Abstract
DysI is identified as the protein that confers specific immunity to dysgalacticin, a plasmid-encoded streptococcal bacteriocin. dysI is transcribed as part of the copG-repB-dysI replication-associated operon. DysI appears to function at the membrane level to prevent the inhibitory effects of dysgalacticin on glucose transport, membrane integrity, and intracellular ATP content.
Dysgalacticin is a 21.5-kDa antimicrobial protein (bacteriocin) secreted by Streptococcus dysgalactiae subsp. equisimilis strain W2580 which exhibits a narrow spectrum of inhibitory activity directed mainly against the human pathogen Streptococcus pyogenes (9). We have previously reported that the mode of action of dysgalacticin is nonlytic and is likely to involve receptor binding to the membrane-bound glucose and/or mannose phosphotransferase system (PTS), followed by disruption of cytoplasmic membrane integrity and then subsequent K+ efflux and dissipation of the membrane potential in S. pyogenes (9, 15).
The dysgalacticin structural gene dysA is located on a 3,043-bp plasmid, pW2580 (9). However, strain W2580C, a plasmid-cured derivative of strain W2580, not only is defective in dysgalacticin production but also is sensitive to exogenously added recombinant dysgalacticin (9). We therefore hypothesized that the gene specifying the dysgalacticin immunity factor must also be present on pW2580. The aims of the present investigation were to identify the dysgalacticin immunity factor and to elucidate its self-protective mechanism.
Immunity to dysgalacticin is plasmid encoded and replication associated.
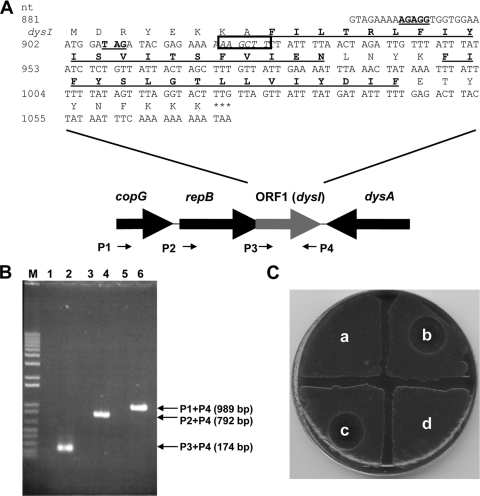
Plasmid pW2580 is a member of the pLS1/pMV158 family, which replicates via a rolling-circle mechanism (9, 10). In pMV158, two genes (copG and repB) are coexpressed, with CopG directly regulating expression of the operon (10). Inspection of the pW2580 nucleotide sequence reveals a 174-bp open reading frame, ORF1 (nucleotides [nt] 902 to 1075) which overlaps the 3′ end of repB, the gene encoding the replication initiation protein (Fig. 1A). While the 57-amino-acid (ca. 7.0-kDa) translational product of ORF1 did not exhibit similarities to any protein of known function, in silico analyses using the SOSUI software tool (http://bp.nuap.nagoya-u.ac.jp/sosui/) and other bioinformatic tools available on the Swiss Institute of Bioinformatics ExPASy server (http://au.ExPASy.org/tools/) predict it to be a cationic polypeptide (pI of 9.26) with a topology containing two hydrophobic transmembrane α-helices (Fig. 1A). These structural characteristics are consistent with those possessed by bacteriocin immunity-conferring proteins of other Gram-positive bacteria (11, 17, 18), for example, NukH (pI 9.94; 10.7 kDa) and SunI (pI 9.2; 12.1 kDa), which confer immunity to nukacin ISK-1 and sublancin 168, respectively (4, 13).
FIG. 1.
(A) Organization and nucleotide sequence of the copG-repB-ORF1 (dysI) region in pW2580. The genes shown are not drawn to scale. The putative ribosome binding site for dysI (AGAGG; nt 889 to 903) and the stop codon for repB (TAG; nt 907 to 909) are underlined. The hydrophobic amino acid residues predicted to form transmembrane helices are in bold text and underlined. The unique HindIII site that was digested and end filled to generate the frameshift mutation in ORF1 is in italics and boxed. The arrows indicate the positions and orientations of the various primers (P1 to P4) used in RT-PCR experiments. (B) RT-PCR amplicons generated from total RNA extracted from S. dysgalactiae W2580 grown to mid-logarithmic phase. Lanes: M, DNA size marker (Fermentas, Maryland); 2, P3 + P4 (dysI only; 174 bp); 4, P2 + P4 (repB-dysI; 792 bp); 6, P1 + P4 (copG-dysI; 989 bp); 1, 3, and 5, negative-control reactions for each of the above lanes in which reverse transcriptase was not added. (C) ORF1 (dysI) confers immunity to dysgalacticin. Twenty-microgram amounts of recombinant dysgalacticin were applied to lawns of S. dysgalactiae W2580C and its dysI-related derivatives in order to determine bacteriocin sensitivity. The indicator strains were as follows: a, wild-type S. dysgalactiae W2580 (immune control); b, strain W2580C (sensitive control); c, DWPS2 (W2580C carrying pWPS2, ΔdysI); d, DWPS1 (W2580C carrying pWPS1, dysI+).
Due to the overlapping nature of repB and ORF1 in pW2580, we hypothesized that copG, repB, and ORF1 may be expressed as a single transcriptional unit. To test this, reverse transcriptase PCR (RT-PCR) was carried out on total RNA extracted from mid-logarithmic-growth-phase (optical density at 600 nm [OD600] = 0.5) cultures of wild-type S. dysgalactiae W2580. Three primer combinations (Table 1 and Fig. 1A) were used to detect the following transcripts: (i) near-full-length copG-ORF1 (989 bp), (ii) repB-ORF1 (792 bp), and (iii) ORF1 alone (174 bp). All three amplicons were generated (Fig. 1B), demonstrating that ORF1 is indeed part of an operon. Interestingly, a PCR product was not generated when dysA-specific primers (Table 1) were used with the same reverse-transcribed cDNA template, supporting previous observations that dysgalacticin is produced not during mid-logarithmic phase but later in the growth cycle, i.e., after 6 h (late logarithmic phase) (20).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequence (5′-3′)a |
|---|---|
| CopGFwd (P1) | AAATTTAGATTGACGATAACGCTCA |
| RepBFwd (P2) | AAATTTATGGCTAAAGAAAAAGCAAGATA |
| DysIFwd (P3) | AAATTTATGGATAGATACGAGAAAAAAG |
| DysIRev (P4) | TTTAAATTTTTTTTTGAAATTATAGTAAGTCTCAAAAATAT |
| DysAFwd | AAATTTAATGAAACAAATAACTTTGCAGAAAC |
| DysARev | TTTAAATTATGATACAGTTGTCGCAC |
| P1F-PstI | ATCCAGTTACTGCAGATAGTGTTAGGTG |
| P1R-His6 | ATGATGATGATGATGATGCATTTCCACCACCTCTTTTTC |
| P2F-His6 | ATGCATCATCATCATCATCATGATAGATACGAGAAAAAAGCT |
| P2R-XbaI | AGATGTCTAGAAGAAGCATGGGGATATG |
The PstI and XbaI sites used for cloning purposes during the construction of pWPS3 are underlined.
In order to further evaluate the function of ORF1 in immunity to dysgalacticin, two recombinant plasmids were derived from pW2580 (Table 2). First, pWPS1 was constructed by insertion (in the same transcriptional orientation) of an erythromycin resistance determinant, ermAM (2), into the dysgalacticin structural gene, dysA. This strategy not only allowed pW2580 to be selectable with erythromycin but also obviated any potential interference from dysgalacticin biosynthesis. Second, pWPS2 was derived from pWPS1 by digestion and filling-in (with a Klenow fragment) of the unique HindIII site (Fig. 1A), thereby generating a frameshift mutation in ORF1. All desired mutations in this study were confirmed by direct sequencing of PCR amplicons spanning the relevant portions of the plasmids (Allan Wilson Centre Genome Service, Palmerston North, New Zealand). Plasmids pWPS1 and pWPS2 were individually introduced by electroporation (12) into both the isogenic S. dysgalactiae strain W2580C and the dysgalacticin-sensitive indicator S. pyogenes FF22. The latter strain was selected as a suitable host because (i) it is plasmid-free, and (ii) S. pyogenes would be compatible, with respect to plasmid replication, due to the discovery of a pW2580-like plasmid, pDN281, in a dysgalacticin-producing S. pyogenes strain, 71-698 (8, 14).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| S. dysgalactiae subsp. equisimilis | ||
| W2580 | Dysgalacticin producer strain; Dysi | 9 |
| W2580C | pW2580-cured derivative of W2580; Dyss | 9 |
| DWPS1 | W2580C carrying pWPS1; Dysi Emr | This study |
| DWPS2 | W2580C carrying pWPS2; Dyss Emr | This study |
| DWPS3 | W2580C carrying pWPS3; Dysi Emr | This study |
| S. pyogenes | ||
| FF22 | Indicator strain for dysgalacticin; Dyss | 9 |
| PWPS1 | FF22 carrying pWPS1; Dysi Emr | This study |
| PWPS2 | FF22 carrying pWPS2; Dyss Emr | This study |
| Plasmids | ||
| pW2580 | Dysgalacticin-encoding indigenous plasmid of strain W2580 | 9 |
| pWPS1 | pW2580 with ΔdysA::ermAMdysI+; Emr | This study |
| pWPS2 | pWPS1 with ΔdysI; Emr | This study |
| pWPS3 | pWPS1 containing C-terminal His6-encoding dysI (His6-dysI); Emr | This study |
Dysi, dysgalacticin immune (resistant); Dyss, dysgalacticin sensitive; Emr, erythromycin resistant.
One erythromycin-resistant transformant from each of the S. dysgalactiae and S. pyogenes transformation experiments (Table 2) was selected for subsequent deferred antagonism (9, 16) and MIC assays (15). S. dysgalactiae W2580C or S. pyogenes FF22 carrying pWPS1 was immune to the effects of dysgalacticin (Fig. 1C), with a concomitant >120-fold increase in the MIC of dysgalacticin for both species (Table 3). On the other hand, pWPS2 (containing the frameshift-mutated ORF1) failed to confer immunity to dysgalacticin when introduced into either S. dysgalactiae or S. pyogenes hosts (Fig. 1C, Table 3). Taken together, these results not only clearly demonstrate that ORF1, now designated dysI, encodes the dysgalacticin immunity factor (DysI) but also that it is genetically linked to the replicative process of pW2580.
TABLE 3.
Agar-based and dysgalacticin MIC assays of wild-type S. dysgalactiae and S. pyogenes strains and their derivatives
DysI interacts with the cytoplasmic membrane.
Based on the predicted topology of DysI and the finding that dysgalacticin is membrane targeted (15), we postulated that DysI exerts its protective effect at the membrane level. To determine whether DysI localizes to, or interacts with, the cytoplasmic membrane, pWPS3, in which dysI was modified by overlap extension recombinant PCR to encode a DysI molecule (DysI-His6) containing a C-terminal hexahistidine (His6) tag, was constructed. S. dysgalactiae W2580C was electrotransformed with pWPS3, and the resulting derivative (strain DWPS3) was dysgalacticin resistant (MIC > 44 μg/ml), indicating that the His6 tag did not interfere with DysI function. DysI-His6 localization was determined by western immunoblotting (6) utilizing an anti-His6 primary antibody and detected using a horseradish peroxidase-based chemiluminescence system (Pierce Biotechnology [Thermo Fisher Scientific], Rockford, IL) on cytoplasmic and membrane fractions of DysI-His6-expressing S. dysgalactiae W2580C. A 7.8-kDa band corresponding to the expected size of DysI-His6 was observed more intensely in the membrane fraction than in the cell lysate and was absent in the cytoplasmic fraction (Fig. 2). These results indicate that DysI is membrane associated and that its C-terminal end is not critical for its function.
FIG. 2.
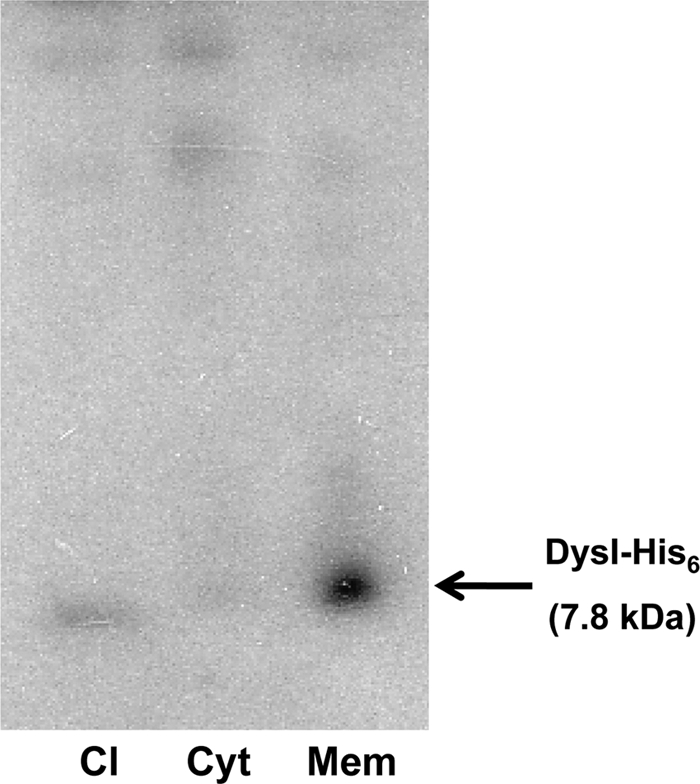
Western blot analysis of DysI-His6 localization. Cell lysate (Cl), cytoplasmic (Cyt), and membrane (Mem) fractions of DysI-His6-expressing S. dysgalactiae strain DWPS3.
DysI prevents the inhibitory effects of dysgalacticin on 2DG uptake, glucose fermentation, membrane integrity, and intracellular ATP.
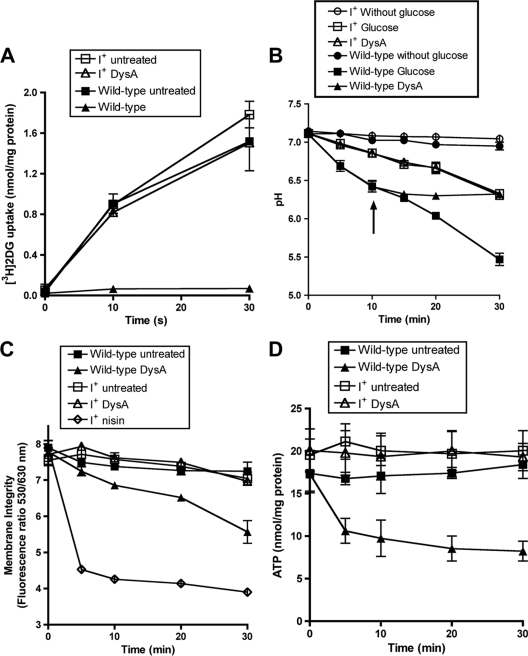
Dysgalacticin is reported to interact with the membrane-bound glucose/mannose PTS in S. pyogenes, inhibiting uptake of glucose as well as the nonmetabolizable analog 2-deoxyglucose (2DG), which in turn adversely affects glucose fermentation (15). To examine the possibility that DysI may prevent dysgalacticin from interacting with the glucose/mannose PTS, [3H]-2DG uptake was measured (15) in the DysI+ S. pyogenes strain PWPS1 (FF22 carrying pWPS1). Whereas the rate of [3H]2DG uptake in wild-type S. pyogenes FF22 exposed to dysgalacticin is very low (0.139 nmol [mg protein]−1 min−1), the rate observed with strain PWPS1 treated under the same conditions, i.e., 3.01 nmol [mg protein]−1 min−1, was comparable to that of untreated cells (3.04 to 3.56 nmol [mg protein]−1 min−1) (Fig. 3A).
FIG. 3.
Protective effect of DysI in S. pyogenes strain PWPS1 (FF22 carrying pWPS1) with respect to [3H]2DG uptake (A), glucose fermentation (B), membrane permeabilization (C), or intracellular ATP content (D). Cells were energized with glucose in all experiments except that analyzed in panel A and a glucose-free control (B). Untreated controls of wild-type S. pyogenes FF22 are shown as filled squares, and cells treated with 11 μg/ml of dysgalacticin are represented by filled triangles. “I+” represents dysI+ S. pyogenes strain PWPS1, which was treated with dysgalacticin (open triangles), untreated controls (open squares), or untreated controls without glucose (open circles). The arrow indicates the time point at which purified recombinant dysgalacticin (9) was added to the cells at a final concentration of 11 μg/ml. Nisin, a known pore-forming bacteriocin, was included as a positive control (open diamonds) in the membrane permeabilization experiments in order to maintain consistency with previous studies (15) and was added at a concentration of 25 μg/ml. The MIC of nisin for S. pyogenes is 0.78 μg/ml.
The glucose fermentation of S. pyogenes PWPS1 was measured as a change in the external pH of the cell suspension (15). As shown in Fig. 3B, the rates of glucose fermentation in strain PWPS1 were comparable between the untreated control and cells treated with dysgalacticin. In contrast, glucose fermentation was inhibited in wild-type S. pyogenes FF22 within 5 min of dysgalacticin addition. Taken together, these results indicate that DysI may function to prevent the interaction of dysgalacticin with its proposed receptor (e.g., the glucose/mannose PTS), yet it does not interfere with the functionality of the glucose/mannose PTS permease, i.e., in glucose uptake.
The subsequent effect of the interaction between dysgalacticin and its cognate receptor has been proposed to involve disruption of cytoplasmic membrane integrity (15). Therefore, the effect of dysgalacticin on membrane integrity was determined in dysI+ S. pyogenes PWPS1. The membrane integrity of strain PWPS1 was intact, while that of wild-type S. pyogenes FF22 was compromised (Fig. 3C). Moreover, since S. pyogenes treated with dysgalacticin was reported to show a reduction in the intracellular ATP level (15), it was hypothesized that DysI may prevent a reduction in intracellular ATP content. The intracellular ATP content of dysI+ S. pyogenes PWPS1 was not affected when the strain was treated with dysgalacticin, whereas a 50% loss (P < 0.01) after 10 min was observed in wild-type S. pyogenes FF22 (Fig. 3D). These results collectively support our hypothesis that DysI interferes with the activity of dysgalacticin at the membrane level.
Concluding remarks.
In the present study, we have not only verified that the immunity factor (DysI) for the plasmid-encoded bacteriocin dysgalacticin is indeed plasmid borne but have also demonstrated that dysI is transcribed along with the genes essential for replication of pW2580. Although the genetic loci of several bacteriocins of Gram-positive bacteria are known to reside on (mega)plasmids (3, 5, 19), this appears to be the first report of a direct transcriptional link between bacteriocin immunity and plasmid replication. Such a link can be regarded as a novel toxin-antitoxin (i.e., dysgalacticin-DysI) plasmid maintenance system (7), since the expression of dysI as part of the copG-repB-dysI operon would ensure that immunity to the bacteriocin is in place well in advance of bacteriocin biosynthesis. This is further supported by possible temporal delays in dysgalacticin production, such as the following: (i) the orientation of dysA being opposite to that of copG-dysI (Fig. 1A), (ii) the observed lack of dysA transcript in the mid-logarithmic phase of cell growth, and (iii) the requirement of Sec-dependent export to yield active mature dysgalacticin (9).
Although we have shown that DysI is membrane associated and its presence protects the target cell from the deleterious effects of dysgalacticin, the details of DysI action have not yet been established. Unlike dysgalacticin, which is actively secreted into the external milieu, a signal peptide was not detected in the deduced amino acid sequence of DysI using the SignalP algorithm (1), indicating that DysI exerts its protective effects from the cytosolic side of the cell membrane. It is therefore tempting to speculate that DysI may function as a “molecular lock,” i.e., by interacting with the putative bacteriocin receptor (e.g., the glucose/mannose PTS) such that the binding of dysgalacticin to the PTS is interfered with but without affecting the functionality of the sugar permease itself. Future studies will be aimed at determining, at the molecular level, how DysI confers bacteriocin immunity.
Acknowledgments
This work was funded by the Otago Medical Research Foundation and the University of Otago Research Grants Committee. P. M. Swe was supported by a Department of Microbiology and Immunology (University of Otago) Ph.D. scholarship.
Footnotes
Published ahead of print on 8 October 2010.
REFERENCES
- 1.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 2.Brehm, J., G. Salmond, and N. Minton. 1987. Sequence of the adenine methylase gene of the Streptococcus faecalis plasmid pAMβ1. Nucleic Acids Res. 15:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claesson, M. J., Y. Li, S. Leahy, C. Canchaya, J. P. van Pijkeren, A. M. Cerdeño-Tárraga, J. Parkhill, S. Flynn, G. C. O'Sullivan, J. K. Collins, D. Higgins, F. Shanahan, G. F. Fitzgerald, D. van Sinderen, and P. W. O'Toole. 2006. Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. U. S. A. 103:6718-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois, J. Y., T. R. Kouwen, A. K. Schurich, C. R. Reis, H. T. Ensing, E. N. Trip, J. C. Zweers, and J. M. van Dijl. 2009. Immunity to the bacteriocin sublancin 168 is determined by the SunI (YolF) protein of Bacillus subtilis. Antimicrob. Agents Chemother. 53:651-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, G., G. K. Guron, J. J. Churey, and R. W. Worobo. 2009. Characterization of mundticin L, a class IIa anti-Listeria bacteriocin from Enterococcus mundtii CUGF08. Appl. Environ. Microbiol. 75:5708-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauntlett, J. C., S. Gebhard, S. Keis, J. M. Manson, K. M. Pos, and G. M. Cook. 2008. Molecular analysis of BcrR, a membrane-bound bacitracin sensor and DNA-binding protein from Enterococcus faecalis. J. Biol. Chem. 283:8591-8600. [DOI] [PubMed] [Google Scholar]
- 7.Hayes, F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496-1499. [DOI] [PubMed] [Google Scholar]
- 8.Heng, N. C. K., A. S. McCaw, and J. R. Tagg. 2006. Prog. Abstr. 7th ASM Conf. Streptococcal Genet., abstr. A90. American Society for Microbiology, Washington, DC.
- 9.Heng, N. C. K., N. L. Ragland, P. M. Swe, H. J. Baird, M. A. Inglis, J. R. Tagg, and R. W. Jack. 2006. Dysgalacticin: a novel, plasmid-encoded antimicrobial protein (bacteriocin) produced by Streptococcus dysgalactiae subsp. equisimilis. Microbiology 152:1991-2001. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Arriaga, A. M., T. S. Rubio-Lepe, M. Espinosa, and G. del Solar. 2009. Repressor CopG prevents access of RNA polymerase to promoter and actively dissociates open complexes. Nucleic Acids Res. 37:4799-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann, A., T. Schneider, U. Pag, and H.-G. Sahl. 2004. Localization and functional analysis of PepI, the immunity peptide of Pep5-producing Staphylococcus epidermidis strain 5. Appl. Environ. Microbiol. 70:3263-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimoto, H., and A. Taketo. 2003. Efficient electrotransformation system and gene targeting in pyogenic streptococci. Biosci. Biotechnol. Biochem. 67:2203-2209. [DOI] [PubMed] [Google Scholar]
- 13.Okuda, K., Y. Aso, J. Nagao, K. Shioya, Y. Kanemasa, J. Nakayama, and K. Sonomoto. 2005. Characterization of functional domains of lantibiotic-binding immunity protein, NukH, from Staphylococcus warneri ISK-1. FEMS Microbiol. Lett. 250:19-25. [DOI] [PubMed] [Google Scholar]
- 14.Simpson, W. J., and J. R. Tagg. 1984. Survey of the plasmid content of group A streptococci. FEMS Microbiol. Lett. 23:195-199. [Google Scholar]
- 15.Swe, P. M., G. M. Cook, J. R. Tagg, and R. W. Jack. 2009. Mode of action of dysgalacticin: a large heat-labile bacteriocin. J. Antimicrob. Chemother. 63:679-686. [DOI] [PubMed] [Google Scholar]
- 16.Tagg, J. R., and L. V. Bannister. 1979. Fingerprinting beta-hemolytic streptococci by their production of and sensitivity to bacteriocine-like inhibitors. J. Med. Microbiol. 12:397-411. [DOI] [PubMed] [Google Scholar]
- 17.van Belkum, M. J., J. Kok, G. Venema, H. Holo, I. F. Nes, W. N. Konings, and T. Abee. 1991. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J. Bacteriol. 173:7934-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venema, K., R. E. Haverkort, T. Abee, A. J. Haandrikman, K. J. Leenhouts, L. de Leij, G. Venema, and J. Kok. 1994. Mode of action of LciA, the lactococcin A immunity protein. Mol. Microbiol. 14:521-532. [DOI] [PubMed] [Google Scholar]
- 19.Wescombe, P. A., J. P. Burton, P. A. Cadieux, N. A. Klesse, O. Hyink, N. C. K. Heng, C. N. Chilcott, G. Reid, and J. R. Tagg. 2006. Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek 90:269-280. [DOI] [PubMed] [Google Scholar]
- 20.Wong, H. K. 1981. Inhibitors active against group A streptococci. M.S. thesis. University of Otago, Dunedin, New Zealand.