Cycle 1 | 29.0 ± 0.6 | 12.7 ± 0.9 | 55.0 ± 5.7 | 7.0 ± 1.2 | 0.25 | 0.14 | |
| Cycle 2 | 24.6 ± 1.1 | 11.3 ± 0.4 | 38.5 ± 6.4 | 4.3 ± 0.9 | 0.23 | 0.09 | |
| Cycle 3 | 19.7 ± 0.5 | 11.0 ± 0.8 | 32.5 ± 3.0 | 3.6 ± 0.6 | 0.22 | 0.07 | |
| Cyclic fed-batch fermentation | 42-liter fermentor | ||||||
| Cycle 1 | 25.2 ± 0.9 | 9.1 ± 0.7 | 40.4 ± 2.8 | 3.7 ± 0.5 | 0.23 | 0.09 | |
| Cycle 2 | 89.0 ± 3.1 | 43.0 ± 1.4 | 39.0 ± 8.5 | 16.8 ± 4.2 | 0.27 | 0.11 | |
| Cycle 3 | 174.6 ± 4.7 | 115.0 ± 4.2 | 11.8 ± 3.8 | 13.7 ± 4.9 | 0.60 | 0.07 |
Fermentation techniques, medium components, feeding solutions, and cultivation conditions were as described in the text. All cultivations were conducted at 50°C. Analyses were done in duplicate; averages and standard deviations are presented.
The substrate conversion factor for cells is given in g CDW/g glucose used.
The substrate conversion factor for poly(3HB) is given in g poly(3HB)/g glucose used.
Flask experiments with 30 g glucose/liter (data not shown).
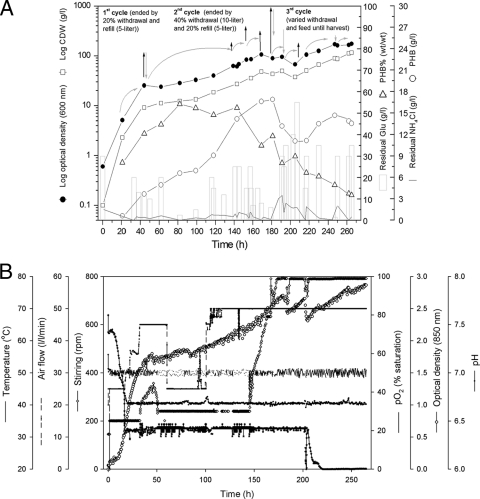
A modified semicontinuous culture technique, cyclic fed-batch fermentation (CFBF), was also conducted at a 42-liter scale (Fig. 3); during this process, variable volumes from the growth culture were withdrawn and replaced with fresh medium as required, thereby partially recycling 20 to 40% of the culture volume. The cycling of culture was performed carefully at different intervals, taking into account the increased culture volume due to the semicontinuous feeding and also the decreased pO2 concentration during HCD FBF. The semicontinuous feeding was performed to maintain adequate concentrations of glucose and ammonium, with the aim of reaching high cell densities and high poly(3HB) contents. Cultivation was started as a batch culture, as described above, in the CBF, with 30 g/liter as the initial glucose concentration. Feeding was started after 21 h of growth using a solution consisting of glucose (30%, wt/vol), MgSO4 (0.5%, wt/vol), trace elements solution (2.5%, vol/vol), CaCl2 (0.25%, wt/vol), and Fe(III)NH4+-citrate (0.25%, wt/vol). The first cycling was done after 44 h of cultivation, when the cells grew at a relatively high growth rate (μ = 0.070/h) and before poly(3HB) degradation was expected to occur. Five liters of the culture was withdrawn (20%, vol/vol) and replaced with the same volume of fresh MSM containing glucose. Following that, continuous feeding was performed, and then withdrawal/refilling cycles were done as needed to reduce the increasing culture volume. The second cycle was finished by withdrawal of 40% of the culture volume (10 liters) and by refilling with only 5 liters fresh medium to prevent severe dilution of the culture broth, which could affect the growth rate and by-product accumulation in the culture broth. After 14 h in the third cycle, the remaining refilling volume (5 liters fresh MSM) was fed. During this cycle, feeding was continued, with the offline analysis of glucose and ammonium concentrations taken into account. The highest poly(3HB) contents (over 50%, wt/wt) were reached between 82 and 143 h during cycle 2 (Fig. 3A). At the end of this cycle (after 181 h of cultivation), a high cell density (43.0 ± 1.4 g CDW/liter), an adequate poly(3HB) content (39.0% ± 8.5%, wt/wt), and the maximal poly(3HB) volumetric productivity [16.8 ± 4.2 g poly(3HB)/liter] were achieved. A drastic increase in cell growth was recorded at the end of the last cycle (cycle 3), when an optical density of 174.6 ± 4.7 at 600 nm and a cell density as high as 115.0 ± 4.3 g CDW/liter were achieved. Although a lower poly(3HB) content was detected at the end of this fermentation (11.8% ± 3.8%, wt/wt), an adequate volumetric poly(3HB) productivity [13.7 ± 4.9 g poly(3HB)/liter] was retained (Fig. 3A; Table 1).
FIG. 3.
Cyclic fed-batch fermentation (CFBF) for HCD cultivation of the thermophile Chelatococcus sp. MW10. Cultivation was done in a Biostat UD-30 stirred-tank reactor containing 25 liters MSM with glucose as the sole carbon source. The bioreactor was inoculated with a 24-h-grown MSM preculture (4% [vol/vol] inoculum size). (A) Glucose was fed semicontinuously, keeping its concentration higher than 20 g/liter. The glucose feeding solution (30%, wt/vol) was supplemented with MgSO4 and other nutrients as described in the text. The cycling of cultures was conducted carefully at different intervals, as indicated by black arrows (withdrawal) and gray arrows (refill). Curved gray arrows indicate the semicontinuous feeding. During the time course of cultivation, samples were withdrawn, and the concentrations of glucose and ammonium as well as the cell dry weight (CDW) and poly(3HB) content of the cells were determined as described in the text. (B) Parameters obtained by online monitoring. The optical density was monitored at 850 nm; aeration and agitation rates were controlled automatically by adjusting the pO2 at 20% saturation; the pH of the medium was controlled at 6.7 using NaOH (5 N); and the fermentation temperature was controlled at 50°C.
Intracellular degradation of poly(3HB) was first attributed to the exhaustion of the carbon source in the cultivation medium, as previously hypothesized (3, 21). However, in the present study a decreased poly(3HB) content was also noticed in spite of the availability of excess glucose during the entire FBF (Fig. 1). In this context, the CBF was performed with the aim of reaching an HCD as high as possible before the culture entered into the decline phase, during which excessive degradation of poly(3HB) usually occurred. The cycling of batch cultures (Fig. 2) is a simple technique for partial or total harvest of the culture to save fermentor installation time, making use of the high temperature at which this fermentation is conducted (50°C), where contamination is unlikely to occur. However, a method for the application of this strategy for high poly(3HB) productivity must still be designed after a detailed study of the effect of cycling time in correlation with the growth rate, as recommended by Bushell et al. (5).
Further modifications were made using CFBF (Fig. 3), a strategy by which series of FBFs can be achieved if aseptic conditions are maintained and if the respective product (cells and/or metabolites) can be attained (10, 11, 23, 43). The volumetric poly(3HB) productivities achieved during CFBF were about five times higher than those attained by the other cultivation techniques investigated in this study (Table 1). Also, a very high cell yield (0.60 g CDW/g glucose) was achieved. The total amount of harvested cells was 4.96 kg (CDW). This cell yield is higher than the cell yield reached by the 2-liter-scale FBF (0.08 g CDW/g glucose, at 53 h) and by the 42-liter-scale CBF (0.25 g CDW/g glucose, at 50 h) (Table 1). Regarding poly(3HB) yields, the maximal yield was attained in the first cycle of CBF [0.14 g poly(3HB)/g glucose]. Comparable yields were also recorded during the 42-liter-scale CFBF, especially during the first and second cycles [0.09 and 0.11 g poly(3HB)/g glucose, respectively] (Table 1).
The semicontinuous feeding regime, which was used during CFBF, minimally controlled the concentrations of glucose and ammonium during the entire fermentation time (Fig. 3A); this may be due to the relatively high evaporation rate at this elevated temperature (42). Obvious pO2 deficiency was also detected in the HCD culture during the third cycle (Fig. 3B).
Bench-scale cyclic fed-batch culture (1.8-liter bioreactor) and repeated batch cultivation (7-liter bioreactor) have been successfully applied previously for the production of human serum albumin by Pichia pastoris GS115 HIS4 (5) and for poly(3HB) production by Ralstonia eutropha NRRL B14690 (23), respectively. More complicated fermentation techniques, such as cell recycle fed-batch fermentation (6.6-liter bioreactor), which was used for cultivation of a recombinant Escherichia coli strain (1), or complete cell recycle continuous cultivation and dialysis fed-batch fermentation (2- and 4-liter bioreactors), which were used for the extremophiles Thermoanaerobium brockii, Pyrococcus furiosus, and Sulfolobus shibatae (15, 25), were all reported to achieve significantly enhanced growth with higher cell densities than those obtained by conventional fermentation techniques.
It should be noted that the decreased content of accumulated poly(3HB) observed with all the cultivation techniques used in this study is still not fully understood. The high cultivation temperature may contribute to this problem. A CFBF with online control of poly(3HB) content via flow cytometry analysis (38) is supposed to be a vital tool during poly(3HB) production; thereby an exact time for culture cycling could be easily determined, achieving high yields. On the other hand, the decreased poly(3HB) productivity or the intracellular mobilization (degradation) of poly(3HB) may be correlated with the production of other polymers such as exopolysaccharides (EPS). Although EPS were not analyzed during this study, formation of EPS is expected for this strain because of its mucous-colony shape, observed previously (19).
The relationship between EPS production and intracellular poly(3HB) accumulation/mobilization has been studied before during balanced and unbalanced growth of many mesophilic bacterial species (9, 27, 45). Interestingly, the degradation of poly(3HB) can be investigated for the in vivo production of enantiomerically pure (R)-3-hydroxybutyric acid, which has various industrial and medical applications (30, 47).
In conclusion, operating CFBF under thermophilic conditions represents an easy-to-apply process with simple fermentor infrastructure and quality control during the withdrawal/refilling processes. This is the first report on the application of CFBF for HCD cultivation of poly(3HB)-accumulating thermophiles in a stirred-tank reactor, with the aim of developing attractive energy-saving processes.
Acknowledgments
Financial support of this study by BASF AG (Ludwigshafen, Germany) is gratefully acknowledged.
Footnotes
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Ahn, W. S., S. J. Park, and S. Y. Lee. 2001. Production of poly(3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol. Lett. 23:235-240. [Google Scholar]
- 2.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 4.Braunegg, G., M. Koller, P. Varila, C. Kutschera, R. Bona, C. Hermann, P. Horvat, J. Neto, and L. Pereira. 2007. Production of plastics from waste derived from agrofood industry, p. 119-135. In M. Graziani and P. Fornasiero (ed.), Renewable resources and renewable energy: a global challenge. CRC Press, Taylor and Francis Group, Boca Raton, FL.
- 5.Bushell, M. E., M. Rowe, C. A. Avignone-Rossa, and J. N. Wardell. 2003. Cyclic fed-batch culture for production of human serum albumin in Pichia pastoris. Biotechnol. Bioeng. 82:678-683. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C. C., D. D. Y. Ryu, C. S. Park, and J. Y. Kim. 1998. Improvement of heterologous protein productivity using recombinant Yarrowia lipolytica and cyclic fed-batch process strategy. Biotechnol. Bioeng. 59:379-385. [DOI] [PubMed] [Google Scholar]
- 7.Choi, J., and S. Y. Lee. 1999. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 51:13-21. [Google Scholar]
- 8.Cinar, A., S. J. Parulekar, C. Ündey, and G. Birol. 2003. Process control, p. 348-410. In A. Cinar et al. (ed.), Batch fermentation: modeling, monitoring, and control. Marcel Dekker, Inc., New York, NY.
- 9.Goff, M., J. Nikodinovic-Runic, and K. E. O'Connor. 2009. Characterization of temperature-sensitive and lipopolysaccharide overproducing transposon mutants of Pseudomonas putida CA-3 affected in PHA accumulation. FEMS Microbiol. Lett. 292:297-305. [DOI] [PubMed] [Google Scholar]
- 10.Gray, P. P., and K. Vu-Trong. 1987. Production of the macrolide antibiotic tylosin in cyclic fed-batch culture. Biotechnol. Bioeng. 29:33-40. [DOI] [PubMed] [Google Scholar]
- 11.Gray, P. P., and K. Vu-Trong. 1987. Extended cyclic fed-batch tylosin fermentations. Biotechnol. Lett. 9:617-620. [DOI] [PubMed] [Google Scholar]
- 12.Gross, R. A., and B. Kalra. 2002. Biodegradable polymers for the environment. Science 297:803-807. [DOI] [PubMed] [Google Scholar]
- 13.Hai, T., S. Hein, and A. Steinbüchel. 2001. Multiple evidence for widespread and general occurrence of type-III PHA synthases in cyanobacteria and molecular characterization of the PHA synthases from two thermophilic cyanobacteria: Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp. strain MA19. Microbiology 147:3047-3060. [DOI] [PubMed] [Google Scholar]
- 14.Hensing, M., H. Vrouwenvelder, C. Hellinga, R. Baartmans, and H. van Dijken. 1994. Production of extracellular inulinase in high-cell-density fed-batch cultures of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 42:516-521. [Google Scholar]
- 15.Holst, O., Å. Manelius, M. Krahe, H. Märkl, N. Raven, and R. Sharp. 1997. Thermophiles and fermentation technology. Comp. Biochem. Physiol. 118A:415-422. [Google Scholar]
- 16.Reference deleted.
- 17.Ibrahim, M. H. A., and A. Steinbüchel. 2009. Poly(3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl. Environ. Microbiol. 75:6222-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim, M. H. A., and A. Steinbüchel. 2010. Zobellella denitrificans strain MW1, a newly isolated bacterium suitable for poly(3-hydroxybutyrate) production from glycerol. J. Appl. Microbiol. 108:214-225. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim, M. H. A., A. Willems, and A. Steinbüchel. 2010. Isolation and characterization of new poly(3HB)-accumulating star-shaped cell-aggregates-forming thermophilic bacteria. J. Appl. Microbiol. 109:1579-1590. [DOI] [PubMed] [Google Scholar]
- 20.Ikada, Y., and H. Tsuji. 2000. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 21:117-132. [Google Scholar]
- 21.Jendrossek, D. 2001. Microbial degradation of polyesters, p. 293-325. In W. Babel and A. Steinbüchel (ed.), Advances in biochemical engineering/biotechnology: biopolyesters, vol. 71. Springer-Verlag Berlin, Heidelberg, Germany. [DOI] [PubMed] [Google Scholar]
- 22.Kessler, B., R. Weusthuis, B. Witholt, and G. Eggink. 2001. Production of microbial polyesters: fermentation and downstream processes, p. 159-182. In W. Babel and A. Steinbüchel (ed.), Advances in biochemical engineering/biotechnology: biopolyesters, vol. 71. Springer-Verlag Berlin, Heidelberg, Germany. [DOI] [PubMed] [Google Scholar]
- 23.Khanna, S., and A. K. Srivastava. 2005. Repeated batch cultivation of Ralstonia eutropha for poly(β-hydroxybutyrate) production. Biotechnol. Lett. 27:1401-1403. [DOI] [PubMed] [Google Scholar]
- 24.Kosaric, N., and F. Vardar-Sukan. 2001. Fermentation modes of industrial interest, p. 139-149. In M. Roehr (ed.), The biotechnology of ethanol: classical and future applications. Wiley-VCH GmbH, Weinheim, Germany.
- 25.Krahe, M., G. Antranikian, and H. Märkl. 1996. Fermentation of extremophilic microorganisms. FEMS Microbiol. Rev. 18:271-285. [Google Scholar]
- 26.Kurdikar, D. L., F. E. Strauser, A. J. Solodar, and M. D. Paster. July 2000. High temperature PHA extraction using PHA-poor solvents. U.S. patent 6,087,471.
- 27.Lama, L., B. Nicolaus, V. Calandrelli, M. C. Manca, I. Romano, and A. Gambacorta. 1996. Effect of growth conditions on endo- and exopolymer biosynthesis in Anabaena cylindrica 10 C. Phytochemistry 42:655-659. [Google Scholar]
- 28.Laska, M. E., and C. L. Cooney. 1999. Bioreactors, continuous stirred-tank reactors, p. 353-371. In M. C. Flickinger and S. W. Drew (ed.), Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation, vol. 1-5. John Wiley and Sons, Inc., New York, NY. [Google Scholar]
- 29.Lee, S. Y., J. I. Choi, and S. H. Lee. 2000. Production of polyhydroxyalkanoates by fermentation of bacteria. Macromol. Symp. 159:259-266. [Google Scholar]
- 30.Lee, S. Y., S. H. Park, Y. Lee, and S. H. Lee. 2002. Production of chiral and other valuable compounds from microbial polyester, p. 375-387. In Y. Doi and A. Steinbüchel (ed.), Biopolymers, vol. 4. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 31.Lynch, H. C., and M. E. Bushell. 1995. The physiology of erythromycin biosynthesis in cyclic fed batch culture. Microbiology 141:3105-3111. [DOI] [PubMed] [Google Scholar]
- 32.Macauley-Patrick, S., and B. Finn. 2008. Modes of fermenter operation, p. 69-95. In B. McNeil and L. M. Harvey (ed.), Practical fermentation technology. John Wiley and Sons, Ltd., West Sussex, England.
- 33.Marchal, R., M. Warzywoda, and B. Chaussepied. March 1999. Process for the production of sophorolipids by cyclic fermentation with feed of fatty acid esters or oils. U.S. patent 5,879,913.
- 34.Miyake, M., M. Erata, and Y. Asada. 1996. A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J. Ferment. Bioeng. 82:512-514. [Google Scholar]
- 35.Nair, L. S., and C. T. Laurencin. 2007. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32:762-798. [Google Scholar]
- 36.Pantazaki, A. A., M. G. Tambaka, V. Langlois, P. Guerin, and D. A. Kyriakidis. 2003. Polyhydroxyalkanoate (PHA) biosynthesis in Thermus thermophilus: purification and biochemical properties of PHA synthase. Mol. Cell. Biochem. 254:173-183. [DOI] [PubMed] [Google Scholar]
- 37.Pantazaki, A. A., A. K. Ioannou, and D. A. Kyriakidis. 2005. A thermostable β-ketothiolase of polyhydroxyalkanoates (PHAs) in Thermus thermophilus: purification and biochemical properties. Mol. Cell. Biochem. 269:27-36. [DOI] [PubMed] [Google Scholar]
- 38.Rieseberg, M., C. Kasper, K. F. Reardon, and T. Scheper. 2001. Flow cytometry in biotechnology. Appl. Microbiol. Biotechnol. 56:350-360. [DOI] [PubMed] [Google Scholar]
- 39.Riesenberg, D., and R. Guthke. 1999. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 51:422-430. [DOI] [PubMed] [Google Scholar]
- 40.Sheu, D. S., W. M. Chen, J. Y. Yang, and R. C. Chang. 2009. Thermophilic bacterium Caldimonas taiwanensis produces poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme Microb. Technol. 44:289-294. [Google Scholar]
- 41.Singh, D., I. M. Banat, P. Nigam, and R. Marchant. 1998. Industrial scale ethanol production using the thermotolerant yeast Kluyveromyces marxianus IMB3 in an Indian distillery. Biotechnol. Lett. 20:753-755. [Google Scholar]
- 42.Sonnleitner, B., and A. Fiechter. 1983. Advantages of using thermophiles in biotechnological processes: expectation and reality. Trends Biotechnol. 1:74-80. [Google Scholar]
- 43.Stanbury, P. F., A. Whitaker, and S. Hall. 1995. Microbial growth kinetics, p. 13-34. In P. F. Stanbury et al. (ed.), Principles of fermentation technology. Butterworth Heinemann, Oxford, United Kingdom.
- 44.Steinbüchel, A., and B. Füchtenbusch. 1998. Bacterial and other biological systems for polyester production. Trends Biotechnol. 16:419-427. [DOI] [PubMed] [Google Scholar]
- 45.Wang, J., and H. Q. Yu. 2007. Biosynthesis of polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Appl. Microbiol. Biotechnol. 75:871-878. [DOI] [PubMed] [Google Scholar]
- 46.Wiegel, J., and L. G. Ljungdahl. 1985. The importance of thermophilic bacteria in biotechnology. Crit. Rev. Biotechnol. 3:39-108. [Google Scholar]
- 47.Xiao, X. Q., Y. Zhao, and G. Q. Chen. 2007. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 28:3608-3616. [DOI] [PubMed] [Google Scholar]
- 48.Yang, S. T. 2007. Bioprocessing—from biotechnology to biorefinery, p. 1-24. In S. T. Yang (ed.), Bioprocessing for value-added products from renewable resources. Elsevier Science B.V., Amsterdam, Netherlands.
- 49.Zeikus, J. G. 1979. Thermophilic bacteria: ecology, physiology and technology. Enzyme Microb. Technol. 1:243-252. [Google Scholar]