Abstract
Dupuytren disease (DD) is a common fibroproliferative disease of unknown etiopathogenesis affecting the palmar aponeurosis, causing reduced hand function and resulting in fixed flexion contractures of the digits. Current gold standard treatment for the management of DD is surgical excision involving removal of the affected palmar fascial tissue. However, there are potential complications associated with surgery as it is costly and a positive surgical outcome is often short-lived because the disease tends to recur. Therefore, there is growing interest in nonsurgical, outpatient-based treatments that could be quicker, cheaper, reduce morbidity, show a decreased rate of recurrence, and give DD patients an improved quality of life when compared with traditional surgical management. Of the available nonsurgical options, injectable Clostridium histolyticum collagenase (CHC) has received recent clinical interest. In this article, a brief overview of DD surgical and nonsurgical treatments utilized is given, followed by a detailed examination of the nine papers published to date on the use of CHC in DD (and similar fibrotic disorders). These papers have investigated safe and efficacious doses for the injection of CHC to treat palpable DD cords in adult patients and have shown significant short- to mid-term results for correction to near-full digital extension (≤5° extension) following CHC injection of DD cords. CHC has been shown to target the collagen-based DD cords while sparing surrounding neurovasculature, with a complication profile that appears comparable to that of the surgical methods currently utilized. In conclusion, clostridial collagenase is a novel nonsurgical treatment option of considerable potential in the management of DD when administered by specialist hand surgeons with detailed knowledge of the disease and the relevant anatomy. Nonetheless, there is a need for further data on long-term results, complications, and rate of recurrence with the use of this emerging treatment option.
Keywords: Dupuytren contracture, enzymatic injection, fibrosis, nonsurgical treatment
Introduction
Dupuytren disease (DD) is a common, fibroproliferative disease affecting the hands and resulting in fixed flexion contractures that are progressive and irreversible.1 This deformity results in considerable disability and can limit patient activities of daily living, manual activities, and sporting hobbies, markedly reducing patients’ quality of life.2 There are a variety of treatments that have been investigated, with the ongoing mainstay being surgical correction involving either release or excision of the affected palmar fascia. However, there has been an increasing interest in the use of and clinical success with the utilization of injectable Clostridium histolyticum collagenase (CHC) for the nonoperative treatment of DD.3–10 Recent clinical trials3,5,9,11–18 have shown encouraging results, to the extent that this novel technique will likely be used frequently in future clinical practice – hence, it is important to explore the efficacy and safety profile of such a technique.
The aims of this review are to give an overview of DD and its current treatment options, with particular focus on the nonsurgical methods trialed historically; to give a synopsis of the history and uses of CHCs to date; to summarize the uses of such CHCs within the field of DD (and associated fibrotic conditions); and, to highlight the growing utility of such collagenases within the treatment options available for DD.
Methods
Searches were undertaken using Medline, PubMed, and Scopus to identify seminal studies and reviews using all keywords relating to DD and CHC. To ensure no studies were missed, the references of each selected study or review were also searched and any relevant articles were included. All relevant European and US patents were reviewed, plus all US Food and Drugs Administration (FDA) documentation relating to the Xiaflex® (Auxilium Pharmaceuticals Inc., Malvern, PA) application for approval (granted in February 2010). Finally, the product specification sheets for the commercially available clostridial collagenases were reviewed.
DD – an overview
DD is a benign yet progressive, fibroproliferative disorder of unknown origin affecting the palmar fascia and extending into the digits of the hands.1 It is commonly bilateral, with a pathological transformation of the fascial tissues into thickened fibrous nodules and cords which, on progression to the advanced stage of the disease, result in irreversible flexion contractures of the involved digits.19 DD can be associated with extra-palmar ectopic fibrosis over the knuckle pads, known as Garrod nodes;20 in the sole of the foot, known as Lederhose disease or plantar fibromatosis;21 formation of fibrotic plaques within the corpora cavernosal tunica albuginea, resulting in penile bending during erections, known as Peyronie disease (PD);22 and, uncommonly, with involvement of the wrist20 or with nodular fasciitis in the popliteal space.19
DD was first described in the medical literature in 1831 by the eponymous Baron Guillaume Dupuytren,23 although Dupuytren was not the first to describe this disease. Henry Cline, in 1777, initially described DD as a palmar fascial disorder through his study of cadaveric specimens: he first noted the contribution of the palmar fascia to the disorder, then, 10 years later, suggested its treatment via palmar fasciotomy.23,24 In addition, Cline’s student, Sir Astley Cooper wrote in 1822 on this disease and demonstrated a procedure similar to needle fasciotomy.25 Dupuytren visited Cooper in 1826 but did not perform his first surgery (on his coach man) until 1831 – this was the operation he later described and published in Lancet.26
The prevalence of DD is thought to be affected by gender, age, ethnicity, and geographical origin.27 The male to female incidence ratio ranges between 5:1 and 15:1,28 although the frequency in women increases substantially with age, catching up to that seen in men in later life.29 Rarely, children as young as 9 years have been diagnosed with the condition.30 More commonly, men tend to present in the fifth decade, whereas women require treatment later23 – irrespectively, the incidence in both sexes increases with age, with the reported prevalence in northern Europe for men older than 65 years being between 18% and 30%.31 DD is primarily a disease of northern European caucasians, with only sporadic case reports of it affecting nonwhite individuals. Where nonwhite-skinned populations are affected, eg, the Japanese (where the prevalence is similar to that seen in northern Europe) and, rarely, African Americans, authors have postulated a prior interracial mixing in the genetic lineage of the affected individuals.23 Geographically, DD is commonest in the United Kingdom, Scandinavia, North America, Australasia, and Japan, with a markedly decreased prevalence in the more southerly Mediterranean regions and South America. DD is considered to be rare in Africa and China.27,32
The etiology of DD remains controversial, with associations of varying significance having been found with heredity,27,33,34 alcohol consumption,35 smoking,36 diabetes mellitus,37,38 epilepsy (or antiepileptic medications),39,40 raised serum lipids,41 local injury (leading to algodystrophy and disuse of the affected hand),42 and occupations involving vibration exposure.43 Of these, genetic heritability undoubtedly has a significant etiological contribution, with some authors suggesting a Mendelian autosomal dominant transmission with variable penetrance44 and others suggesting DD to be a complex trait (like diabetes, hypertension, or cancer).31 The associations with smoking, diabetes, raised serum lipids, and alcohol consumption have been suggested to be related to the microangiopathic ischemia that underlies these conditions – this results in increased free radical production, which stimulates fibroblast proliferation and cytokine release, potentially leading to the development of DD.45
The pathophysiology of DD is complex and multifactorial, to date eluding the establishment of an overarching pathophysiological model despite substantial investigative efforts. The disease affects the palmar fascial tissues, causing abnormalities in fibroblast proliferation, myofibroblast production, and collagen deposition that result in the development of the structurally distinct nodule and cord disease components.46 Three distinct histological phases have been described.23 The proliferative stage, the most biologically active phase of the disease, is characterized by local fascial fibroplasia and myofibroblast proliferation resulting in nodule formation. Next follows the involutional stage in which the myofibroblasts become aligned along lines of tissue mechanical stress, demonstrating progressive contractile behavior triggered by the effect of a variety of cytokines on the intracellular actin microfilaments – this results in the formation of the progressively shortening fascial cords. In the final disease stage, termed the residual phase, nodules regress leaving the tendon-like, inelastic, relatively acellular cords that cause the clinically apparent fixed flexion contractures.23 Normal palmar fascial tissue is primarily composed of type I collagen – in DD, the increased density and decreased apoptosis of fibroblasts, plus an imbalance in matrix metalloproteinase activity, result in excessive type III collagen production.45
Irrespective of the underlying etiological factors, what primarily concerns patients is the degree of manual functional disability that DD imposes.47 Studies that objectively attempt to link severity of DD contractures and manual disability have shown little to no correlation between the severity of contracture and the degree of manual disability (as assessed by the disability of the arm, shoulder and hand [DASH] questionnaire).48 This may be unsurprising because the DASH is neither DD-specific nor hand-specific, with an emphasis on pain (not a cardinal feature of DD) – a DD-specific hand function questionnaire has yet to be validated. Also, there are no objective papers to date examining the impact of DD on patients’ quality of life. Nevertheless, the authors’ experience is that patients commonly complain of problems with shaking hands, putting on gloves, typing, performing recreational activities (eg, golf and gardening), and performing basic personal care activities (eg, washing their face).1 Despite a lack of formal documentation of the impact of such problems, it is obvious that such activity impairment must affect patients’ quality of life and thus stimulate the drive to seek correction of the imposed deformities.
Management issues in DD
DD classically progresses in two distinct manners. In the less-common disease form associated with the term diathesis and usually found in patients with a strong familial predisposition to the disease, the progression tends to be more aggressive with contractures developing over a few months to years and with rapid disease recurrence after surgical interventions. 49 The more common disease form not associated with diathesis, where there is no obvious familial preponderance, usually progresses more slowly, often allowing the patient to maintain sufficient hand function to live with the disease for years prior to seeking treatment, following which there is a lower risk of disease recurrence.49 To date, there is no known cure for either form of the disease, but its impact has been commonly managed with appropriate surgical intervention.
In view of the variability in the rate of disease progression and the extent of disability caused to patients’ hand function, initial treatment of DD usually involves watchful waiting to observe the rate of progression of the disease until the contractures are significantly affecting a patient’s hand function or their activities of daily living.1 Currently, the gold standard treatment in DD is surgical, with a choice of selective, segmental, limited, or modified fasciectomy; radical or total aponeurectomy; or dermofasciectomy to excise the affected fascial tissue and restore digital extension1 (the operation is chosen at the surgeon’s discretion and depending on the extent of the disease – dermofasciectomy is most commonly used in recurrent cases, particularly those involving the proximal interphalangeal joint [PIPJ]). Other less-invasive surgical treatment options include closed fasciotomy and needle aponeurotomy, both of which incise the fascial bands, causing rupture of the cord.50–52 These methods are much less invasive and can achieve digital extension, but they have been associated with higher rates of recurrence, generally being reserved for elderly or infirm patients in whom the risks of more extensive surgery outweigh the improved recurrence rate benefit.53 Overall, the development of a nonsurgical treatment option for this disease may improve morbidity and recovery time and enhance patients’ quality of life.
To date, a variety of nonsurgical treatments have been investigated with variable success. These have included the administration of steroids,54,55 dimethyl sulfoxide,56 vitamin E (tocopherol),57 radiotherapy,58–60 ultrasonic therapy,61 physical therapy,47 methylhydrazine,62 allopurinol,63 enzymatic fasciotomy (using a combination of trypsin, hyaluronidase, and lidocaine),64 and collagenase3,5,9,11–18 (see Table 1). Steroid injection of the nodules has shown some clinical promise in modifying the disease progression (97% of nodules injected showed some softening or flattening; however, 50% of these patients exhibited recurrence within less than 3 years)54 and merits further investigation. However, to date, the most clinically promising of these treatments has been the use of injectable CHC to locally degrade the collagen structures, allowing rupture of the fibrous cords and restoration of complete or near-complete digital extension (see CHCs and fibrotic disease).
Table 1.
All papers retrieved relating to CHC use in the management of fibrotic disorders, in their chronological order of publication
| Authors/refs | Disorder | Aim/end point | Key findings |
|---|---|---|---|
| Gelbard11 | PD | Effect of CHC on: healthy vs PD-affected tunica albuginea surrounding tissues following injection into rat femoral canal | No significant difference in the degree of collagen degradation between the diseased and healthy tissues Visible tissue clearing within the injection zone but not outside the radius of the zone Collagenolysis with preservation of elastin and no evidence of injury to local neurovasculature Macroscopic hematoma; microscopic venule and perineurium degradation with sparing of arterioles and endoneurium |
| Starkweather12 | DD | Effect of injected CHC on tensile strength of DD cords | Phase I: 3,600 U injection; 10 CHC vs 10 control injections; 93% decrease in tensile modulus Phase II: dose response (150/300/600 U/control); curvilinear trend between increased dose and decreased tensile strength |
| Badalamente13 | DD | In vivo effect of CHC on joint contractures – correction to ≤5° extension | Phase I: dose trial (300, 600, 1,200, 4,800, 9,600 U) – no cord rupture Phase II: 10,000 U; 29 patients – 88% correction of MCPJ, 44% full correction of PIPJ; 2 MCPJ, 2 PIPJ no response – surgery required; No major adverse reactions, no clinical systemic immune response |
| Badalamente14 | DD | In vivo effect of CHC on joint contractures – correction to ≤5° extension (PCRT) | Phase I: MCPJ – 14/18 CHC-injected joints achieved primary end point vs 2/18 placebo; 4/18 recurrence at 4 y PIPJ – 5/7 CHC-injected joints achieved primary end point vs 0/7 placebo; 4/7 recurrence at 4 y Phase II: dose-response (2,500, 5,000, 10,000, placebo): significantly dose related, 10,000 U most effective; 90% MCPJ and 70% PIPJ achieved primary end point; 1 MCPJ and 1 PIPJ of 80 patients showed recurrence at 2 y Substudy 1: pharmacokinetics of CHC – no CHC in serum; 28% of administered CHC dose found in urine 1 h after CHC injection in DD cord Substudy 2: addition of lidocaine for joint manipulation in 5 patients; well tolerated and no effect on end point |
| Kang16 | Keloid | Effects of CHC/triamcinolone/ combination of both on scar | Mixed keloid and hypertrophic scars (7 patients): keloid – 33% reduction in scar volume in first 6 mo, then effect lost; hypertrophic scars – no effect Patient-perceived severe reactions to treatment |
| Badalamente9 | DD | In vivo effect of CHC on joint contractures – correction to ≤5° extension (PCRT) | Blinded PCRT: 55 joints; 10,000 U/injection; multiple injections to achieve primary end point; 91% CHC joints achieved primary end point vs 0% placebo Open phase: 35 joints, given CHC injection; 77% achieved primary end point; 5/35 showed disease recurrence at 2 y |
| Del Carlo17 | PD and DD | In vitro effect of CHC when injected into PD plaques and DD cords | Enzymatic effect maximal at 4 h; near-complete digestion at 12 h; no cellular death; noncollagenous tissues unaffected |
| Hurst5 | DD | In vivo effect of CHC on joint contractures – correction to ≤5° extension (PCRT) | 512 joints: 64% CHC joints achieved primary end point vs 6.8% placebo joints 96.6% CHC joints developed ≥1 treatment-related adverse event vs 21.2% placebo joints 3 patients with severe CHC-related adverse events: 1 complex regional pain syndrome; 2 tendon ruptures Biochemical immune response in ≥85.8% of patients with 1 CHC injection and 100% with 3 CHC injections; no clinically apparent systemic immune responses |
| Watt3 | DD | Recurrence rate 8 y after single CHC injection – correction to ≤5° extension | MCPJ – 2/6 no recurrence; 4/6 recurrence PIPJ – 2/2 recurrence Patient perceived subjective success of treatment – 60% successful; 7/8 would consider repeat treatment |
Abbreviations: CHC, Clostridium histolyticum collagenase; PD, Peyronie disease; DD, Dupuytren disease; MCPJ, metacarpophalangeal joint; PIPJ, proximal interphalangeal joint; PCRT, placebo-controlled, randomized trial.
In view of the emergence of, and potential for, increasing clinical use of CHCs, this review gives an overview of the development, mode of action, pharmacokinetics, and current uses of the commercially available collagenases and details the issues related to its application in the nonsurgical treatment of DD.
Collagenase C. histolyticum
The history of the proteolytic enzymes derived from Clostridium bacterial species dates back to the early 20th century when it was observed that culture filtrates from Clostridium perfringens had the ability to digest healthy muscle tissue. 65 However, it was not until the 1940s when the ability of such culture filtrates to break down the collagen framework of healthy Achilles tendon was observed, that this novel enzyme was named collagenase.65 Since then, an increasing number of bacterial species, often those that are pathogenic in humans, have been shown to produce collagenases, which contribute to their pathogenicity.66 Collagen, which comprises nearly 30% of all proteins within mammalian bodies, is an insoluble structural matrix protein composed of three helically wound polypeptide fibrils – it is resistant to degradation by the majority of proteases due to its triple helical structure which requires unwinding before proteolytic degradation can occur.66,67
Degradation is key to a variety of both physiological (eg, embryological bone development and wound repair) and pathological conditions (eg, tumor invasiveness and soft tissue bacterial infection), so the human body also produces its own endogenous collagenase as part of the family of zinc-dependent matrix metalloproteineases.68
To date, CHCs have been investigated for clinical use in the treatment of herniated intervertebral discs,69–71 retained placenta,72 frozen shoulder adhesive capsulitis,73 DD (see section “CHC and fibrotic disease”), PD,11,17,74 wound healing, 75–77 in the debridement of burns,76,77 and in the preparation of pancreatic islet cells for transplantation.16,78 It has also been suggested that CHC could be used in the treatment of keloid scars,16 for recannalization of chronically occluded arterial vessels or stents within peripheral arterial angioplasty procedures79 and in the amelioration of plantar fasciitis,7 lateral epicondylitis,7 and cellulite removal.10 This review focuses on the uses of clostridial collagenases in the treatment of fibrotic disorders, with the primary focus on its use in the nonsurgical management of DD.
Pharmacology
Seven collagenases have been isolated from the culture filtrate of the bacteria C. histolyticum, namely α, β, γ, δ, ɛ, ξ, and η collagenases.80 They are all zinc-dependent matrix metalloproteinases whose unique specificity is in the hydrolysis of collagen molecules via the peptide bond between the Gly-Pro and residue X of the tri-peptide repeat unit (where residue X is often proline or hydroxyproline).68 The seven collagenases can be divided into two classes, I and II, which are classified based on their bacterial gene of origin (colG and ColH, respectively) and on their point of hydrolytic attack on the collagen molecule – class I enzymes act at loci near the ends of the collagen triple-helical domains, whereas class II enzymes make internal initial cleavages80–82 (see Figure 1) – their combined action at many sites along the peptide chain results in the sequential cleavage of short segments. Both classes have specific binding domains that enable them to recognize and bind to triple helical collagen.83,84 The biochemical properties of CHCs mark them out as potentially useful targets for in vivo clinical applications: these include their ability to remain active over a wide pH range (the optimum being pH 7.4); a high degree of substrate specificity, with degradation of only the collagen components of connective tissue structures; a good enzymatic activity at body temperature (37°C); a lack of autodigestion; and, that they can be lyophilized safely and then reconstituted directly prior to use allowing for easy storage and a good stability profile.11 With regard to inhibitors of CHC activity, CHCs are dependent on the presence of Ca2+ ions and, thus, must be diluted in buffer solution containing Ca2+ prior to their utilization – interestingly, a dose-dependent, predominantly noncompetitive inhibition of CHC activity has been noted with the aloin compounds contained within the mucilaginous sap of the Aloe barbadensis plant, commonly referred to as Aloe vera gel (however, this is via direct interaction with the collagenase molecule and not via its catalytic domain).85
Figure 1.
Illustration of classes of Clostridium histolyticum collagenase (CHC) based on their hydrolytic point of attack on collagen molecule: class I CHCs act at loci near the N and C terminals of the collagen domain; class II CHCs cleave within the central collagen domain – the combined action of both classes synergistically effects thorough degradation of the collagen triple helix.
CHCs are manufactured via bacterial fermentation, following which the different collagenases can be separated off chromatographically – the majority of commercially available CHCs are produced in this manner. However, a drawback of this method is that the ratio and activity of class I and class II CHCs yielded is unreliable, so each CHC batch can have differing biological effects.7 There are a large variety of commercially available CHC preparations, each with differing selectivity of included CHCs, and this allows for the different uses to which CHCs are put in the laboratory setting. For in vivo human clinical trials, the investigators have been careful to select highly purified preparations in order to maintain maximal reproducibility. However, more recent work has utilized a novel CHC, designed specifically for medical therapeutic applications and marketed as Xiaflex ®, which gained US FDA approval on February 2, 2010 and is expected to achieve European approval in January 2011. This bypasses the issues of unreliable interbatch reproducibility by highly purifying the initial culture filtrate to select a 1:1 mass ratio of class I to class II CHCs, thereby guaranteeing a reproducible collagenolytic effect.7
CHCs and fibrotic disease
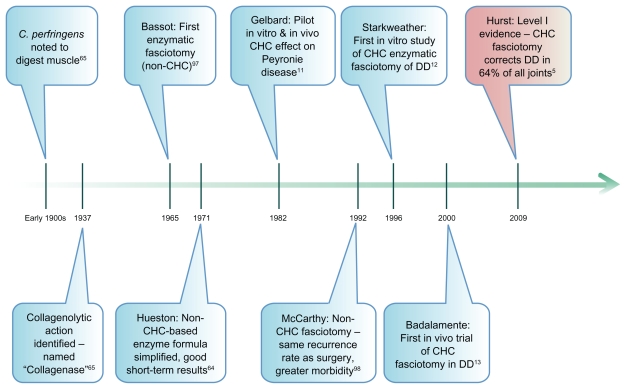
A timeline of key events in the development of CHC use in DD is illustrated in Figure 2. Literature searches retrieved all papers relating to CHC use in the management of fibrotic disease (ie, DD, PD, and keloid scarring), and these are listed for ease of reference in Table 1 and discussed in chronological order of publication hereafter.
Figure 2.
Timeline of key milestones in the history of Clostridium histolyticum collagenases (CHCs) and their use in Dupuytren disease (DD).
The initial pilot study in the examined series was a multifaceted pilot by Gelbard et al11 who looked at the in vitro collagenolytic effect of commercially available CHC on human PD tissue, healthy tunica albuginea, pericardium, and corpora cavernosal sections, plus the in vivo effect of CHC on the femoral neurovascular bundle of an anesthetized rat. First, they compared CHC activity (via measurement of desiccated tissue weight and release of free α-amino acid groups) on PD plaque tissue and healthy tunica albuginea tissue, with tissue derived from three patients for each tissue type. They showed there was no significant difference in the degree of collagen degradation between the diseased and healthy tissues, ie, CHC did not selectively degrade PD plaque collagen but would affect surrounding healthy tissues. To look into the effect of local injection of CHC and dispersion into surrounding tissues they investigated injecting CHC at a variety of unit strengths into human pericardium and demonstrated that when CHC was injected locally into tissue, there was a dose-related response, but this was always limited to the injection site – with a dose of 400 U, there was visible tissue clearing within the injection zone but not outside the radius of the zone. They suggested this confinement of digestion to be related to the mucopolysaccharide “ground substance” in which the collagen is embedded and which does not undergo digestion itself. Next, they injected 400 U into sections of PD-affected and healthy corpora cavernosa – this caused collagenolysis (varying on histological examination from decreased stain uptake to total disappearance of collagen bundles) but with preservation of elastin and with no evidence of injury to the local neurovasculature. Finally, they injected 600 U of CHC into the adventitia surrounding the left femoral neurovascular bundle of an anesthetized rat – 24 hours later, the rat was sacrificed, and the treated and untreated femoral neurovascular bundles were compared. Macroscopically, a moderate hematoma was noted with no signs of active bleeding. Microscopically, venules and the fibrous perineurium underwent degradation, but there was no effect on arterioles or the nerve fibers themselves – this was postulated to be due to the smooth muscle in the arteriolar walls and the myelin sheaths enveloping and thus protecting the nerves. Based on Garvin’s experiments82 on the cytotoxicity of intravenous CHC in rats (see Discussion for greater detail), the authors extrapolated that the cytotoxic dose in a 70 kg human might be around 89,040 U, which was 356 times more potent than the doses they had found to be required for adequate collagenolysis at a local injection level.
In the first of a number of papers submitted by the same US-based group of investigators, Starkweather et al12 analyzed the effect of injected CHC on the tensile strength of DD cords obtained from patients who had undergone fasciectomy. All cords were subjected to controlled stretching within a mechanical testing device that recorded stress and strain effects within the cords, following which they underwent histological examination. In the first phase of the study, the authors compared the effect of 3,600 U CHC injections in 10 DD cord samples against 10 cords injected with a control buffer solution. Cords injected with CHC showed a statistically significant 93% decrease in tensile modulus when compared with the control buffer-injected cords. In the second phase of the study, 20 DD cord samples were randomly allocated into groups and subjected to an injection of 150, 300, or 600 U of CHC or a control buffer solution. Each cord was stressed to failure, ie, until cord rupture was achieved, with a curvilinear trend being demonstrated between increasing CHC dose and decreasing stress required to achieve cord rupture – 89% of the trend was directly attributable (following regression analysis) to the CHC dose administered. The stress to failure values were then compared with the average stress normally generated by the active extension of each of the fingers in a healthy hand. This allowed extrapolation that a CHC injection of 300 U was the minimum potential dose for in vivo clinical use that would result in DD cord rupture (and hence correction of the fixed flexion deformity) following normal active digital extension. One point to note was that the authors believed that it was important to inject CHC into DD cords (these being in the residual disease stage and relatively acellular when compared with the more active nodular areas of disease) as it was suggested this might minimize early recurrence or the risk of worsening the flexion contractures.
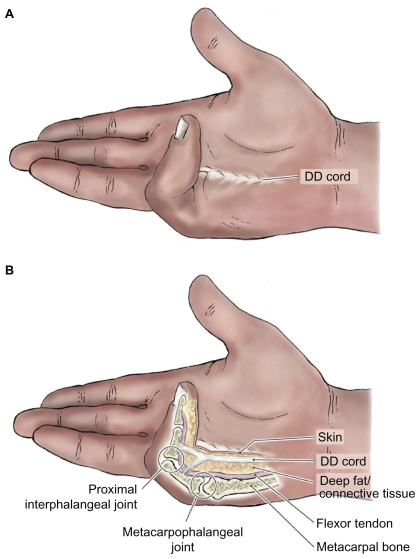
Investigation of the utility of CHC in the treatment of DD was next progressed by Badalamente and Hurst13 who assessed the in vivo efficacy and, importantly, safety of CHC injection within a 32-patient Phase II open-label trial. The study end point was correction of metacarpophalangeal joint (MCPJ) or PIPJ fixed flexion contractures to full extension (0°–5°) and assessment of any related adverse reactions (see Figure 3 for an illustration of the anatomical landmarks associated with CHC in vivo injections). The immunological effect of CHC injection was also assessed via serial serum immunoglobulin E (IgE) assays at days 1 and 14 postinjection. Follow-up was maintained between 2 and 5 years postprocedure to assess disease recurrence. Based on the prior in vitro determination of 300 U being a safe and effective CHC dose for cord rupture, the first patient was given an injection of 300 U – this failed to achieve cord rupture. The dose was then escalated, with trials of 600, 1,200, 4,800, and 9,600 U each in a single patient – again cord rupture was not achieved. The remaining 29 patients (ie, 34 MCPJ, 9 PIPJ, and 1 thumb joint contractures) had 10,000 U CHC injected into each contracture-contributing DD cord with passive digital extension 24 hours later to effect cord rupture. Following rupture, participants were asked to undertake patient-guided extension exercises and application of night extension splints for 4 months following the procedure to maintain maximal digital extension. Within 2 weeks of the procedures (some of which required repeat injections to achieve cord rupture), an 88% success rate was noted in the correction of MCPJ contractures and a 44% success rate in PIPJ contracture correction (to 0°–5° of extension). Two patients with MCPJ disease and 2 with PIPJ involvement failed to achieve cord rupture and digital extension so required surgical correction via fasciectomy. There were no major adverse reactions, but there were transient (1–2 week) minor reactions local to the injection site, including tenderness, palmar and/or dorsal edema, small local hematoma formation, forearm tenderness, and elbow/axilla lymphadenopathy. Three MCPJ contractures relapsed at 2-year postprocedure follow-up along with one PIPJ recurrence 3 months following treatment. Finally, IgE titers were seen to increase, especially in patients requiring repeat treatments; however, they were not associated with any adverse clinical effects and decreased with time postprocedure.
Figure 3.
Diagram illustrating: A) hand with palpable “bow-stringed” Dupuytren disease (DD) cord. B) anatomical landmarks in DD-affected hand.
This work was followed in 2002 by another Phase II trial undertaken by the same group of authors, Badalamente et al.14 The first trial leg was a single-center, randomized, placebo-controlled, double-blinded trial comparing the effect of injection of 10,000 U of CHC against placebo in patients with MCPJ or PIPJ DD-related contractures. 36 MCPJ and 13 PIPJ contracture patients were recruited and split equally between the treatment and placebo groups. Again, the primary end point of the study was correction of MCPJ or PIPJ fixed flexion contractures to full extension (0°–5°), the secondary end point was the percentage change from the preinjection baseline of range of motion and grip strength, and the final goal was identification of any related adverse reactions. In patients with multidigit involvement, the injections were repeated as required up to a maximum of 50,000 U. In the MCPJ group, there was a statistically significant difference between the treatment and placebo groups with 14 of 18 treated patients achieving the primary end point within 2 weeks, compared with two of 18 placebo patients. Within the 4-year follow-up period, four treated patients had disease recurrence. In the PIPJ group, five of seven treated patients achieved the primary end point within 4 weeks, compared with none of six placebo patients. Within the 3.8-year follow-up period, four treated patients had disease recurrence. Range of motion and grip strength showed no significant decline from baseline in either group. Adverse effects of CHC injection were the same as detailed in the authors’ previous paper, remaining locally confined and well tolerated, with the addition of three superficial skin tears secondary to rapid stretching of the overlying skin during cord rupture. The next study leg was a dual-center, randomized, placebo-controlled, double-blinded trial investigating the optimum dose of CHC required and involving 80 patients, evenly randomized to placebo, 2,500, 5,000, or 10,000 U doses. The end points were identical to the first trial leg. The success rate in restoration of full extension at 1-month postprocedure was significantly dose related, with MCPJ joints injected with 10,000 U showing a 90% success rate and PIPJ joints 70% success. Flexion and grip strength showed no significant deviation from the baseline and adverse effects were similar to those shown in the first trial leg. One MCPJ and one PIPJ had a disease recurrence within the follow-up period of 2 years. Finally, there were two substudies undertaken. The first investigated CHC pharmacokinetics – four participants given the 10,000 U CHC treatment had serial blood and urine samples taken over an 180-minute period and 24-hours postinjection for analysis of their collagenase content. There was no collagenase detected within the serum (likely due to its slow release into the bloodstream); however, levels were detectable in the urine primarily in the first hour postinjection, with up to 28% of the administered dose recovered from the urine. The second substudy examined five patients receiving 10,000 U but who had lidocaine injected prior to the passive digital manipulation to cause cord rupture 24 hours postinjection. This was well tolerated and demonstrated no effect on the study end points. Overall, the authors concluded CHC injection to be safe, effective, and less-invasive treatment option for the management of DD contractures.
Following these impressive results, the use of CHC in the treatment of keloid and hypertrophic scarring was piloted by Kang et al.16 They injected CHC, triamcinolone, or a combination into the keloid or hypertrophic scars of seven patients, and the response was measured by scar volume studies, visual subjective evaluation of response, serial photographs, and diarized patient experiences. A 33% reduction in proportional keloid scar volume was noted in the first 6 months with collagenase, but this effect disappeared thereafter. CHC produced no alteration in volume in the injected hypertrophic scars. CHC injection was associated with moderate local pain, severe blistering, skin ulceration, bruising, and swelling – one participant had severe enough symptoms to necessitate hospital admission for antipyretics and supportive measures. Overall, the authors concluded that intralesional injection of collagenase is ineffective and, coupled with the possibility of serious adverse reactions, has little scope as a clinical treatment for keloid or hypertrophic scars. However, it must be noted that the study sample size was not large enough, nor the scar group selected of a sufficient homogeneity, to draw any strong conclusions for or against the technique.
Returning to the previously discussed US group, the next article by Badalamente and Hurst9 continues on from their earlier studies with a Phase III randomized, placebo-controlled, double-blinded trial followed by an open-label extension. A total of 55 DD-contracted digits requiring treatment were recruited for injection with 10,000 U of a novel, mixed subtype CHC preparation. Within the main trial, patients had up to three joints injected, with a maximum of three injections into the primary joint followed by up to two in any remaining joints. A further three injections were allowed in the extension study if required. The primary end point was overall clinical success ie, reduction in deformity to ≤5° within 30 days of primary joint injection(s) – again, adverse reactions were recorded. Within the blinded phase, 91% of CHC-treated joints (86% of MCPJ and 100% of PIPJ joints) vs 0% of placebo group achieved clinical success (with up to three injections given and a mean number of injections to success of 1.4). 35 joints were entered into the open phase, where all were treated with CHC – 77% achieved clinical success. Over the 2-year followup period, one MCPJ and four PIPJ joints exhibited a disease recurrence – of these, the four male patients had a family history of DD, but the single female patient had de novo disease. Adverse events were of the same nature as detailed in the earlier Badalamente et al studies, with no summative systemic enzymatic effect or change in event severity demonstrated with repeated injections. There were no adverse immune events.
Xiaflex® was then utilized in a study by Del Carlo et al17 examining its in vitro effect when PD plaques and DD cords were injected with increasing concentrations of the CHC. Maximal enzymatic activity occurred within the first 4 hours, and near-complete digestion of the samples occurred following 12 hours of CHC exposure – small fibrils were preferentially degraded in the first 4 hours, following which the degradation of the larger fibrils proceeded. No cell death was observed at any concentration of CHC and all noncollagenous tissue structures remained histologically intact.
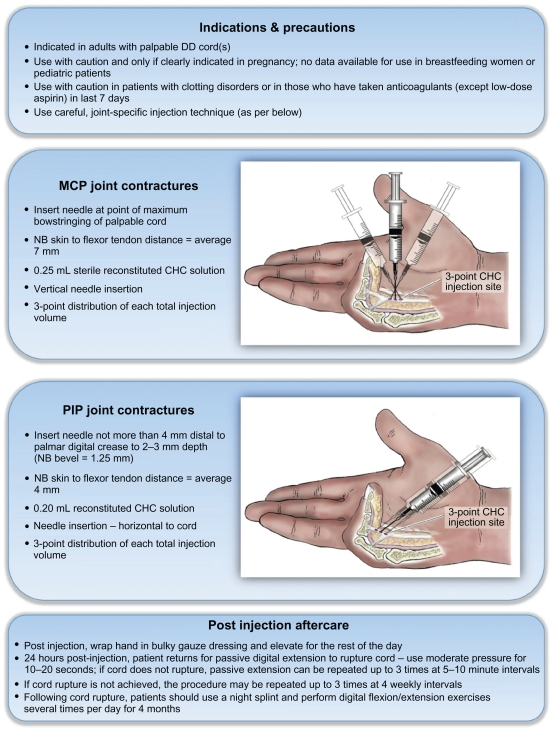
The two most recent CHC papers published originated from members of the same US-based group. The first, by Hurst et al5 (who make up the Collagenase Option for Reduction of Dupuytren’s [CORD] I study group) expands on the earlier Phase III study by Badalamente and Hurst. However, this 90-day Phase III prospective, randomized, placebo-controlled, double-blinded trial extended across 16 centers and had a substantially larger patient sample group (of 308 participants or 512 DD-affected joints) than previous studies. DD cords were injected with 0.58 mg of CHC (Xiaflex®) – a diagrammatic overview of the precise injection methodology utilized can be seen in Figure 4. As with previous studies, following injection the cords were ruptured 24 hours later via passive digital extension and the participants were asked to wear a night splint for 4 months. Three treatment cycles per DD cord were allowed. The primary end point remained extension to ≤5° within 30 days of the final injection alongside recording of all adverse reactions. The diseased joints were split into two baseline-characteristic matched groups, with 204 receiving CHC and 104 receiving placebo injections. The primary end point was achieved in 64% of the CHC group compared with 6.8% of joints in the placebo group. CHC-treated joints also showed a greater improvement in range of movement when compared with placebo-injected joints and all of the successfully treated joints showed no evidence of recurrence at the 90-day assessment. Of those CHC joints that did not achieve the primary end point, more than half did not receive the maximum allowable number (3) of CHC injections, either because the cord was no longer palpable (and thus could not safely be injected) or the patient was happy with the result despite lacking restoration of complete digital extension. However, preinjection baseline severity of contracture affected the likelihood of achieving the primary end point: 88.9% of CHC-injected MCPJ joints with a baseline contracture of 50° or less met the primary end point compared with 57.7% of those joints with a contracture greater than 50°; this effect was also seen in PIPJ joints (80.9% in the smaller than 40° group when compared with 22.4% in the greater than 40° group). This would suggest that CHC treatment may be more applicable for use in less severely affected joints, whereas perhaps those that are more severely contracted might derive greater benefit from traditional surgical correction. 96.6% of all CHC-treated joints developed one or more treatment-related adverse event as compared with 21.2% of those receiving the placebo injections; however, most were rated as mild to moderate with resolution without any medical intervention within a median of 10 days. Twenty-two patients (20 receiving CHC and two receiving placebo) reported adverse reactions, which they rated to be severe in nature, all of which were deemed to be treatment related except the single reports of contact dermatitis, muscular spasms, myocardial infarction (each in a CHC-treated patient), acute cholecystitis, nasopharyngitis, and radius fracture (each of which occurred in a placebo-group patient). The reported treatment-related events were primarily localized to the injection sites (edema [2%], pain [2%], hemorrhage [1.5%], contusion [1%], tenderness [1%], ecchymosis [0.5%], cellulitis [0.5%], and skin laceration [0.5%]) or to the injected side of the body (upper limb pain [2%] and chest-wall pain [0.5%]). Two participants deemed their reaction to be too severe to continue with the CHC treatment: one due to local pain secondary to the first CHC injection and the other due to dizziness 28 days post-CHC treatment which the patient attributed to the treatment. However, the authors deemed that three participants who received CHC injections suffered serious adverse events directly related to the treatment: two cases of tendon rupture (requiring surgical reparation) and a case of complex regional pain syndrome. There were no clinically observed systemic allergic reactions, alterations in grip strength, nerve injuries, or deaths associated with the treatment. With regard to biochemical or immunological response to the CHC, baseline blood results were compared with samples from 30 and 90 days posttreatment: ≥85.8% of those receiving a single injection developed anti-CHC antibodies, with 100% of patients testing positive following three injections. There were no clinically relevant blood result changes.
Figure 4.
Diagram illustrating indications, relative contraindications, injection technique with adjustments for metacarpophalangeal (MCP) vs proximal interphalangeal (PIP) joints and suggested aftercare following successful Dupuytren disease (DD) cord rupture (as per Hurst et al5).
Abbreviations: CHC, Clostridium histolyticum collagenase.
The final paper published to date, by Watt et al3 reviewed participants from an earlier study undertaken 8 years previously (published by Badalamente and Hurst and discussed previously). Twenty-three patients from a single study arm were contacted 8 years after their initial treatment. Of these 23, only eight were alive, contactable at the same address, available within the study period and received a CHC injection and thus were eligible for reevaluation to assess the state of their DD since the study. Notably, seven of the eight subjects had a family history of DD making them more likely to have aggressive disease and be at higher risk of relapse. All patients had a single CHC injection, six into MCPJ and two into PIPJ; however, varying injection dosages were administered, and not all patients achieved the primary goal of extension to ≤5%. Of the MCPJ joints, two had no evidence of DD recurrence and four had recurrent disease, although the extent of contracture seen at the 8-year assessment was still less than that previously noted for pre-CHC injection in all four patients (60%–20%; 70%–40%; 52%–20%; and 55%–55%, respectively). In the two PIPJ patients, there was a 100% recurrence rate with the degree of contracture (which initially demonstrated a marked improvement at the 1-year assessment) being more extensive than prior to the treatment (35% pretreatment, 0% at 1 year, and 50% at 8 years; and 55% pretreatment, 25% at 1 year, and 70% at 8 years, respectively). Patients subjectively ranked the treatment as being 60% successful, and seven out of eight stated they would consider having the treatment again.
Discussion
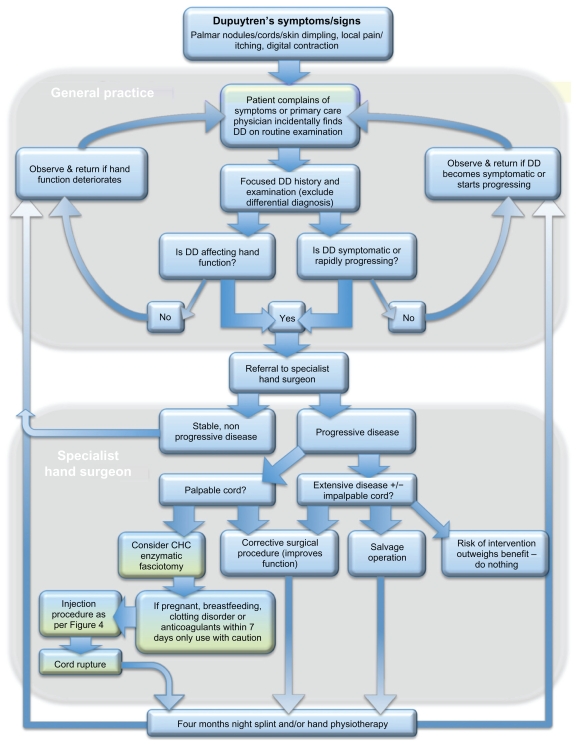
DD is a prevalent disease that causes considerable impairment to patients’ hand function. It has a complex, multifactorial etiology and pathophysiology, a complete understanding of which remains elusive. Despite explorative studies of a broad variety of alternative nonsurgical therapies, few of these have yet shown sufficient promise for them to be considered a valid alternative to the gold standard surgical methods that currently remain the mainstay of DD management. However, the developments discussed earlier in the use of CHC injection therapy indicate that this is likely to become a significant clinically applicable alternative method for the management of DD. Surgery is, however, likely to remain a valid treatment option, especially for recurrent cases following CHC injection, in patients with the aggressive form of the disease (strong diathesis), and in those patients with PIPJ involvement (which appears to not respond as favorably to the CHC-based technique when compared with simple MCPJ disease). Figure 5 presents a treatment algorithm, illustrating where CHC injection is likely to contribute for the management of DD.
Figure 5.
Flowchart for managing patients with Dupuytren disease (DD) from initial presentation to treatment, demonstrating the role of Clostridium histolyticum collagenase (CHC) injection within the treatment algorithm of DD.
The CHC studies undertaken to date have clearly demonstrated that injection of CHC is a valid alternative to the traditional surgical options, despite neither treatment facilitating a definitive cure. CHC demonstrates an impressive degree of digital extension with low rates of disease recurrence – certainly the benefits it offers, such as reduced invasiveness and procedure time, rapidity of recovery of premorbid hand function and the likely reduction in cost of administration (being an office-based method rather than surgical theater-based technique) could be instrumental in its likely increase in popularity both with patients and surgeons over the coming years.
A precise comparison of procedure risk vs derived health benefit between the gold standard selective fasciectomy and CHC injection is difficult. Surgery, with its reported surgeon-dependent and technique-dependent complication rate of 17%–50%,24 still carries a significant morbidity. Some of the local complications are similar to those seen in CHC injection (local pain, tenderness, ecchymosis, edema, risk of infection, and hematoma are expected sequelae), but surgery is also associated, especially those more complex revision operations in which there is often considerable local scarring, with risk of flexor tendon or pulley injury, digital nerve damage, and reflex sympathetic dystrophy.87 CHC injection has also been noted to be associated with tendon rupture and complex regional pain syndrome (two of 204 and one of 204 patients, respectively, in Hurst et al’s most recent study5), but the authors suggested that the risk of these serious complications might be minimized by careful adherence to the proposed injection technique, especially when injecting distal PIPJ contractures (see Figure 4). In view of this requirement for meticulous technique, it is clear that CHC injection will remain, based on the need for sound anatomical knowledge and manual dexterity, within the surgeons’ remit.
As with any novel therapy, the primary concerns that must be addressed prior to its use in the clinical environment are its efficacy – this has been illustrated in the previously mentioned studies – and whether the safety risks it imposes are outweighed by the health benefit it provides. As CHCs act via the degradation of collagen and 25%–33% of the total protein within humans is comprised of collagen,66 it is clear that it is essential to ensure CHCs act only on the diseased DD collagen against which they are therapeutically targeted.
The toxicity of intravenously injected CHC in mice was explored by Garvin Jr86 who showed a median lethal dose of 1,176 ± 208 U/kg – the main signs of toxicity were pulmonary hemorrhage and disseminated tissue digestion. The authors extrapolated this to an average 70 kg human, suggesting a lethal dose of 89,040 U (although this is with direct intravenous administration which is likely to be substantially more systemically damaging when compared with contained local tissue injection). However, the manufacturers of Xiaflex® reported much higher estimated safety margins for the inadvertent intravenous administration of the drug, suggesting a minimum lethal dose of more than 43 times the recommended dosage. Rydevik et al88–91 undertook a number of studies into the effect of CHC on neural, vascular, epidural, and intrathecal tissues in animal models (rabbit ears and hamster cheek pouches). They demonstrated a dose-related short-term and long-term response within the microvasculature of rabbit ears to local CHC injection. At low doses (120 U/mL), acutely, there was a slight reduction in blood flow and functional recruitment of capillaries with a localized hyperemic area persisting at 7 days postinjection and evidence of small vessel proliferation but not of revascularization. At high doses (3000 U/mL), an immediate transient hyperemia was seen, with stagnant flow in venules and capillaries at 60 minutes postinjection and evidence of microbleeding increasing over time. This progressed by day 3 to evidence of marked local hemorrhage extending beyond the injection site with a central area of necrosis observable – microangiograms displayed a central vessel-free zone with peripheral proliferation of wide, tortuous vessels typical of those seen in granulation tissue. In another study, they showed that CHC injected epidurally resulted in local dural thinning at higher concentrations, whereas even small concentrations of CHC injected intrathecally resulted in intrathecal hemorrhage and acute paraplegia. It might be assumed that such CHC-related neurological damage infers that a similar effect that might occur in digital nerves exposed to CHC, resulting in transient neuropraxia or persistent nerve damage. However, none of the clinical studies described in this review have noted any such clinical deficits. Perhaps this is due to the limited follow-up length and relatively small numbers treated to date or that, rather than directly damaging the neuronal fibers, the intrathecally injected CHC created a similar effect on the microvasculature as in the rabbit ear study, inducing a cord ischemia secondary to the local hyperemia, hemorrhage, and resultant edema. Interestingly, at a cellular level, Del Carlo et al17 noted no evidence of cell death or damage to nerve fibers at any of the CHC concentrations tested. Despite this lack of cellular sequelae, the manufacturers of the CHC Xiaflex® advise that CHC injection has not been tested in pregnant, breast-feeding, or pediatric populations in whom its safety and efficacy have not yet been established.
The ability to localize the effect of injected CHC has been demonstrated by Badalamente and Hurst92 who injected CHC in vivo into anesthetized rat tail tendons and demonstrated that no adverse extravasation of CHCs (at doses between 150–300 U) occurred into adjacent muscle, nerve, other tendons, vessels, bone, or skin. Gelbard et al11 in their study investigating the effect of CHC in vivo, also used an anesthetized rat. CHC was injected into the left femoral adventitial tissues surrounding the femoral neurovascular bundle: histological examination revealed that the collagenolytic effect was limited purely to the injection site – only small local venules were degraded with sparing of the nerves and smooth muscle–containing arterioles. Indeed, Mandl93 described the use of CHC to selectively remove connective tissue from the surrounding of nerves before histological studies of the intact nerves.
As CHC is a foreign protein, its injection would be presumed to evoke an immunological response. Indeed, Hamilton et al74 demonstrated such an immune response to injection of CHC for the treatment of PD plaques: pretreatment, anticollagenase IgG was detected in 34% of the healthy controls vs 58% of untreated PD patients. Following intralesional CHC injection of 3,000–12,650 U, an increase of twofold to 10-fold in IgG levels was seen at 1–2 months postinjection in 88% of patients. Only 0.5% showed detectable collagenase-specific IgE antibody. Braun94 described a contact dermatitis in two patients in whom a topical ointment composed of CHC and chloramphenicol (Iruxol™; Smith & Nephew) was applied – although a positive local response was observed, no specific allergen was detected. Finally, Hurst et al5 demonstrated that ≥85.8% of those receiving a single in vivo CHC injection into DD-affected cords developed anti-CHC antibodies, with 100% of patients testing positive following three injections. However, they showed no clinical evidence of systemic allergic response.
With respect to the systemic biochemical and hematological response to local tissue injection of CHCs, Sussmann and Mann69,70,95,96 demonstrated no adverse changes in red blood cell, white blood cell, or platelet counts in rats receiving >100 times the effective dose required for intervertebral disc lysis. Hurst et al5 reported no clinically meaningful changes in hematological or biochemical variables following CHC injection in DD cords. Badalamente et al14 also reviewed the pharmacokinetics of locally injected CHC, showing that despite no evidence of CHC circulating within serum, an estimated 28% of the dose injected was concentrated and excreted in the urine with no evidence of damage to the renal function. They suggested the absence of CHC within serum to be due to the CHC not being released into the circulation as a bolus but rather via incremental leaching from the injection site – thus, the tiny volumes slowly released over the sampling period were below the 4 ng/mL test threshold. However, the manufacturers of Xiaflex® later repeated the pharmacokinetic studies and detected no systemic levels of CHC following a single-dose Xiaflex® injection. This lack of quantifiable systemic exposure negated the need for tissue distribution, metabolism, or excretion studies.99
Overall, it can be concluded that the efficacy, safety, and complication profiles of CHCs appear to compare favorably with the current surgical techniques in adult patients with a palpable DD cord. However, this is still a method relatively within its infancy and thus there is no evidence relating to the possible long-term sequelae of CHC injections, nor any indication as to its safety in pregnancy, in breast-feeding women or in pediatric patients – further studies will be required to elucidate any long-term issues with the technique.
Conclusion
DD is a prevalent disease that has a marked effect on patients’ hand function and for which there is still no curative treatment. CHC is a novel nonsurgical treatment option of considerable potential in the management of DD when administered by specialist hand surgeons with detailed knowledge of the disease and its relevant anatomy. CHC injection has been shown to have a good efficacy and safety profile when compared with the current gold standard of surgery and appears likely to have a role in the therapeutic arsenal for the management of DD.
Nonetheless, there is a need for further data on long-term results, complications, and rate of recurrence with the use of this emerging treatment option.
Acknowledgments
The authors would like to thank Helen Carruthers for professional illustration of Figures 3 and 4. The senior author is a recipient of NIHR (UK) funding.
Footnotes
Disclosure
No authors have conflicting financial interests and no funds have been obtained for the completion of this work.
References
- 1.Bayat A, McGrouther DA. Management of Dupuytren’s disease – clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88(1):3–8. doi: 10.1308/003588406X83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayat A, Cunliffe EJ, McGrouther DA. Assessment of clinical severity in Dupuytren’s disease. Br J Hosp Med. 2007;68(11):604–609. doi: 10.12968/hmed.2007.68.11.27683. [DOI] [PubMed] [Google Scholar]
- 3.Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am. 2010;35(4):534–539. doi: 10.1016/j.jhsa.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Barbir A, Michalek AJ, Abbott RD, Iatridis JC. Effects of enzymatic digestion on compressive properties of rat intervertebral discs. J Biomech. 2010;43(6):1067–1073. doi: 10.1016/j.jbiomech.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 6.Badalamente MA, Wang E. Methods for treatment using collagenase. 20090010918. US patent. 2009 Jan 8;
- 7.Sabatino GL, Del Tito JRBJ, Bassett PJ, Tharia HA, Hitchcock AG. Compositions and methods for treating collagen-mediated diseases. 20070224183. US patent. 2007 Jan 29;
- 8.Badalemente MA, Dagum AB. Collagenase for treating cellulite. World intellectual property organisation patent. 2007100675 (A2) WO. 2007 Sep 7;
- 9.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32(6):767–774. doi: 10.1016/j.jhsa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Badalamente MA. Methods for treating cellulite. World intellectual property organisation patent. 2007100675 (A2) WO. 2007 Sep 7;
- 11.Gelbard MK, Walsh R, Kaufman JJ. Collagenase for Peyronie’s disease experimental studies. Urol Res. 1982;10(3):135–140. doi: 10.1007/BF00255956. [DOI] [PubMed] [Google Scholar]
- 12.Starkweather KD, Lattuga S, Hurst LC, et al. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg Am. 1996;21(3):490–495. doi: 10.1016/S0363-5023(96)80368-6. [DOI] [PubMed] [Google Scholar]
- 13.Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629–636. doi: 10.1053/jhsu.2000.6918. [DOI] [PubMed] [Google Scholar]
- 14.Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788–798. doi: 10.1053/jhsu.2002.35299. [DOI] [PubMed] [Google Scholar]
- 15.Badalamente MA, Stern L, Hurst LC. The pathogenesis of Dupuytren’s contracture: contractile mechanisms of the myofibroblasts. J Hand Surg Am. 1983;8(3):235–243. doi: 10.1016/s0363-5023(83)80150-6. [DOI] [PubMed] [Google Scholar]
- 16.Kang N, Sivakumar B, Sanders R, Nduka C, Gault D. Intra-lesional injections of collagenase are ineffective in the treatment of keloid and hypertrophic scars. J Plast Reconstr Aesthet Surg. 2006;59(7):693–699. doi: 10.1016/j.bjps.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Del Carlo M, Cole AA, Hart SGE, Levine LA. Comparative analysis of collagen degradation in Peyronie’s disease plaque and Dupuytren’s contracture cord tissues injected with mixed collagenase subtypes. J Urol. 2009;181(4 Suppl 1):S279–279. [Google Scholar]
- 18.Hurst LC, Badalamente M, Smith T. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. The authors’ reply. N Engl J Med. 2009;361(26):2578–2580. doi: 10.1056/NEJMc0909497. [DOI] [PubMed] [Google Scholar]
- 19.Rayan GM. Dupuytren’s disease: anatomy, pathology, presentation, and treatment. Instr Course Lect. 2007;56:101–111. [PubMed] [Google Scholar]
- 20.Sinha A. Dupuytren’s disease may extend beyond the wrist crease in continuity. J Bone Joint Surg Br. 1997;79(2):211–212. doi: 10.1302/0301-620x.79b2.6670. [DOI] [PubMed] [Google Scholar]
- 21.Lee TH, Wapner KL, Hecht PJ. Plantar fibromatosis. J Bone Joint Surg A. 1993;75(7):1080–1084. doi: 10.2106/00004623-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Sasso F, Gulino G, Falabella R, et al. Peyronie’s disease: lights and shadows. Urol Int. 2007;78(1):1–9. doi: 10.1159/000096927. [DOI] [PubMed] [Google Scholar]
- 23.Thurston AJ. Dupuytren’s disease. J Bone Joint Surg B. 2003;85(4):469–477. doi: 10.1302/0301-620x.85b4.14215. [DOI] [PubMed] [Google Scholar]
- 24.Caufield RJ, Edwards SG. Dupuytren disease: an update on recent literature. Curr Orthop Pract. 2008;19(5):499–502. [Google Scholar]
- 25.Elliot D. The early history of contracture of the palmar fascia. Part 1: the origin of the disease: the curse of the MacCrimmons: the hand of benediction: Cline’s contracture. J Hand Surg Br. 1988;13(3):246–253. doi: 10.1016/0266-7681_88_90078-2. [DOI] [PubMed] [Google Scholar]
- 26.Dupuytren G. Permanent retraction of the fingers, produced by an affection of the palmar fascia. Lancet. 1834;2:222–225. [Google Scholar]
- 27.Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (NY) 2009;4(3):256–269. doi: 10.1007/s11552-008-9160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degreef I, Steeno P, de Smet L. A survey of clinical manifestations and risk factors in women with Dupuytren’s disease. Acta Orthop Belg. 2008;74(4):456–460. [PubMed] [Google Scholar]
- 29.Stahl S, Calif E. Dupuytren’s palmar contracture in women. Isr Med Assoc J. 2008;10(6):445–447. [PubMed] [Google Scholar]
- 30.Urban M, Feldberg L, Janssen A, Elliot D. Dupuytren’s disease in children. J Hand Surg Br. 1996;21(1):112–116. doi: 10.1016/s0266-7681(96)80024-6. [DOI] [PubMed] [Google Scholar]
- 31.Burge P. Genetics of Dupuytren’s disease. Hand Clin. 1999;15(1):63–71. [PubMed] [Google Scholar]
- 32.Murrell GA, Hueston JT. Aetiology of Dupuytren’s contracture. Aust N Z J Surg. 1990;60(4):247–252. doi: 10.1111/j.1445-2197.1990.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 33.Hindocha S, John S, Stanley JK, Watson SJ, Bayat A. The heritability of Dupuytren’s disease: familial aggregation and its clinical significance. J Hand Surg Am. 2006;31(2):204–210. doi: 10.1016/j.jhsa.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Bayat A, Watson JS, Stanley JK, et al. Genetic susceptibility in Dupuytren’s disease. J Bone Joint Surg B. 2002;84(2):211–215. doi: 10.1302/0301-620x.84b2.12083. [DOI] [PubMed] [Google Scholar]
- 35.Attali P, Ink O, Pelletier G, et al. Dupuytren’s contracture, alcohol consumption, and chronic liver disease. Arch Intern Med. 1987;147(6):1065–1067. [PubMed] [Google Scholar]
- 36.An HS, Southworth SR, Jackson WT, Russ B. Cigarette smoking and Dupuytren’s contracture of the hand. J Hand Surg Am. 1988;13(6):872–874. doi: 10.1016/0363-5023(88)90262-6. [DOI] [PubMed] [Google Scholar]
- 37.Gudmundsson KG, Arngrimsson R, Sigfusson N, Bjornsson A, Jonsson T. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study . J Clin Epidemiol. 2000;53(3):291–296. doi: 10.1016/s0895-4356(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 38.Arkkila PET, Kantola IM, Viikari JSA. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24(1):153–159. [PubMed] [Google Scholar]
- 39.Strzelczyk A, Vogt H, Hamer HM, Kramer G. Continuous phenobarbital treatment leads to recurrent plantar fibromatosis. Epilepsia. 2008;49(11):1965–1968. doi: 10.1111/j.1528-1167.2008.01684.x. [DOI] [PubMed] [Google Scholar]
- 40.Hart MG, Hooper G. Clinical associations of Dupuytren’s disease. Postgrad Med J. 2005;81(957):425–428. doi: 10.1136/pgmj.2004.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson PL, Morris MA, Stanley JK, Fahmy NR. Lipids and Dupuytren’s disease. J Bone Joint Surg Br. 1992;74(6):923–927. doi: 10.1302/0301-620X.74B6.1447259. [DOI] [PubMed] [Google Scholar]
- 42.Livingstone JA, Field J. Algodystrophy and its association with Dupuytren’s disease. J Hand Surg Br. 1999;24(2):199–202. doi: 10.1054/jhsb.1998.0011. [DOI] [PubMed] [Google Scholar]
- 43.Bovenzi M. Hand-arm vibration syndrome and dose-response relation for vibration induced white finger among quarry drillers and stonecarvers. Occup Environ Med. 1994;51(9):603–611. doi: 10.1136/oem.51.9.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling RSM, Edinburgh S. The genetic factor in Dupuytren’s disease. J Bone Joint Surg Br. 1963;45(4):709–718. [PubMed] [Google Scholar]
- 45.Al-Qattan MM. Factors in the pathogenesis of Dupuytren’s contracture. J Hand Surg Am. 2006;31(9):1527–1534. doi: 10.1016/j.jhsa.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren’s contracture unfolded. Br Med J. 2006;332(7538):397–400. doi: 10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engstrand C, Boren L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren’s contracture three months after fasciectomy and hand therapy interventions. J Hand Ther. 2009;22(1):21–27. doi: 10.1016/j.jht.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Degreef I, Vererfve PB, de Smet L. Effect of severity of Dupuytren contracture on disability. Scand J Plast Reconstr Surg Hand Surg. 2009;43(1):41–42. doi: 10.1080/02844310802410125. [DOI] [PubMed] [Google Scholar]
- 49.Hindocha S, Stanley JK, Watson S, Bayat A. Dupuytren’s diathesis revisited: evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg. 2006;31(10):1626–1634. doi: 10.1016/j.jhsa.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Cheng HS, Hung LK, Tse WL, Ho PC. Needle aponeurotomy for Dupuytren’s contracture. J Orthop Surg. 2008;16(1):88–90. doi: 10.1177/230949900801600120. [DOI] [PubMed] [Google Scholar]
- 51.Foucher G, Medina J, Navarro R. Percutaneous needle aponeurotomy: complications and results. J Hand Surg Br. 2003;28(5):427–431. doi: 10.1016/s0266-7681(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 52.van Rijssen AL, Werker PMN. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Br. 2006;31(5):498–501. doi: 10.1016/j.jhsb.2006.03.174. [DOI] [PubMed] [Google Scholar]
- 53.Rayan GM. Nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2008;33(7):1208–1210. doi: 10.1016/j.jhsa.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25(6):1157–1162. doi: 10.1053/jhsu.2000.18493. [DOI] [PubMed] [Google Scholar]
- 55.Al-Qattan MM, Ketchum LD. The injection of nodules of Dupuytren’s disease with triamcinalone acetonide. J Hand Surg Am. 2001;26(3):560–561. doi: 10.1053/jhsu.2001.24150. [DOI] [PubMed] [Google Scholar]
- 56.Vuopala U, Kaipainen WJ. DMOS in the treatment of Dupuytren’s contracture. A therapeutic experiment. Acta Rheumatol Scand. 1971;17(1):61–62. doi: 10.3109/rhe1.1971.17.issue-1-4.09. [DOI] [PubMed] [Google Scholar]
- 57.Kirk JE, Chieffi M. Tocopherol administration to patients with Dupuytren’s contracture; effect on plasma tocopherol levels and degree of contracture. Proc Soc Exp Biol Med. 1952;80(4):565–568. doi: 10.3181/00379727-80-19692. [DOI] [PubMed] [Google Scholar]
- 58.Keilholz L, Seegenschmiedt MH, Sauer R. Radiotherapy for prevention of disease progression in early-stage Dupuytren’s contracture: initial and long-term results. Int J Radiat Oncol Biol Phys. 1996;36(4):891–897. doi: 10.1016/s0360-3016(96)00421-x. [DOI] [PubMed] [Google Scholar]
- 59.Seegenschmiedt MH, Olschewski T, Guntrum F. Radiotherapy optimization in early-stage Dupuytren’s contracture: first results of a randomized clinical study. Int J Radiat Oncol Biol Phys. 2001;49(3):785–798. doi: 10.1016/s0360-3016(00)00745-8. [DOI] [PubMed] [Google Scholar]
- 60.Betz N, Ott OJ, Adamietz B, Sauer R, Fietkau R, Keilholz L. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186(2):82–90. doi: 10.1007/s00066-010-2063-z. [DOI] [PubMed] [Google Scholar]
- 61.Stiles PJ. Ultrasonic therapy in Dupuytren’s contracture. J Bone Joint Surg B. 1966;48(3):452–454. [PubMed] [Google Scholar]
- 62.Duperrat B, Colliard H. [Hypertrophic aponeurosis, peculiar variation of Dupuytren’s disease? Excellent therapeutic result with a cytostatic agent (methylhydrazine)]. Une aponévrosite hypertrophique, variante particulière de la maladie de Dupuytren? Excellent résultat thérapeutique d’un agent cytostatique (méthyl-hydrazine) Bull Soc Fr Dermatol Syphiligr. 1969;76(1):14–15. [PubMed] [Google Scholar]
- 63.Murrell GAC, Francis MJO, Bromley L. Free radicals and Dupuytren’s contracture. Br Med J. 1987;295(6610):1373–1375. doi: 10.1136/bmj.295.6610.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hueston JT. Enzymic fasciotomy. Hand. 1971;3(1):38–40. doi: 10.1016/0072-968x(71)90010-6. [DOI] [PubMed] [Google Scholar]
- 65.Maclennan JD, Mandl I, Howes EL. Bacterial digestion of collagen. J Clin Invest. 1953;32(12):1317–1322. doi: 10.1172/JCI102860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrington DJ. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun. 1996;64(6):1885–1891. doi: 10.1128/iai.64.6.1885-1891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung L, Dinakarpandian D, Yoshida N, et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520–526. doi: 10.1007/s00253-003-1442-0. [DOI] [PubMed] [Google Scholar]
- 69.Sussman BJ, Mann M. Experimental intervertebral discolysis with collagenase. J Neurosurg. 1969;31(6):628–635. doi: 10.3171/jns.1969.31.6.0628. [DOI] [PubMed] [Google Scholar]
- 70.Sussman BJ. Experimental intervertebral discolysis. A critique of collagenase and chymopapain applications. Clin Orthop Relat Res. 1971;80:181–190. [PubMed] [Google Scholar]
- 71.Bromley JW, Varma AO, Santoro AJ. Double-blind evaluation of collagenase injections for herniated lumbar discs. Spine. 1984;9(5):486–488. doi: 10.1097/00007632-198407000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Fecteau KA, Haffner JC, Eiler H. The potential of collagenase as a new therapy for separation of human retained placenta: hydrolytic potency on human, equine and bovine placentae. Placenta. 1998;19:5–6. 379–383. doi: 10.1016/s0143-4004(98)90077-7. [DOI] [PubMed] [Google Scholar]
- 73.Badalamente M. Methods for treating adhesive capsulitis. World intellectual property organisation patent. 2006078870 (A2) WO. 2006 Jul 27;
- 74.Hamilton RG, Mintz GR, Gelbard MK. Humoral immune responses in Peyronie’s disease patients receiving clostridial collagenase therapy. J Urol. 1986;135(3):641–647. doi: 10.1016/s0022-5347(17)45768-5. [DOI] [PubMed] [Google Scholar]
- 75.Yavuzer R, Latifoglu O, Ayhan S, Edali N, Celik B, Atabay K. Enhanced wound healing using collagenase in guinea pig. Gazi Med J. 1997;8(3):110–113. [Google Scholar]
- 76.Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26(3):207–222. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 77.Vaccaro S, Gennari G, Callegaro L, Giannelli A, Caruso S. New pharmaceutical compositions containing hyaluronic acid and collagenase for the topical treatment of wounds, burns and ulcers. World intellectual property organisation patent. 2007006484 (A1) WO. 2007 Jan 18;
- 78.Johnson PRV, White SA, London NJM. Collagenase and human islet isolation. Cell Transplant. 1996;5(4):437–452. doi: 10.1177/096368979600500403. [DOI] [PubMed] [Google Scholar]
- 79.Strauss BH, Segev A. Use of collagenase to facilitate guide wire crossing in total arterial occlusions. World intellectual property organisation patent. 2005097177 (A1) WO. 2005 Oct 20;
- 80.Mookhtiar KA, van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116–126. [PubMed] [Google Scholar]
- 81.Mallya SK, Mookhtiar KA, van Wart HE. Kinetics of hydrolysis of type I, II, and III collagens by the class I and II Clostridium histolyticum collagenases. J Protein Chem. 1992;11(1):99–107. doi: 10.1007/BF01025096. [DOI] [PubMed] [Google Scholar]
- 82.French MF, Mookhtiar KA, van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by Class I and Class II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681–687. doi: 10.1021/bi00377a004. [DOI] [PubMed] [Google Scholar]
- 83.Matsushita O, Koide T, Kobayashi R, Nagata K, Okabe A, Itano T. Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res. 2001;42(4):281–290. doi: 10.3109/03008200109016842. [DOI] [PubMed] [Google Scholar]
- 84.Toyoshima T, Matsushita O, Minami J, Nishi N, Okabe A, Itano T. Collagen-binding domain of a Clostridium histolyticum collagenase exhibits a broad substrate spectrum both in vitro and in vivo. Connect Tissue Res. 201;42(4):281–290. doi: 10.3109/03008200109016842. [DOI] [PubMed] [Google Scholar]
- 85.Barrantes E, Guinea M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003;72(7):843–850. doi: 10.1016/s0024-3205(02)02308-1. [DOI] [PubMed] [Google Scholar]
- 86.Garvin PJ., Jr Toxicity of collagenase: the relation to enzyme therapy of disk herniation. Clin Orthop Relat Res. 1974;(101):286–291. [PubMed] [Google Scholar]
- 87.Bulstrode NW, Jemec B, Smith PJ. The complications of Dupuytren’s contracture surgery. J Hand Surg Am. 2005;30(5):1021–1025. doi: 10.1016/j.jhsa.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Olmarker K, Rydevik B, Dahlin LB. Effects of epidural and intrathecal application of collagenase in the lumbar spine: an experimental study in rabbits. Spine. 1987;12(5):477–482. doi: 10.1097/00007632-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 89.Rydevik B, Brown MD, Ehira T, Nordborg C. Effects of collagenase on nerve tissue: an experimental study on acute and long-term effects in rabbits. Spine. 1985;10(6):562–566. doi: 10.1097/00007632-198507000-00010. [DOI] [PubMed] [Google Scholar]
- 90.Rydevik B, Ehira T, Linder L, Olmarker K, Romanus M, Branemark PI. Microvascular response to locally injected collagenase. An experimental investigation in hamsters and rabbits. Scand J Plast Reconstr Surg Hand Surg. 1989;23(1):17–21. doi: 10.3109/02844318909067503. [DOI] [PubMed] [Google Scholar]
- 91.Rydevik M, Bergström F, Mitts C, Danielsen N. Locally-applied collagenase and regeneration of transsected and repaired rat sciatic nerves. Scand JPlast Reconstr Surg Hand Surg. 2002;36(4):193–196. doi: 10.1080/02844310260259833. [DOI] [PubMed] [Google Scholar]
- 92.Badalamente MA, Hurst LC. Enzyme injection as a nonoperative treatment for Dupuytren’s disease. Drug Delivery: Journal of Delivery and Targeting of Therapeutic Agents. 1996;3(1):35–40. [Google Scholar]
- 93.Mandl I. Collagenase. Science. 1970;169(951):1234–1238. doi: 10.1126/science.169.3951.1234. [DOI] [PubMed] [Google Scholar]
- 94.Braun WPH. Contact allergy to collagenase mixture (Iruxol) Contact Derm. 1975;1(4):241–242. doi: 10.1111/j.1600-0536.1975.tb05392.x. [DOI] [PubMed] [Google Scholar]
- 95.Sussman BJ. Intervertebral discolysis with collagenase. J Natl Med Assoc. 1968;60(3):184–187. [PMC free article] [PubMed] [Google Scholar]
- 96.Sussman BJ, Bromley JW, Gomez JC. Injection of collagenase in the treatment of herniated lumbar disk. Initial clinical report. JAMA. 1981;245(7):730–732. [PubMed] [Google Scholar]
- 97.Bassot J. Traitement de la maladie de Dupuytren par “exerese” pharmaco-dynamique isolee ou completee par un temps plastique uniquement cutane. 1965;20:38–44. [PubMed] [Google Scholar]
- 98.McCarthy DM. The long-term results of enzymic fasciotomy. J Hand Surg Br. 1992;17(3):356. doi: 10.1016/0266-7681(92)90129-p. [DOI] [PubMed] [Google Scholar]
- 99.US Food and Drug Administration. Meeting of the Arthritis Drugs Advisory Committee; September 16, 2009; [Accessed 2010 Oct 3]. Briefing information. Available from http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/ucm182012.htm. [Google Scholar]