Abstract
Soil microorganisms drive critical functions in plant-soil systems. As such, various microbial properties have been proposed as indicators of soil functioning, making them potentially useful in evaluating the recovery of polluted soils via phytoremediation strategies. To evaluate microbial responses to metal phytoextraction using hyperaccumulators, a microcosm experiment was carried out to study the impacts of Zn and/or Cd pollution and Thlaspi caerulescens growth on key soil microbial properties: basal respiration; substrate-induced respiration (SIR); bacterial community structure as assessed by PCR-denaturing gradient gel electrophoresis (DGGE); community sizes of total bacteria, ammonia-oxidizing bacteria, and chitin-degrading bacteria as assessed by quantitative PCR (Q-PCR); and functional gene distributions as determined by functional gene arrays (GeoChip). T. caerulescens proved to be suitable for Zn and Cd phytoextraction: shoots accumulated up to 8,211 and 1,763 mg kg−1 (dry weight [DW]) of Zn and Cd, respectively. In general, Zn pollution led to decreased levels of basal respiration and ammonia-oxidizing bacteria, while T. caerulescens growth increased the values of substrate-induced respiration (SIR) and total bacteria. In soils polluted with 1,000 mg Zn kg−1 and 250 mg Cd kg−1 (DW), soil bacterial community profiles and the distribution of microbial functional genes were most affected by the presence of metals. Metal-polluted and planted soils had the highest percentage of unique genes detected via the GeoChip (35%). It was possible to track microbial responses to planting with T. caerulescens and to gain insight into the effects of metal pollution on soilborne microbial communities.
Phytoremediation, the use of green plants to remove pollutants from the environment or to render them harmless (4), has great potential for the remediation of polluted soils. “Phytoextraction” refers to the utilization of plants to transport and concentrate metals from the soil into the aboveground shoots (34). Thlaspi caerulescens, a hyperaccumulating plant extensively studied due to its remarkable capacity to phytoextract zinc (Zn) and cadmium (Cd) from polluted soils (8, 15, 16), has been suggested as a model species for research on metal phytoextraction (1).
Traditionally, when evaluating the success of a phytoextraction process, emphasis has mostly been placed on metal removal from soil. However, most importantly, the ultimate goal of any phytoextraction process must be not only to remove the metal(s) from the soil, but also to restore soil health/functioning. Soil microbial properties, particularly those reflecting the biomass, activity, and diversity of the soil microbial communities, have great potential as bioindicators of the effects of disturbances (e.g., metal pollution) on soil functioning (7).
DNA microarray technologies are rapidly becoming important tools for the analysis of complex microbial communities inhabiting various environments (14, 44, 47, 50, 56, 57). A recently developed functional gene array, termed GeoChip (14), has proven to be a valuable tool in the assessment of microbial-community responses to a variety of environmental conditions, including metal pollution (42). Since this platform contains probes for genes with key biological functions (e.g., those involved in nitrogen, carbon, sulfur, and phosphorus cycling; metal reduction and resistance; and degradation of organic contaminants), it is particularly well suited for assessing the recovery of soilborne microbial communities from perturbations such as metal pollution. On the other hand, ammonia-oxidizing and chitin-degrading bacteria perform important roles within the soil ecosystem: the amoA gene codes for the α subunit of ammonia monooxygenase, a key enzyme in aerobic ammonia-oxidizing bacteria that catalyzes the rate-limiting process of nitrification, i.e., the oxidation of ammonia to hydroxylamine (53); in turn, chitin (poly-N-acetyl-glucosamine) is one of the most abundant biopolymers on Earth (39).
The objective of this work was to determine the impact of metal (Zn and Cd) pollution and T. caerulescens growth on soil functioning through the determination of soil microbial properties with potential as bioindicators of soil health: basal respiration; substrate-induced respiration; bacterial community structure as assessed by PCR-denaturing gradient gel electrophoresis (DGGE); community sizes of total bacteria, ammonia-oxidizing bacteria, and chitin-degrading bacteria as assessed by quantitative PCR (Q-PCR); and functional gene distributions via GeoChip analysis. The capacity of T. caerulescens to phytoextract Zn and Cd from polluted soil was also assessed.
MATERIALS AND METHODS
Experimental design.
A 4-month microcosm phytoextraction experiment was carried out with soil collected from the top layer (0 to 30 cm) of a natural grassland located in Derio (northern Spain). Immediately after collection, the soil was sieved to <4 mm. Small root particles were removed manually as far as possible. A soil subsample was air dried, passed through a 2-mm sieve, and subjected to physicochemical characterization (30). The water content in soil samples was calculated by drying an aliquot of soil at 105°C for 24 h; soil parameters were corrected for water content. The soil was a clay loam with a pH of 5.2, an organic matter (OM) content of 4.12%, a total nitrogen (N) content of 0.23%, a C/N ratio of 10.4, a phosphorus (P) content of 26.4 mg kg−1, an electrical conductivity of 0.08 dS m−1, a Zn concentration of 70.8 mg kg−1 (dry weight [DW]), and a Cd concentration of <0.80 mg kg−1 (DW). Subsequently, the soil was artificially polluted with combinations of different concentrations of Zn (250, 500, and 1,000 mg kg−1 [DW]) in the form of ZnCl2 and Cd (0, 50, and 250 mg kg−1 [DW]) in the form of CdCl2. The following 9 combinations of metal pollution were studied (in mg kg−1 [DW]): (i) 250 Zn plus 0 Cd (control-unpolluted; this small concentration of Zn was added to support T. caerulescens growth), (ii) 250 Zn plus 50 Cd, (iii) 250 Zn plus 250 Cd, (iv) 500 Zn plus 0 Cd, (v) 500 Zn plus 50 Cd, (vi) 500 Zn plus 250 Cd, (vii) 1,000 Zn plus 0 Cd, (viii) 1,000 Zn plus 50 Cd, and (ix) 1,000 Zn plus 250 Cd. Metal-polluted soils were stored at a constant room temperature of 16°C and an average 15% relative humidity for 6 months. Study pots (17-cm diameter by 13-cm height) were filled with 1.5 kg (DW) of each of these polluted soils, which were then fertilized with 120 mg kg−1 of N, P, and K+ and subsequently allowed to precondition, under the greenhouse conditions described below, for 1 week.
Concurrently, T. caerulescens J. and C. Presl. seeds of a local ecotype, named Lanestosa, were germinated for 2 weeks (on a mixture of perlite and vermiculite, 2:3 [vol/vol], moistened with deionized water) in a growth chamber under the following controlled conditions: 20/16°C day/night temperatures, 70% relative humidity, and a photosynthetic photon flux density of 300 μmol photons m−2 s−1 by supplementing natural illumination with white cold lamps. Then, half of the study pots were planted with five T. caerulescens seedlings per pot, and the other half were kept unplanted as controls. A total of 18 treatments were studied (each in quadruplicate) in this experiment: 9 metal combinations (see above) for planted pots and 9 metal combinations for unplanted pots. The plants were then allowed to grow for 4 months in a soft polyethylene-covered greenhouse (Venlo type) located in Derio (northern Spain) at latitude 43°17′N, longitude 2°52′W, and an altitude of 65 m above sea level. The climate in this region is Atlantic temperate. The minimal temperature set points controlling air heating were 14/18°C night/day, and the maximal temperature set points were 18/20°C night/day. Vent opening temperatures were 20/25°C night/day. During the experiment, the average temperatures were 15/24°C night/day, with an average relative humidity of 60% and an average photosynthetically active radiation of 459 μmol photon m−2 s−1. Throughout the experiment, plants were bottom watered periodically (as required to maintain a 1-cm depth in the pot plates).
After 4 months, shoots and roots were harvested by cutting the shoots exactly at the swelling formed in the root-shoot junction, and the metal contents were determined (see below). Half of the soil present in the study pots was sampled, and soil physicochemical and microbial properties were determined (see below). The remaining half was used to grow alfalfa (Medicago sativa L.) in order to assess the capacity of the soil to support plant growth at the end of the experiment: 1 g of alfalfa seeds (approximately 100 seeds) was sown in each study pot. The alfalfa plants were then allowed to grow under the same greenhouse conditions described above for 2 months (the plants were bottom watered periodically as described above). Finally, shoots were harvested, washed thoroughly with deionized water, and oven dried at 70°C for 48 h, and their dry weights were recorded.
Soil physicochemical parameters.
Soil was sampled at the end of the experiment, i.e., 4 months after T. caerulescens plant transplantation. For soil physicochemical analysis, soils were air dried and sieved to <2 mm. Soil pH, OM content, total N, nitrate content, extractable P, and potassium (K+) were measured following standard methods (30). Total concentrations of metals in soil were determined using flame atomic absorption spectrometry (AAS) (Varian) following digestion with a mixture of HNO3/HClO4 (55). The CaCl2-extractable (0.01 M CaCl2) fraction of metals was determined according to the method of Houba et al. (18) and analyzed using AAS.
Soil microbial parameters.
For microbial parameters, soils were sieved to <2 mm and stored fresh at 4°C until they were analyzed. Soil basal respiration was determined according to International Organization for Standardization (ISO) norm 16072 (Laboratory Methods for Determination of Microbial Soil Respiration) at 30°C and a water holding capacity (WHC) of 60%. Substrate-induced respiration (SIR) was determined, with glucose as a substrate, following ISO norm 17155 (Determination of Abundance and Activity of Soil Microflora Using Respiration Curves). The microbial respiration quotient (QR) was calculated (QR = basal respiration/SIR).
Soil samples for DNA analysis were sieved to <2 mm and stored fresh at −20°C. DNA was extracted from soil samples (0.25 g soil) using a Power Soil DNA Isolation Kit (MO Bio Laboratories, CA) according to the manufacturer's specifications. Prior to DNA extraction, soil samples were washed twice in 120 mM K2HPO4 (pH 8.0) to wash away extracellular DNA (21). The amounts of DNA in our samples were determined on an ND-1000 spectrophotometer.
Q-PCR measurements of gene copy abundance were carried out according to the method of Yergeau et al. (51, 52). The primers and amplification regimes used to assess (i) 16S rRNA gene fragments for total bacteria, (ii) ammonia monooxygenase genes (amoA) for ammonia-oxidizing bacteria, and (iii) group A bacterial chitinases for chitin-degrading bacteria are summarized in Table 1.
TABLE 1.
Primers and amplification regimes used in Q-PCR
DGGE and GeoChip analyses were performed for only the four most extreme treatments of the study (in mg kg−1 [DW]): 250 Zn plus 0 Cd planted, 250 Zn plus 0 Cd unplanted, 1,000 Zn plus 250 Cd planted, and 1,000 Zn plus 250 Cd unplanted. For DGGE, bacterial 16S rRNA gene fragments were amplified by using the primer pair F968-GC/R1378 (17). PCR and DGGE analyses were carried out following the method of Yergeau et al. (51). The resulting binary (presence-absence) matrices from the DGGE were used to calculate Jaccard similarity indexes (CJ) among treatments (CJ = a/a + b + c, where a is the total number of bands present in both treatments, b is the number of bands present only in the first treatment, and c is the number of bands present only in the second treatment) (19).
Before GeoChip analysis, a desalting protocol was applied to the DNA in order to remove contaminants and improve 260/230 ratios. Samples were precipitated with 2.5× volumes of 100% ice-cold ethanol and a 1:10 volume of NaOAc (3 M; pH 5.2). After 3 h of incubation at room temperature, the samples were centrifuged (13,000 × g; 30 min), and the resulting pellet was washed with 70% ethanol. The samples were then centrifuged (13,000 × g; 10 min), decanted, dried at room temperature, and finally resuspended in 0.1 ml of nuclease-free water. The rest of the protocol for the GeoChip analyses is a modification of the one described by Yergeau et al. (52). Due to the small amounts of DNA recovered from several samples, rolling-circle amplification was carried out prior to GeoChip analysis, using the TempliPhi kit (Amersham, Piscataway, NJ) according to the manufacturer's specifications. Spermidine (0.1 μg μl−1) and single-stranded DNA binding protein (∼260 ng μl−1) were added to the reaction mixtures to facilitate amplification. The reaction mixtures were incubated at 30°C for 6 h, and the enzyme was then inactivated by incubation at 65°C for 10 min and cooled at 4°C. The rolling-circle amplification products were labeled with Cy5 dye, and amplification products were denatured for 5 min at 99.9°C in a solution containing 1× random octamer primer mixture (Invitrogen, Carlsbad, CA) and immediately chilled on ice. Following denaturation, the following components were added: 50 nmol dATP, dGTP, and dCTP; 25 nmol dTTP; 25 nM Cy5 dye-inserted dTTP; and 80 U of the large Klenow fragment (the large fragment of DNA polymerase I; Invitrogen). The reaction mixtures were then incubated at 37°C for 6 h. Labeled target DNA was purified with a QIAquick PCR kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, measured on an ND-1000 spectrophotometer, and dried in a Speed Vac at 45°C for 45 min. Prior to hybridization, dried, labeled DNA was resuspended in a solution containing 50% formamide, 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.3% SDS, 0.7 μg μl−1 herring sperm DNA, and 0.8 nM dithiothreitol (DTT). This solution was incubated at 98°C for 3 min and kept at 65°C until it was hybridized. Hybridizations were performed using an HS4800 Hybridization Station (Tecan U.S., Durham, NC) at 42°C for 10 h.
Microarrays were scanned using a ProScanArray microarray scanner (Perkin Elmer, Boston, MA). The emitted fluorescent signal was detected by a photomultiplier tube (PMT) at 633 nm using a laser power of 95% and a PMT gain of 64%. The images were then processed with ImaGene 6.0 software (BioDiscovery, El Segundo, CA), with which a grid of individual circles defining the location of each DNA spot on the array was superimposed on the image in order to designate each fluorescent spot to be quantified. Hybridization spots with a signal-noise ratio (SNR) of ≥2 (13) were kept for further analysis. Normalization among slides was done based on the mean signals of all spots (49). Outliers of a gene within replicate slides (>2 standard deviations) were removed, and gene detection was considered positive when a positive hybridization signal was obtained for at least 33% of spots targeting the gene among all replicates.
Plant parameters.
T. caerulescens plants were harvested at the end of the experiment (4 months after being transplanted). For metal analyses, shoots and roots were washed thoroughly with deionized water to remove soil particles. For metal desorption, roots were also washed with 1% HNO3. Subsequently, shoots and roots were oven dried at 70°C for 48 h, and their dry weights were recorded. Subsamples of dried plant tissue were digested with a mixture of HNO3 and HClO4 (55), and subsequently, Zn and Cd in the digest were determined using AAS.
Statistical analysis.
Significant differences (P < 0.05) between treatments were analyzed by two-way analysis of variance (ANOVA), using Microsoft Stat View software (SAS Institute), with either the soil metal concentration and plant presence or soil Zn and Cd as factors. The Tukey-Kramer test was used to establish the significance (P < 0.05) of the differences among means. Relationships between soil microbial and physicochemical parameters were examined by means of principal-component analysis (PCA) applied to the correlation matrix of these variables. In this way, PCA not only revealed the multivariate relationships between data, but was also used as a dimension reduction technique.
To choose between linear and unimodal models of response in DGGE binary matrices and GeoChip data, a detrended correspondence analysis (DCA) was applied: the length of the first and the remaining axes of this DCA were short (eigenvalue [λ], <2.5 standard deviations), suggesting low beta diversity in our data along ordination axes; therefore, a linear response model was selected. Then, a redundancy analysis (RDA) was applied to explain DGGE and GeoChip data as a function of linear combinations of two variables: T. caerulescens shoot biomass and soil metal concentration (40). To explore the individual and shared effects of shoot biomass versus soil metal concentration on patterns in DGGE and GeoChip data, variation partitioning procedures were employed (23). RDAs were also performed to identify the physicochemical and microbial soil parameters with the most significant influence on DGGE and GeoChip data. All multivariate analyses were performed in Canoco for Windows 4.5 (41). Pearson's correlations between Q-PCR and GeoChip results were calculated using the SPSS program (38).
RESULTS
Plant parameters.
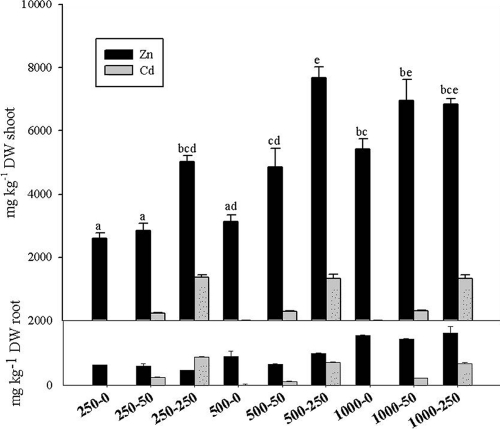
Regarding shoot biomass at the end of the experiment (Table 2), neither the Zn nor the Cd concentration resulted in significant differences among treatments. No significant interaction was found between soil Zn and Cd concentrations for root Zn concentrations and shoot and root Cd concentrations (Fig. 1). Increasing soil metal concentrations led to significantly higher values of their respective tissue metal concentrations (metal concentrations were higher in shoots than in roots). In contrast, there was a significant interaction between soil Zn and Cd concentrations for shoot Zn concentrations. In soils polluted with 250 and 500 mg Zn kg−1 (DW), the shoot Zn concentration was significantly higher in the presence of 250 mg Cd kg−1 (DW).
TABLE 2.
T. caerulescens shoot biomass, amount of metal phytoextracted from soil, BF, and amount of metal remaining in the soil at the end of the experiment in pots subjected to different soil Zn and Cd concentrationsa
| Treatment (Zn/Cd) (mg kg−1 [DW]) | Shoot biomass (g [DW] tissue) | Amt phytoextracted (mg) |
BF |
Amt remaining (mg) |
|||
|---|---|---|---|---|---|---|---|
| Zn | Cd | Zn | Cd | Zn | Cd | ||
| 250/0 | 3.61 ± 0.21 | 9.31 ± 0.71A | 8.4 ± 0.6ABC | 463 ± 6A | |||
| 250/50 | 5.59 ± 0.42 | 16.24 ± 2.61AB | 1.24 ± 0.14A | 9.8 ± 0.9AB | 5.5 ± 0.4 | 442 ± 35A | 61 ± 3A |
| 250/250 | 7.62 ± 0.88 | 38.97 ± 5.77CD | 10.57 ± 1.46B | 17.4 ± 0.8D | 8.0 ± 0.4 | 435 ± 4A | 257 ± 0B |
| 500/0 | 5.89 ± 0.95 | 18.28 ± 2.96AB | 6 ± 0.5AC | 778 ± 16B | |||
| 500/50 | 5.69 ± 0.54 | 27.01 ± 2.89BCD | 1.61 ± 0.11AC | 10.1 ± 1.1B | 7.2 ± 0.3 | 720 ± 7B | 60 ± 2A |
| 500/250 | 3.63 ± 0.23 | 28.03 ± 2.53BCD | 4.93 ± 0.73CD | 15.2 ± 0.7D | 7.1 ± 0.8 | 759 ± 10B | 284 ± 10C |
| 1,000/0 | 4.83 ± 0.28 | 25.90 ± 1.03ABC | 5.4 ± 0.3C | 1,517 ± 13C | |||
| 1,000/50 | 4.57 ± 0.18 | 31.78 ± 3.42BCD | 1.37 ± 0.11AC | 7.8 ± 0.8ABC | 7.5 ± 0.6 | 1,349 ± 16D | 60 ± 3A |
| 1,000/250 | 6.35 ± 0.48 | 43.39 ± 3.20D | 8.56 ± 1.13BD | 8.3 ± 0.2ABC | 8.0 ± 0.9 | 1,236 ± 13E | 254 ± 6B |
Values labeled with different letters are significantly different (P < 0.05) according to the Tukey-Kramer test. Shown are mean values (n = 4) ± standard errors. ANOVA for columns from left to right (*, significantly different at a P value of <0.05): Zn, 0.5699, 0.0008*, 0.0302*, <0.0001*, 0.3543, <0.0001*, and 0.0461*; Cd, 0.1159, <0.0001*, <0.0001*, <0.0001*, 0.0871, <0.0001*, and <0.0001*; Zn × Cd, 0.0004*, 0.0430*, 0.0041*, 0.0008*, 0.1532, <0.0001*, and 0.0373*.
FIG. 1.
Shoot and root metal concentrations at the end of the experiment in T. caerulescens plants subjected to different Zn (first value) and Cd (second value) soil concentrations (mg kg−1 [DW]). Bars labeled with different letters are significantly different (P < 0.05) according to the Tukey-Kramer test (statistics are shown only when, according to two-way ANOVA, a significant interaction was found between soil Zn and Cd concentrations). Mean values (n = 4) and standard errors are shown.
With respect to translocation factors (TF) (TF = shoot metal concentration/root metal concentration) (data not shown), a significant interaction was observed between soil Zn and Cd concentrations. The highest values of TF were found in treatments with (in mg kg−1 [DW]) 250/250 and 1,000/250 for Zn (TF = 11.1) and Cd (TF = 3.8), respectively. Similarly, a significant interaction was found between soil Zn and Cd concentrations in relation to the amount of metal phytoextracted (shoot metal concentration × shoot biomass) (Table 2): the highest values for phytoextracted Zn and Cd were observed in treatments with 1,000/250 (43.4 mg) and 250/250 (10.6 mg), respectively.
A significant interaction was found between the soil metal concentration and plant presence for values of total and CaCl2-extractable soil Zn and Cd concentrations (Table 3). Considering the single metals separately, soil Zn and Cd concentrations led to significant differences between treatments (apart from the soil Cd concentration and values for total Zn). A significant interaction was found between the soil Cd and Zn concentrations in planted and unplanted pots, except for CaCl2-extractable Zn in the absence of T. caerulescens. In unplanted pots, CaCl2-extractable Cd was significantly higher in treatments with (in mg kg−1 [DW]) 500/250 and 1,000/250 (Table 3). In planted pots, total and CaCl2-extractable Zn levels were lower in treatments with 1,000/250 than in treatments with 1,000/0 and 1.000/50. Although, in general, the differences were not statistically significant, planted soils showed lower total and CaCl2-extractable metal concentrations than unplanted soils.
TABLE 3.
Total and CaCl2-extractable metal concentrations in soils polluted with different Zn and Cd concentrations at the end of the experimenta
| Soil | Treatment (Zn/Cd) (mg kg−1 [DW]) | Concn (mg kg−1 [DW] soil) |
|||
|---|---|---|---|---|---|
| Total Zn | Extractable Zn | Total Cd | Extractable Cd | ||
| Unplanted | 250/0 | 334 ± 3AB | 116 ± 2ABC | 0 ± 0A | 0 ± 0AB |
| 250/50 | 321 ± 6AB | 111 ± 2AB | 41 ± 1B | 19 ± 0AC | |
| 250/250 | 368 ± 8A | 99 ± 4AB | 186 ± 3C | 77 ± 3D | |
| 500/0 | 529 ± 9CD | 201 ± 1D | 1 ± 0A | 0 ± 0AB | |
| 500/50 | 520 ± 4CD | 212 ± 8D | 41 ± 0B | 20 ± 0C | |
| 500/250 | 551 ± 15C | 189 ± 1CD | 224 ± 3D | 106 ± 1EF | |
| 1,000/0 | 1,049 ± 16E | 395 ± 3EF | 2 ± 0A | 1 ± 0AB | |
| 1,000/50 | 1,005 ± 14EF | 405 ± 9E | 43 ± 0B | 22 ± 0C | |
| 1,000/250 | 959 ± 20FG | 353 ± 17EF | 236 ± 11D | 122 ± 10E | |
| Planted | 250/0 | 308 ± 4AB | 95 ± 2AB | 0 ± 0A | 0 ± 0B |
| 250/50 | 294 ± 23B | 79 ± 2A | 40 ± 2B | 16 ± 0ABC | |
| 250/250 | 290 ± 3B | 79 ± 3A | 171 ± 0C | 61 ± 1D | |
| 500/0 | 519 ± 11CD | 167 ± 3BCD | 1 ± 0A | 0 ± 0AB | |
| 500/50 | 480 ± 5D | 162 ± 4BCD | 40 ± 1B | 17 ± 0ABC | |
| 500/250 | 506 ± 7CD | 224 ± 43D | 189 ± 7C | 96 ± 5F | |
| 1,000/0 | 1,011 ± 9EF | 390 ± 11EF | 1 ± 0A | 0 ± 0B | |
| 1,000/50 | 899 ± 11G | 329 ± 10F | 40 ± 2B | 17 ± 1ABC | |
| 1,000/250 | 824 ± 9H | 232 ± 10D | 169 ± 4C | 76 ± 6D | |
Values labeled with different letters are significantly different (P < 0.05) according to the Tukey-Kramer test. Shown are mean values (n = 4) ± standard errors. ANOVA for columns from left to right (*, significantly different at a P value of < 0.05): plant, <0.0001*, <0.0001*, <0.0001*, <0.0001*; metal, <0.0001*, <0.0001*, <0.0001*, <0.0001*; plant × metal, 0.0001*, 0.0002*, <0.0001*, <0.0001*; unplanted, Zn, <0.001*, <0.0001*, <0.0001*, 0.0002*; unplanted, Cd, 0.1702, 0.0006*, <0.0001*, <0.0001*; unplanted, Zn × Cd, 0.0008*, 0.1738, <0.0001*, <0.0001*; planted, Zn, <0.0001*, 0.0002*, 0.0331*, <0.0001*; planted, Cd, <0.0001*, <0.0001*, <0.0001*, 0.0415*; planted, Zn × Cd, <0.0001*, <0.0001*, 0.0137*, <0.0001*.
For Zn bioconcentration factors (BF) (BF = shoot metal concentration/soil metal concentration) (Table 2), a significant interaction was observed between the soil Zn and Cd concentrations. The highest values of BF for Zn were found in treatments with (in mg kg−1 [DW]) 250/250 (17.4) and 500/250 (15.2). In contrast, soil Zn and Cd concentrations did not result in significant differences between treatments regarding Cd BF.
Regarding the amount of metal remaining in the soil at the end of the experiment (Table 2), there was a significant interaction between soil Zn and Cd concentrations. In soils polluted with 500 mg Zn kg−1 (DW) soil, the amount of Zn remaining in the soil was 2 to 8% lower in planted versus unplanted pots; in soils polluted with 1,000 mg Zn kg−1 (DW) soil, the amount of Zn remaining in the soil was 4 to 14% lower in planted versus unplanted pots (the differences between planted and unplanted soils were higher at higher soil Cd concentrations). Similarly, in soils polluted with 50 mg Cd kg−1 (DW) soil, the amount of Cd remaining in the soil was 1 to 6% lower in planted versus unplanted pots; in soils polluted with 250 mg Cd kg−1 (DW) soil, the amount of Cd remaining in the soil was 8 to 28% lower in planted versus unplanted pots. Treatment with (in mg kg−1 [DW]) 1,000/250 showed the greatest differences between planted and unplanted pots regarding the amount of metal remaining in the soil: 1,236 and 254 mg of Zn and Cd, respectively, for planted pots versus 1,439 and 354 mg of Zn and Cd, respectively, for unplanted pots.
Plant productivity.
Alfalfa growth was highly inhibited by the presence of both metals (actually, growth was observed only in treatments with (in mg kg−1 [DW]) 250/0, 250/50, and 500/0). In previously unplanted soils, the following values of alfalfa shoot biomass were found for treatments with 250/0, 250/50, and 500/0, respectively: 1.80 ± 0.33, 0.14 ± 0.04, and 0.16 ± 0.05 g (DW) shoots. In previously planted (with T. caerulescens) soils, the following values of alfalfa shoot biomass were found for treatments with 250/0, 250/50, and 500/0, respectively: 0.88 ± 0.21, 0.18 ± 0.06, and 0.21 ± 0.10 g (DW) shoots. For 250/0 treatment, values of alfalfa shoot biomass were 49% lower in previously planted versus previously unplanted pots (conversely, under treatment with 250/50 and 500/0, values of alfalfa shoot biomass were 26% and 34% higher, respectively, in previously planted versus unplanted soils).
Soil microbial and physicochemical parameters.
Table 4 shows values of basal respiration, SIR, and QR at the end of the experiment. For basal respiration, two-way ANOVA indicated a significant interaction between the soil metal concentration and plant presence. Considering the single metals separately, the presence of Zn in unplanted soils led to significant differences between treatments regarding basal respiration: lower values were found at higher Zn concentrations (in planted soils, a significant interaction was found between soil Zn and Cd concentrations). No significant interaction was observed between the soil metal concentration and plant presence for SIR and QR: values of SIR in treatments with 250/50 and 250-250 were significantly higher than in treatments with 250/0. Also, planted soils showed significantly higher values of SIR than unplanted soils. The highest and lowest values of QR were found under treatments with 250/0 and 1,000/250, respectively. Considering the single metals separately, a significant interaction was found between soil Zn and Cd concentrations in planted and unplanted pots for both SIR and QR.
TABLE 4.
Soil basal respiration, SIR, and QR in soils polluted with different Zn and Cd concentrations at the end of the experimenta
| Soil | Treatment (Zn/Cd) (mg kg−1 [DW]) | Basal respiration (μg C g−1 [DW] soil h−1) | SIR (μg C g−1 [DW] soil h−1) | QR |
|---|---|---|---|---|
| Unplanted | 250/0 | 1.33 ± 0.07AB | 3.38 ± 0.31 | 0.41 ± 0.06 |
| 250/50 | 1.19 ± 0.08BCDE | 6.78 ± 0.17 | 0.18 ± 0.01 | |
| 250/250 | 1.27 ± 0.11ABC | 6.03 ± 0.32 | 0.21 ± 0.01 | |
| 500/0 | 0.88 ± 0.03EFG | 2.96 ± 0.49 | 0.34 ± 0.07 | |
| 500/50 | 0.87 ± 0.01FG | 3.78 ± 0.68 | 0.26 ± 0.05 | |
| 500/250 | 0.95 ± 0.01DEFG | 4.93 ± 0.26 | 0.19 ± 0.01 | |
| 1,000/0 | 0.55 ± 0.07HI | 4.79 ± 0.08 | 0.12 ± 0.01 | |
| 1,000/50 | 0.55 ± 0.04HI | 4.32 ± 0.76 | 0.14 ± 0.02 | |
| 1,000/250 | 0.40 ± 0.02I | 3.75 ± 0.39 | 0.11 ± 0.02 | |
| Planted | 250/0 | 1.51 ± 0.08A | 4.18 ± 0.44 | 0.39 ± 0.07 |
| 250/50 | 1.21 ± 0.03ABCD | 8.46 ± 0.47 | 0.15 ± 0.01 | |
| 250/250 | 0.97 ± 0.01CDEFG | 8.08 ± 0.08 | 0.12 ± 0.00 | |
| 500/0 | 0.95 ± 0.02DEFG | 6.85 ± 1.40 | 0.16 ± 0.03 | |
| 500/50 | 1.23 ± 0.03ABCD | 6.55 ± 1.22 | 0.21 ± 0.03 | |
| 500/250 | 0.81 ± 0.06FGH | 5.94 ± 0.67 | 0.14 ± 0.02 | |
| 1,000/0 | 1.06 ± 0.03BCDEF | 5.92 ± 0.68 | 0.19 ± 0.03 | |
| 1,000/50 | 0.85 ± 0.02FGH | 5.47 ± 0.17 | 0.16 ± 0.01 | |
| 1,000/250 | 0.69 ± 0.05GHI | 7.62 ± 0.92 | 0.10 ± 0.01 |
Values labeled with different letters are significantly different (P < 0.05) according to the Tukey-Kramer test (statistics are shown only when, according to two-way ANOVA, a significant interaction was found between the metal concentration and plant presence). Shown are mean values (n = 4) ± standard errors. ANOVA for columns from left to right (*, significantly different at a P value of < 0.05): plant, <0.0001*, 0.0001*, <0.0001*; metal, <0.0001*, <0.0001*, 0.0340*; plant × metal, <0.0001*, 0.2497, 0.1433; unplanted, Zn, <0.0001*, 0.0033*, 0.0003*; unplanted, Cd, 0.5987, 0.0078*, 0.0050*; unplanted, Zn × Cd, 0.3174, 0.0018*, 0.0404*; planted, Zn, <0.0001*, 0.7233, 0.0562; planted, Cd, <0.0001*, 0.1121, 0.0005*; planted, Zn × Cd, <0.0001*, 0.0341*, 0.0025*.
The abundance of bacterial 16S rRNA gene fragments (total bacteria) did not show a significant interaction between the soil metal concentration and plant presence (planted soils showed higher values than unplanted soils) (Table 5). Considering the single metals separately, in unplanted pots, a significant interaction was found between soil Zn and Cd concentrations; in planted pots, the presence of Cd led to significant differences between treatments. Regarding the abundance of chitinase and ammonia monooxygenase gene copies, a significant interaction was found between the soil metal concentration and plant presence (Table 5). Values of ammonia-oxidizing gene abundance were higher in treatments with 250/50 unplanted versus 1,000/250 unplanted and 1,000/50 planted. Considering the single metals separately, a significant interaction was observed between soil Zn and Cd concentrations in unplanted (not in planted) pots.
TABLE 5.
Total bacteria, group A bacterial chitinases, and ammonia monooxygenase gene abundance in soils polluted with different Zn and Cd concentrations at the end of the experimenta
| Soil | Treatment (Zn/Cd) (mg kg−1 [DW]) | Total bacteria (105 copies g−1 [DW] soil) | Chitinase group A (103 copies g−1 [DW] soil) | amoA (101 copies g−1 [DW] soil) |
|---|---|---|---|---|
| Unplanted | 250/0 | 1,244 ± 332 | 135 ± 23 | 1,900 ± 77AB |
| 250/50 | 1,376 ± 545 | 253 ± 7 | 3,689 ± 134C | |
| 250/250 | 228 ± 62 | 157 ± 26 | 1,667 ± 237AB | |
| 500/0 | 102 ± 4 | 112 ± 25 | 1,360 ± 77AB | |
| 500/50 | 646 ± 189 | 147 ± 20 | 1,690 ± 178AB | |
| 500/250 | 389 ± 67 | 156 ± 19 | 1,907 ± 114AB | |
| 1000/0 | 311 ± 51 | 110 ± 9 | 2,332 ± 481AC | |
| 1000/50 | 335 ± 6 | 160 ± 22 | 815 ± 151AB | |
| 1000/250 | 593 ± 96 | 212 ± 12 | 308 ± 77B | |
| Planted | 250/0 | 2,059 ± 499 | 179 ± 14 | 1,938 ± 445AB |
| 250/50 | 631 ± 76 | 118 ± 29 | 1,842 ± 326AB | |
| 250/250 | 664 ± 201 | 161 ± 24 | 1,374 ± 206AB | |
| 500/0 | 1,023 ± 295 | 151 ± 30 | 2,378 ± 454AC | |
| 500/50 | 492 ± 61 | 180 ± 13 | 1,330 ± 135AB | |
| 500/250 | 444 ± 132 | 102 ± 27 | 1,160 ± 393AB | |
| 1000/0 | 894 ± 81 | 158 ± 8 | 1,563 ± 553AB | |
| 1000/50 | 1,023 ± 254 | 166 ± 32 | 408 ± 54B | |
| 1000/250 | 1,026 ± 139 | 156 ± 11 | 703 ± 124AB |
Values labeled with different letters are significantly different (P < 0.05) according to the Tukey-Kramer test (statistics are shown only when, according to two-way ANOVA, a significant interaction was found between metal concentration and plant presence). Shown are mean values (n = 4) ± standard errors. ANOVA for columns from left to right (*, significantly different at a P value of < 0.05): plant, 0.0006*, 0.1778, <0.0001*; metal, 0.0096*, 0.4956, 0.0365*; plant × metal, 0.0644, 0.0046*, 0.0071*; unplanted, Zn, 0.0210*, 0.0785, <0.0001*; unplanted, Cd, 0.2152, 0.0022*, 0.0016*; unplanted, Zn × Cd, 0.0469*, 0.0310*, <0.0001*; planted, Zn, 0.1155, 0.7630, 0.0315*; planted, Cd, 0.0131*, 0.5622, 0.0218*; planted, Zn × Cd, 0.0501, 0.2055, 0.6405.
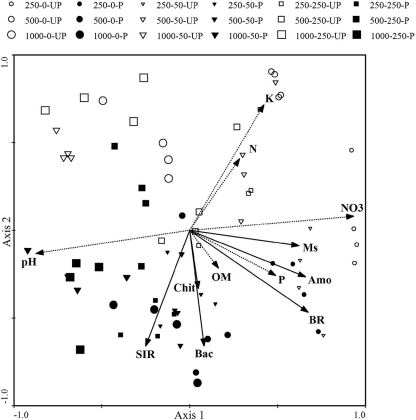
The application of PCA to all soil physicochemical and microbial parameters (Fig. 2) separated soils subjected to different levels of Zn pollution along axis 1, which accounted for 29% of the variance. Soils treated with 250 mg Zn kg−1 (DW) showed higher values for nitrate, alfalfa growth, amoA gene abundance, extractable P content, and basal respiration. These five parameters were positively correlated with each other and negatively correlated with soil pH. No clear patterns were observed when the soil Cd concentration and physicochemical or microbial parameters were compared. Planted and unplanted pots were also separated. Planted pots showed higher values for SIR, bacterial 16S rRNA gene copies, and group A bacterial chitinase gene copies and lower values for total N and K+ content.
FIG. 2.
Principal-component analysis of soil physicochemical and microbial properties in planted (P) and unplanted (UP) soils polluted with different Zn (first value) and Cd (second value) concentrations at the end of the experiment. Axis 1 and axis 2 account for 29 and 17% of the variance, respectively. Chit, group A bacterial chitinase gene abundance; Bac, total bacterial-gene abundance; BR, basal respiration; P, extractable phosphorus content; Amo, ammonia monooxygenase gene abundance; Ms, Medicago sativa growth; NO3, nitrate content; N, total N content; K, extractable potassium content.
Concerning the values of soil pH at the end of the experiment, a significant interaction was observed between soil Zn and Cd concentrations and between the soil Zn concentration and plant presence: in soils treated with 50 mg Cd kg−1 (DW), pH values were significantly higher in soils treated with 1,000 mg Zn kg−1 (DW) (4.78 ± 0.03) than with 250 (4.57 ± 0.04) and 500 (4.55 ± 0.03) mg Zn kg−1 (DW). Higher values of pH were found at higher values of soil Cd: 4.54 ± 0.02, 4.63 ± 0.03, and 4.70 ± 0.02 in soils treated with 0, 50, and 250 mg Cd kg−1 (DW), respectively. Finally, soil pH increased from 4.58 ± 0.02 to 4.67 ± 0.02 as a result of the presence of T. caerulescens.
In the comparison of treatments based upon Jaccard indices of bacterial-specific PCR-DGGE analysis, the treatment pairs 250/0 unplanted-250/0 planted and 1,000/250 unplanted-1,000/250 planted showed the highest levels of similarity (CJ = 0.64 and CJ = 0.66, respectively). All other pairwise comparisons yielded Jaccard index values ranging between 0.40 and 0.46. According to RDA, the soil metal concentration and T. caerulescens shoot biomass explained 41% of the total inertia present in the DGGE data. After the influence of selected sets of covariables was partitioned out, most of the explained variation in DGGE data was attributable to changes in the soil metal concentration (31.7%) and only 7.0% could be attributed to changes in T. caerulescens shoot biomass. In further RDA analysis, the soil properties that yielded P values of <0.05 after forward selection were basal respiration (P < 0.001) and pH (P < 0.008).
Table 6 shows a summary of the genes detected in the GeoChip for each treatment. In every gene category, significantly more genes were detected in polluted (1,000/250) than in nonpolluted (250/0) soils, and gene detection was higher in planted than in unplanted soils (although this difference was significant only for the C-cycle and heavy-metal gene categories in metal-polluted and nonpolluted pots, respectively). RDA explained a high percentage (above 54%) of the total inertia present in the GeoChip data. After the influence of selected covariables (T. caerulescens shoot biomass and metal concentration) was partitioned out, most of the explained variation was due to changes in the soil metal concentration. Indeed, for every gene category, the soil metal concentration explained from 35.2 to 46.7% of the data variation, while T. caerulescens shoot biomass explained from 14.7 to 16.1%.
TABLE 6.
Genes detected in the GeoChip functional-gene array, together with the variation partitioning obtained by RDA, in planted and unplanted soils polluted with different Zn and Cd concentrations at the end of the experimenta
| Parameterb | Value |
|||||
|---|---|---|---|---|---|---|
| N cycle | C cycle | Sulfate | Heavy metals | Organic compounds | Total | |
| Treatment (Zn/Cd) | ||||||
| 250/0-UP | 22 ± 6A | 9 ± 2A | 4 ± 1A | 18 ± 1A | 37 ± 5A | 37 ± 5A |
| 250/0-P | 41 ± 2AB | 27 ± 1A | 8 ± 1A | 51 ± 2B | 70 ± 3A | 204 ± 5A |
| 1,000/250-UP | 70 ± 1BC | 56 ± 1B | 16 ± 0B | 91 ± 1C | 156 ± 4B | 404 ± 8B |
| 1,000/250-P | 91 ± 10C | 82 ± 7C | 22 ± 3B | 108 ± 6C | 176 ± 12B | 502 ± 41B |
| % Explained | 54.6 | 63.3 | 55.8 | 60.9 | 59.3 | |
| % Unexplained | 45.4 | 36.7 | 44.2 | 39.1 | 40.7 | |
| Explaining factorsc | ||||||
| Shoot biomass | 15.4 | 15.4 | 16.1 | 14.7 | 14.7 | |
| Soil metal concn | 39.9 | 44.0 | 35.2 | 46.7 | 41.6 | |
| Interaction | 0.0 | 3.9 | 4.5 | 0.0 | 3.0 | |
Mean values ± standard errors; values followed by different letters are significantly different (P < 0.05) according to the Tukey-Kramer test.
P, planted; UP, unplanted.
The numbers quantify the effect of each set of explaining factors (T. caerulescens shoot biomass and soil metal concentration) after the effects of the other variables were partitioned out, expressed as a percent contribution to the total inertia in the response variable.
Metal-polluted (1,000/250) planted soils had the highest percentage of unique genes detected via GeoChip (genes that were found only in that treatment), representing 35.0% of all genes detected in the treatment. In contrast, nonpolluted soils showed a low percentage of unique genes (4.4% and 5.0% in unplanted and planted soils, respectively). Also, the two metal-polluted treatments had the highest degree of overlap in detected genes among pairwise comparisons between treatments (52.2%).
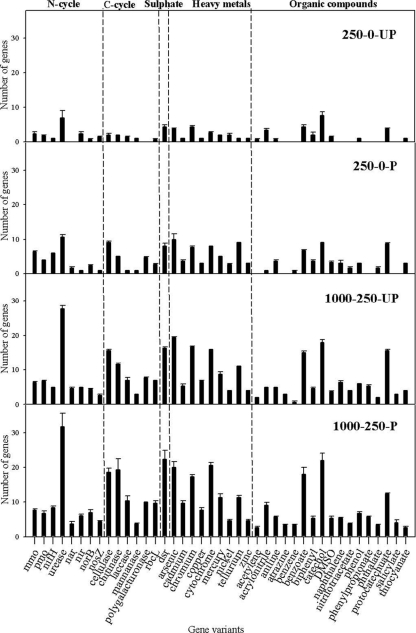
In order to gain insight into the level of functional redundancy across soils with different treatments, binary presence-absence data for detected gene variants were compared. The gene families that were well represented (with a minimum of 5 gene variants detected) and that showed significant (P < 0.05) differences between treatments according to ANOVA are shown in Fig. 3. As a general pattern, metal polluted (1,000/250) and planted soils showed larger numbers of gene variants than nonpolluted and unplanted soils, respectively.
FIG. 3.
Number of gene variants detected in the GeoChip functional gene array in planted (P) and unplanted (UP) soils polluted with different Zn (first value) and Cd (second value) concentrations at the end of the experiment. Only those gene variants that were well represented (with a minimum of 5 gene variants) and that showed significant differences (P < 0.05) among treatments, according to ANOVA, are presented.
All 12 of the gene targets on the GeoChip associated with Zn and/or Cd resistance genes were detected in metal-polluted (1,000/250) planted soils, while only 7 of them were found in metal-polluted (1,000/250) unplanted soils. Five were detected in 250/0 planted soils and just 1 in the nonpolluted unplanted soils.
For some groups of genes, it was possible to compare GeoChip results with gene family-specific quantitative PCR. However, no significant correlations were found in the detected abundances of ammonia monooxygenase (amoA) and group A bacterial chitinase genes in comparisons across these two platforms.
RDAs were also performed for each gene category in order to identify the soil physicochemical and microbial properties that had the most significant influence on functional-gene detection. The soil properties that yielded P values of <0.05 after forward selection were SIR and K+ content for the C cycle, sulfate reduction, and organic-contaminant degradation gene categories. In contrast, SIR and nitrate content yielded P values of <0.05 after forward selection for the N cycle and metal reduction and resistance gene categories.
DISCUSSION
Metal phytoextraction.
The T. caerulescens Lanestosa ecotype proved to have a great capacity for Zn and Cd hyperaccumulation. Shoot Cd concentrations reached values above the hyperaccumulation threshold of 100 mg Cd kg−1 (DW) (2), as previously observed (8). T. caerulescens plants did not show any phytotoxicity symptoms in the presence of high levels of metals. Our results confirm that the T. caerulescens Lanestosa ecotype has an elevated tolerance for Cd, a heavy metal for which few hyperaccumulator species are known (2). At soil Zn concentrations of 250 and 500 mg kg−1 (DW), the presence of 250 mg Cd kg−1 (DW) soil significantly stimulated Zn shoot accumulation (and also Zn translocation at 250 mg Zn kg−1 [DW]). Translocation factors for both metals were >1, as is usually the case with hyperaccumulators (28). T. caerulescens plants showed promising bioconcentration factors for Cd (up to 8.0) and Zn (up to 17.4), considering that 10 is the threshold value reported for a phytoextraction process to be considered feasible (27). For phytoextraction, bioconcentration factors are more important than shoot metal concentrations (54).
If one assumes that metal pollution is restricted to the active rooting zone of T. caerulescens (the top 20-cm soil layer), which would give a total soil mass to be remediated of 2,600 tons ha−1 (considering a bulk density of 1.3 tons m−3) and a T. caerulescens biomass yield of 5 tons ha−1 (55), according to our highest soil and shoot metal concentration values, it would take 44 and 72 consecutive crops to reduce the initial soil Zn and Cd concentrations in treatment with 1,000/250 to 300 mg Zn kg−1 and 3 mg Cd kg−1, the target endpoint concentrations used by Lombi et al. (24). Thus, assuming 2 crops per year, 36 years would be needed to reduce both metals below permissible levels. However, as the metal concentration in shoots might decrease with decreasing metal content in soil, this calculation represents only a theoretical abstraction.
Impact of metal pollution on soil properties.
Biological parameters, especially those related to the activity, size, and diversity of soil microbial communities, are increasingly being used to assess the impact of pollutants on soil functioning due to their sensitivity, rapid response, and capacity to provide information that integrates many environmental factors (6, 8, 16). Basal respiration has been shown to be negatively affected by the presence of heavy metals (5, 20). In our study, Zn concentrations were negatively correlated with basal respiration, abundance of ammonia oxidizer genes, and alfalfa growth (i.e., the capacity of the phytoremediated soil to support plant growth). Mertens et al. (29) reported that ammonia oxidizer populations were sensitive to increasing Zn concentrations in artificially contaminated soils. In our study, these three parameters were also negatively correlated with soil pH. Thus, soil pH and not Zn could be responsible for these responses. However, Wang et al. (43) reported a positive correlation between soil biological activities and pH. Finally, it must be kept in mind that the abundance of ammonia oxidizer genes was also positively correlated with the soil nitrate content.
In treatment with (in mg kg−1 [DW]) 1,000/250, heavy metals had clear effects on the soil bacterial community profiles, in accordance with previous studies that have detected metal-induced shifts in the soil bacterial communities using DGGE and other molecular techniques (12, 35).
The distribution of microbial functional genes, as determined by the GeoChip, was also most affected by the presence of metals. Unlike soil properties related to microbial activity or biomass, the number of genes detected significantly increased as a result of metal pollution, regardless of the gene category. Similarly, functional redundancy appeared higher as a response to metal pollution, and lower numbers of unique genes were found in the nonpolluted soils. We did not find significant correlations between Q-PCR data and the corresponding gene abundance detected by the GeoChip. This is not surprising, as these discrepancies may be due to differences in coverage of the respective gene families between the two approaches (52) or biases due to technical issues, such as rolling-circle amplification efficiency (33, 37). On the other hand, Zn and Cd resistance genes increased considerably in polluted soils, suggesting that 10 months of exposure (6 months of aging plus 4 months of the experiment) are enough for the soil microbial communities to develop genotypic responses to Zn and Cd pollution. In any case, short-term and long-term exposure to heavy metals may cause different quantitative and qualitative effects on soil bacteria (32).
The soil toxicity test, based on the quantification of alfalfa productivity at the end of the experiment, showed that residual Zn and Cd levels were still very toxic for this plant species (growth was observed only under treatments with 250/0, 250/50, and 500/0, with much higher growth under 250/0). Thus, phytoremediated soils retained phytotoxic properties, although one must keep in mind that such assays are influenced by a variety of factors, including the species used, the type and levels of pollution, and the edaphic and environmental factors of the assay.
Effect of T. caerulescens on soil properties.
Planted soils showed higher values of microbial biomass data, which were all positively correlated with each other despite targeting different portions of the soil microbial community (e.g., SIR, an indicator of potentially active microbial biomass, was positively correlated with total and chitin-degrading bacterial-gene abundances). The lower values of total N and extractable K+ observed in planted versus unplanted soils were most likely due to plant uptake. A positive effect of vegetation on these properties has previously been attributed to associated improvements in soil conditions due to the presence of greater quantities of organic compounds and surfaces for microbial colonization (10). In similar phytoextraction experiments with T. caerulescens, we found higher values of microbial activity in rhizosphere versus nonrhizosphere soil (8, 16).
In treatment with (in mg kg−1 [DW]) 250/0, alfalfa growth was lower in previously planted soils, probably due to the reduction in soil nutrient levels caused by T. caerulescens growth. However, in treatments with 250/50 and 500/0, alfalfa growth was higher in previously planted soils, possibly owing to the reduction in soil metal concentrations caused by T. caerulescens, which more than compensated for the negative effect of decreased nutrient levels.
With respect to bacterial community profiles, no major differences were found between planted and unplanted soils. Similarly, in a phytoremediation study with poplars, Frey et al. (9) reported that the DGGE patterns of Pseudomonas populations of unplanted and planted treatments were similar. However, Wang et al. (45) found differences in bacterial community compositions (also measured with DGGE) between Elsholtzia splendens- and Trifolium repens-planted and unplanted Cu-polluted soils.
In our study, although the presence of plants explained a small percentage of the variability of the GeoChip data, the number of genes detected and the functional redundancy in planted soils were higher (but rarely significantly) for every gene category than in unplanted soils. Current knowledge suggests a high functional redundancy in the soil ecosystem (48). A higher functional redundancy is a priori associated with higher stability, which comprises both resilience, the ability of the system to recover after disturbance, and resistance, the inherent capacity of the system to withstand disturbance (22). Frey et al. (9) found that bacterial populations (Pseudomonas- and amoA-containing populations) were able to recover after the relief of metal stress by phytoextraction practices, whereas bulk microbial activities still remained depressed.
T. caerulescens-planted and metal-polluted (1,000/250) soils showed the highest number of unique genes. Moreover, the abundance of Zn and/or Cd resistance genes detected was higher in T. caerulescens-planted than unplanted soils, even in the absence of metals. Out of the 12 Zn and/or Cd resistance genes detected, 3 belonged to Staphylococcus spp. There are quite a few reports on the effects of metals on microbial communities in bulk soils (3, 36), but information on the microbial community present in the rhizosphere of heavy-metal-accumulating plants is still scarce. In particular, it is unclear if heavy-metal-accumulating plants selectively affect bacterial community composition in heavy-metal-contaminated soils. Addressing this issue, Gremion et al. (11) studied rRNA-based clone libraries from T. caerulescens rhizosphere samples, finding that Actinobacteria (and more specifically Rubrobacteria) might be a dominant part of the metabolically active bacteria in the rhizosphere of this hyperaccumulator.
As proven here, although effective phytoextraction can take unacceptably long periods of time, the growth of the phytoremediating plant might have a beneficial effect on soil health in a very short time, actually enhancing the activity and functionality of the soil microbial communities. For instance, 14 and 28% reductions in the amounts of soil Zn and Cd, respectively, were observed in treatment with (in mg kg−1 [DW]) 1,000/250 as a result of the phytoextraction process; nonetheless, T. caerulescens growth led to 103 and 73% increases in the SIR and abundance of bacterial 16S rRNA gene fragments, respectively. In consequence, the ultimate goal of a phytoextraction process (the recovery of soil health) can be achieved much earlier than the removal of the metal(s) from the soil. This kind of consideration must be taken into account when carrying out ecological risk assessments. It was concluded that soil microbial properties have great potential for the assessment of the efficiency of phytoextraction processes.
Acknowledgments
This work has been supported by the Basque Government through BERRILUR II and III projects and by the Biscay County Council through the EKOGEN and LURCHIP projects. The GeoChip portion was supported by the Genomics:GTL program through the Virtual Institute of Microbial Stress and Survival (VIMSS; http://vimss.lbl.gov) as part of contract no. DE-AC02-05CH11231 between the U.S. Department of Energy and Lawrence Berkeley National Laboratory.
We thank Eiko Kuramae and Joy D. Van Nostrand for their helpful assistance.
Footnotes
Published ahead of print on 8 October 2010.
REFERENCES
- 1.Assunção, A. G. L., H. Schat, and M. G. M. Aarts. 2003. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol. 159:351-360. [DOI] [PubMed] [Google Scholar]
- 2.Baker, A. J. M., S. P. McGrath, R. D. Reeves, and J. A. C. Smith. 2000. Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils, p. 85-107. In N. Terry and G. Bañuelos (ed.), Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, FL.
- 3.Brim, H., H. Heuer, E. Krögerrecklenfort, M. Mergeay, and K. Smalla. 1999. Characterization of the bacterial community of a zinc-polluted soil. Can. J. Microbiol. 45:326-338. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham, S. D., and W. R. Berti. 1993. Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev. Biol. 29:207-212. [Google Scholar]
- 5.Duxbury, T. 1985. Ecological aspects of heavy metal responses in microorganisms, p. 185-236. In K. C. Marshall (ed.), Advances in microbial ecology, vol. 8. Plenum, New York, NY. [Google Scholar]
- 6.Epelde, L., I. Mijangos, J. M. Becerril, and C. Garbisu. 2008. Soil microbial community as bioindicator of the recovery of soil functioning derived from metal phytoextraction with sorghum. Soil Biol. Biochem. 41:505-513. [Google Scholar]
- 7.Epelde, L., J. M. Becerril, I. Alkorta, and C. Garbisu. 2009. Heavy metal phytoremediation: microbial indicators of soil health for the assessment of remediation efficiency, p. 299-313. In A. Singh, R. C. Kuhad, and O. P. Ward (ed.), Advances in applied bioremediation. Springer-Verlag, Berlin, Germany.
- 8.Epelde, L., J. M. Becerril, J. Hernández-Allica, O. Barrutia, and C. Garbisu. 2008. Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Appl. Soil Ecol. 39:299-310. [Google Scholar]
- 9.Frey, B., M. Pesaro, A. Rüdt, and F. Widmer. 2008. Resilience of the rhizosphere Pseudomonas and ammonia-oxidizing bacterial populations during phytoextraction of heavy metal polluted soil with poplar. Environ. Microbiol. 10:1433-1449. [DOI] [PubMed] [Google Scholar]
- 10.Grayston, S. J., D. Vaughan, and D. Jones. 1997. Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5:29-56. [Google Scholar]
- 11.Gremion, F., A. Chatzinotas, and H. Harms. 2003. Comparative 16S rDNA and 16S rRNA sequence analysis indicates that Actinobacteria might be a dominant part of the metabolically active bacteria in heavy metal-contaminated bulk and rhizosphere soil. Environ. Microbiol. 5:896-907. [DOI] [PubMed] [Google Scholar]
- 12.Gremion, F., A. Chatzinotas, K. Kaufmann, W. Von Sigler, and H. Harms. 2004. Impacts of heavy metal contamination and phytoremediation on a microbial community during a twelve month microcosm experiment. FEMS Microbiol. Ecol. 48:273-283. [DOI] [PubMed] [Google Scholar]
- 13.He, Z., and J. Zhou. 2008. Empirical evaluation of a new method for calculating signal-to-noise ratio for microarray data analysis. Appl. Environ. Microbiol. 74:2957-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, Z., T. J. Gentry, C. W. Schadt, L. Wu, J. Liebich, S. C. Chong, Z. Huang, W. Wu, P. Jardine, C. Criddle, and J. Zhou. 2007. GeoChip: a comprehensive microarray for investigating biogeochemical, ecological and environmental processes. ISME J. 1:67-77. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Allica, J., C. Garbisu, J. M. Becerril, O. Barrutia, J. I. García-Plazaola, F. J. Zhao, and S. P. McGrath. 2006. Synthesis of low molecular weight thiols in response to Cd exposure in Thlaspi caerulescens. Plant Cell Environ. 29:1422-1429. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Allica, J., J. M. Becerril, O. Zárate, and C. Garbisu. 2006. Assessment of the efficiency of a metal phytoextraction process with biological indicators of soil health. Plant Soil 281:147-158. [Google Scholar]
- 17.Heuer, H., M. Krsek, P. Baker, K. Smalla, and E. Wellington. 1997. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 63:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houba, V. J. G., E. J. M. Temminghoff, G. A. Gaikhorst, and W. van Vark. 2000. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant. Anal. 31:1299-1396. [Google Scholar]
- 19.Jacard, P. 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44:223-270. [Google Scholar]
- 20.Kandeler, E., C. Kampichler, and O. Horak. 1996. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fert. Soils 23:299-306. [Google Scholar]
- 21.Kowalchuk, G. A., L. R. Stephen, W. De Boer, J. I. Prosser, T. M. Embley, and J. W. Woldendorp. 1997. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzaro, A., R. Schulin, F. Widmer, and B. Frey. 2006. Changes in lead availability affect bacterial community structure but not basal respiration in a microcosm study with forest soil. Sci. Total Environ. 371:110-124. [DOI] [PubMed] [Google Scholar]
- 23.Leps, J., and P. Šmilauer. 2003. Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge, United Kingdom.
- 24.Lombi, E., F. J. Zhao, S. J. Dunham, and S. P. McGrath. 2001. Phytoremediation of heavy-metal contaminated soils: natural hyperaccumulation versus chemically enhanced phytoextraction. J. Environ. Qual. 30:1919-1926. [DOI] [PubMed] [Google Scholar]
- 25.Lueders, T., B. Wagner, P. Claus, and M. W. Friedrich. 2004. Stable isotope probing of rRNA and DNA reveals a dynamic methylotroph community and trophic interactions with fungi and protozoa in oxic rice field soil. Environ. Microbiol. 6:60-72. [DOI] [PubMed] [Google Scholar]
- 26.Lueders, T., M. Manefield, and M. W. Friedrich. 2004. Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6:73-78. [DOI] [PubMed] [Google Scholar]
- 27.McGrath, S. P., E. Lombi, C. W. Gray, N. Calille, S. J. Dunham, and F. J. Zhao. 2006. Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri. Environ. Pollut. 141:115-125. [DOI] [PubMed] [Google Scholar]
- 28.McGrath, S. P., F. J. Zhao, and E. Lombi. 2002. Phytoremediation of metals, metalloids, and radionuclides. Adv. Agron. 75:1-56. [Google Scholar]
- 29.Mertens, J., D. Springael, I. DeTroyer, K. Cheyns, P. Wattiau, and E. Smolders. 2006. Long term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Environ. Microbiol. 8:2170-2178. [DOI] [PubMed] [Google Scholar]
- 30.Ministerio de Agricultura, Pesca y Alimentación. 1994. Métodos oficiales de análisis de suelos y aguas para riego, p. 205-285. In Ministerio de Agricultura, Pesca y Alimentación (ed.), Métodos oficiales de análisis, vol. III. Ministerio de Agricultura, Pesca y Alimentación, Madrid, Spain. [Google Scholar]
- 31.Nicolaisen, M. H., and N. B. Ramsing. 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J. Microbiol. Methods 50:186-203. [DOI] [PubMed] [Google Scholar]
- 32.Piotrowska-Seget, Z., and J. Kozdroj. 2008. Changes in culturable bacterial community of soil treated with high dosages of Cu or Cd. Plant Soil Environ. 54:520-528. [Google Scholar]
- 33.Reysenbach, A. L., L. J. Giver, G. S. Wickman, and N. R. Pace. 1992. Differential amplification of rRNA genes by polymerase chain reaction. Appl. Environ. Microbiol. 58:3417-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salt, D. E., M. Blaylock, N. P. B. A. Kumar, V. Dushenkov, B. D. Ensley, I. Chet, and I. Raskin. 1995. Phytoremediation: a novel strategy for removal of toxic metals from the environment using plants. Biotechnology 13:468-474. [DOI] [PubMed] [Google Scholar]
- 35.Sandaa, R. A., O. Enger, and V. Torsvik. 1999. Abundance and diversity of archaea in heavy-metal-contaminated soil. Appl. Environ. Microbiol. 65:3293-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandaa, R. A., V. Torsvik, O. Enger, F. L. Daae, T. Castberg, and D. Hahn. 1999. Analysis of bacterial communities in heavy metal-contaminated soils at different levels of resolution. FEMS Microbiol. Ecol. 30:237-251. [DOI] [PubMed] [Google Scholar]
- 37.Smith, C. J., and A. M. Osborn. 2009. Advantages and limitations of quantitative PCR (Q.-PCR)-based approaches in microbial ecology. FEMS Microbiol. Ecol. 67:6-20. [DOI] [PubMed] [Google Scholar]
- 38.SPSS. 1989. SPSS for Windows. SPSS Inc., Chicago, IL.
- 39.Stryer, L. 1988. Biochemistry, 3rd ed. Freeman, New York, NY.
- 40.Ter Braak, C. J. F. 1994. Canonical community ordination. Part I: basic theory and linear methods. Ecoscience 1:127-140. [Google Scholar]
- 41.Ter Braak, C. J. F., and P. Šmilauer. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination. Microcomputer Power, Ithaca, NY.
- 42.Van Nostrand, J. D., W. M. Wu, L. Y. Wu, Y. Deng, J. Carley, S. Carroll, Z. L. He, B. H. Gu, J. Luo, C. S. Criddle, D. B. Watson, P. M. Jardine, T. L. Marsh, J. M. Tiedje, T. C. Hazen, and J. Z. Zhou. 2009. GeoChip-based analysis of functional microbial communities during the reoxidation of a bioreduced uranium-contaminated aquifer. Environ. Microbiol. 11:2611-2626. [DOI] [PubMed] [Google Scholar]
- 43.Wang, A. S., J. S. Angle, R. L. Chaney, T. A. Delorme, and M. McIntosh. 2006. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 38:1451-1461. [Google Scholar]
- 44.Wang, F., H. Zhou, J. Meng, X. Peng, L. Jiang, P. Sun, C. Zhang, J. D. Van Nostrand, Y. Deng, Z. He, L. Wu, J. Zhou, and X. Xiao. 2009. GeoChip-based analysis of metabolic diversity of microbial communities at the Juan de Fuca Ridge hydrothermal vent. Proc. Natl. Acad. Sci. U. S. A. 106:4840-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y. P., Q. B. Li, J. Y. Shia, Q. Lin, X. C. Chen, W. Wu, and Y. X. Chen. 2008. Assessment of microbial activity and bacterial community composition in the rhizosphere of a copper accumulator and a non-accumulator. Soil Biol. Biochem. 40:1167-1177. [Google Scholar]
- 46.Williamson, N., P. Brian, and E. M. H. Wellington. 2000. Molecular detection of bacterial and streptomycete chitinases in the environment. Antonie Van Leeuwenhoek 78:315-321. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, K. H., W. J. Wilson, J. L. Radosevich, T. Z. DeSantis, V. S. Viswanathan, T. A. Kuczmarski, and G. L. Andersen. 2002. High-density microarray of small-subunit ribosomal DNA probes. Appl. Environ. Microbiol. 68:2535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolters, V. 2001. Biodiversity of soil animals and its function. Eur. J. Soil Biol. 37:221-227. [Google Scholar]
- 49.Wu, L., L. Kellogg, A. H. Devol, J. M. Tiedje, and J. Zhou. 2008. Microarray-based characterization of microbial community functional structure and heterogeneity in marine sediments from the Gulf of Mexico. Appl. Environ. Microbiol. 74:4516-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yergeau, E., S. A. Schoondermark-Stolk, E. L. Brodie, S. Dejean, T. Z. DeSantis, O. Goncalves, Y. M. Piceno, G. L. Andersen, and G. A. Kowalchuk. 2009. Environmental microarray analyses of Antarctic soil microbial communities. ISME J. 3:340-351. [DOI] [PubMed] [Google Scholar]
- 51.Yergeau, E., S. Bokhorst, A. H. L. Huiskes, H. T. S. Boshker, R. Aerts, and G. A. Kowalchuk. 2007. Size and structure of bacterial, fungal and nematode communities along an Antarctic environmental gradient. FEMS Microbiol. Ecol. 59:436-451. [DOI] [PubMed] [Google Scholar]
- 52.Yergeau, E., S. Kang, Z. He, J. Zhou, and G. Kowalchuk. 2007. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. ISME J. 1:163-179. [DOI] [PubMed] [Google Scholar]
- 53.Yuan, F., W. Ran, Q. Shen, and D. Wang. 2005. Characterization of nitrifying bacteria communities of soils from different ecological regions of China by molecular and conventional methods. Biol. Fertil. Soils 41:22-27. [Google Scholar]
- 54.Zhao, F. J., E. Lombi, and S. P. McGrath. 2003. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37-43. [Google Scholar]
- 55.Zhao, F. J., S. P. McGrath, and A. R. Crosland. 1994. Comparison of three wet digestion methods for the determination of plant sulphur by inductively coupled plasma atomic emission spectrometry (ICP-AES). Commun. Soil Sci. Plant Anal. 25:407-418. [Google Scholar]
- 56.Zhou, J. 2003. Microarrays for bacterial detection and microbial community analysis. Curr. Opin. Microbiol. 6:288-294. [DOI] [PubMed] [Google Scholar]
- 57.Zhou, J., S. Kang, C. W. Schadt, and C. T. Garten. 2008. Spatial scaling of functional gene diversity across various microbial taxa. Proc. Natl. Acad. Sci. U. S. A. 105:7768-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]