Abstract
Large-scale tissue engineering is limited by nutrient perfusion and mass transport limitations, especially oxygen diffusion, which restrict construct development to smaller than clinically relevant dimensions and limit the ability for in vivo integration. The goal of this work was to develop a modular approach to tissue engineering, where scaffold and tissue size, transport issues, and surgical implantation in vivo are considered from the outset. Human mesenchymal stem cells (hMSCs) were used as the model cell type, as their differentiation has been studied for several different cell lineages and often with conflicting results. Changes in the expression profiles of hMSCs differentiated under varied oxygen tensions are presented, demonstrating tissue-specific oxygen requirements for both adipogenic (20% O2) and chondrogenic (5% O2) differentiation. Oxygen and nutrient transport were enhanced by developing a bioreactor system for perfusing hMSC-seeded collagen gels using porous silk tubes, resulting in enhanced oxygen transport and cell viability within the gels. These systems are simple to use and scaled for versatility, to allow for the systematic study of relationships between cell content, oxygen, and cell function. The data may be combined with oxygen transport modeling to derive minimally sized modular units for construction of clinically relevant tissue-engineered constructs, a generic strategy that may be employed for vascularized target tissues.
Introduction
The field of tissue engineering is currently limited by an inability to adequately vascularize tissue constructs in vitro. This challenge exists across all cell and tissue types, with the specific metabolic demands of the system's cellular components dictating the overall size of the scaffold. The ability of these scaffolds to allow gas and nutrients to diffuse throughout the construct remains critical, as most cells in vivo exist within 100–200 μm of a capillary,1 and previous work in vitro has shown that insufficient nutrient and oxygen delivery throughout a scaffold leads to a substantial loss of cell viability and proliferation capacity in the core.2 Attempts by tissue engineers to improve mass transport throughout the scaffold have focused on the development of different bioreactor designs or microelectromechanical systems (MEMs) techniques to facilitate improved nutrient and oxygen transport within hydrogels or other materials.3–8 These techniques, however, often represent a “trial-and-error” approach toward perfusion, without fundamental insight into oxygen transport issues.9 Only recently have researchers focused on systems for quantitatively correlating cell viability with oxygen/nutrient concentrations in tissue constructs in vitro.2,10–13 Further, many of the current systems fail to consider the connection to host blood vessels in vivo, making oxygen and nutrient supply a diffusion-limited process and resulting in loss of cell viability at the core of the engineered tissue. By designing a prevascularized tissue with connections to host vasculature, the cells within the construct may be immediately supplied with the required nutrients from the moment of implantation. This incomplete understanding and consideration of the transport limitations associated with forming tissues of clinically relevant thickness has limited the field of tissue engineering, remaining as arguably its biggest challenge.
In this study, we present a system for culturing and differentiating human mesenchymal stem cells (hMSCs) seeded within perfused collagen gels for quantifying the effects of oxygen on cell viability and differentiation. We used our previously described bioreactor system, which was modified to accommodate an oxygen probe and made capable of being connected to host vasculature for in vivo implantation.14 Cell-seeded collagen gels were perfused using a small-diameter silk tube (∼500 μm inner diameter), producing an engineered surrogate tissue that was cultured at different oxygen tensions (5% and 20% O2) in vitro. Focus was placed on differentiation of the hMSCs into two lineages with different oxygen requirements in vivo, adipogenic (highly vascularized) and chondrogenic (avascular). Cellular differentiation was evaluated using quantitative real-time reverse transcriptase polymerase chain reaction (RT–PCR) in static well plates. Perfused and static gels in the bioreactor system were assessed for cell viability, and oxygen gradients within the gel were measured using an oxygen probe, allowing for quantitative evaluation of the oxygen tensions within the gel as a function of distance from the perfused silk tube. Further, these individual bioreactors may be combined together as individual modules and connected to host vasculature via the silk tubes, serving as the basis for forming engineered tissues both in vitro and in vivo with thicknesses beyond the current limitations.
Materials and Methods
Cell culture
hMSCs were harvested and cultured according to previously described protocols and all reagents were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO), unless otherwise noted.15 Briefly, hMSCs (Lonza, Walkersville, MD) were cultured in growth medium consisting of control medium (90% Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 0.1 mM nonessential amino acids, 100 U/mL penicillin, 1000 U/mL streptomycin, 0.2% fungizone antimycotic) with a supplement of 1 ng/mL basic fibroblast growth factor. Cells were cultured in an incubator either at 37°C with 5% CO2 and 95% air (20% O2) or at 37°C with 5% CO2, 90% N2, and 5% O2, both with 95% relative humidity. Cell culture medium was replenished every 2–3 days, and cells were passaged at ∼80% confluence using trypsin–EDTA (0.25% trypsin with 1 mM EDTA · 4Na). Adipogenic medium consisted of growth medium supplemented with 50 μM indometacin, 0.5 mM isobutyl xanthine, 1 μM dexamethasone, and 5 μg/mL insulin. Chondrogenic medium consisted of growth medium without 10% fetal bovine serum, supplemented with 6.25 μg/mL ITS+1 liquid media supplement (insulin, transferrin, selenium), 100 nM dexamethasone, and 10 ng/mL transforming growth factor-β1. Cells were frozen in cryogenic media consisting of growth medium described above, supplemented with 8% (v/v) dimethyl sulfoxide.
Preparation of collagen gels
Collagen gels seeded with hMSCs were prepared based on a previously developed method with minor changes based on company protocols.16 Briefly, hMSCs were harvested as described above, resuspended in growth media, and counted using a hemocytometer. Collagen gels were prepared on ice by mixing volumes of type I rat tail liquid collagen (∼9 mg/mL in 0.02 N acetic acid; BD Biosciences, Franklin Lakes, NJ), 10 × phosphate-buffered saline (PBS), 1 M NaOH, and sterile distilled water to bring the pH of the collagen to neutral and to attain final concentrations of 1× PBS, 0.011 M NaOH, and 4 mg/mL collagen. Neutralized collagen gels were then mixed with differentiation (adipogenic or chondrogenic) or control media with 1.33 × 106 hMSCs/mL media for a final collagen concentration of ∼2.5 mg/mL, and final cell concentration of 0.5 × 106 hMSCs/mL gel. This collagen–hMSC suspension was then aliquot in 400 or 800 μL volumes into each well of the bioreactor (for three-well or two-well configurations, respectively) or 500 μL into each well of a 24-well plate and maintained at 25°C for 15–30 min to allow for even gelation before being placed in the incubator. Within 1 h, medium was added to the static gels to help maintain their hydrated state by covering the top of the gel surface (∼100 μL of medium for gels in bioreactors and ∼300 μL of medium for each gel in the 24-well plates).
Total RNA extraction, cDNA synthesis, and real-time quantitative RT-PCR
Cells cultured in the collagen gels were harvested by aspirating media from the tops of the gels, washing with PBS, and adding collagenase (100 U/mL) to the tops of each gel. The gels were minced and incubated at 37°C for 2–3 h before lysing the cells in Buffer RLT (Qiagen, Valencia, CA) with 1% β-mercaptoethanol, and their RNA was extracted using an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. For reverse transcription, 5 μL of RNA sample with 45 μL of RNase-free water was used to synthesize cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. The real-time RT-PCR reactions were conducted using an ABI Prism 7000 Sequence Detection System (Applied Biosystems) or a Stratagene Mx3000P QPCR system (Stratagene, La Jolla, CA). TaqMan® Gene Expression Assay kits (Applied Biosystems) were used for transcript levels of adipogenic-related genes (PPARG, SLC2A4, FABP4, LPL) and chondrogenic-related genes (COL2A1, COL10A1, ACAN), with GAPDH used as a housekeeping gene (2ΔCt formula, Perkin Elmer User Bulletin No. 2).
Bioreactor design
To perfuse the hMSC-seeded collagen gels, our previously described bioreactor system was modified, allowing for multiple replicates and simple imaging to evaluate experimental outcomes.14 Briefly, the bioreactors comprised of a polydimethylsiloxane (Sylgard 184; Ellsworth Adhesives, Germantown, WI) body with two to three sample wells for perfusion and a cover glass bottom (Goldseal Cover Glass, No. 1, 24 × 60 mm; Ted Pella, Redding, CA) for imaging of the system. Dimensions of the bioreactor wells were 10 × 15 × 5 mm for the three-well configuration and 20 × 15 × 5 mm for the two-well configuration. The bioreactors were cultured in a custom housing system designed to control oxygen conditions and to maintain sterility. Tubing connections allowed perfusion (4 μL/min) of the silk tube embedded within the collagen gel using a programmable syringe pump (Remote PHD Push/Pull Programmable Pump; Harvard Apparatus, Holliston, MA).
For oxygen measurements, a custom housing for the bioreactor and oxygen measurement probe was designed in standard CAD program (Solidworks, Concord, MA), with resultant parts machined from Delrin (McMaster-Carr, Elmhurst, IL). In this design, the oxygen probe was mounted on a one-axis micromanipulator (Edmund Optics, Barrington, NJ), which allowed precise positioning of the probe, all inline with the silk tube in the collagen gel in the bioreactor.
Preparation of aqueous silk fibroin solutions
A 6%–8% (w/v) silk fibroin aqueous solution was obtained from Bombyx mori silkworm cocoons using previously described procedures.17,18 Briefly, the silkworm cocoons (supplied by Tajima Shoji Co., Ltd., Yokohama, Japan) were extracted in 0.02 M sodium carbonate solution for 30 min, rinsed in distilled water, dissolved in 9.3 M lithium bromide, and dialyzed against distilled water using a Slide-a-Lyzer dialysis cassette (molecular weight cutoff: 3500; Pierce, Rockford, IL) for 48 h. The resulting 6%–8% (w/v) fibroin solution was then concentrated by dialyzing 10 mL of this solution against 10 wt% poly(ethylene glycol) for ∼22 h, resulting in a 20%–30% (w/v) silk fibroin aqueous solution. All silk fibroin solutions were stored at 4°C until used to make silk tubes.
Preparation of silk tubes
Small-diameter silk tubes were prepared by dipping a stainless steel wire (0.026" diameter, Type 302/304; McMaster-Carr) into 20%–30% (w/v) silk fibroin according to previously described protocols.14 Briefly, stainless steel rods were alternately dipped in concentrated aqueous silk fibroin and methanol for three to four times or until evenly coated. Porous tubes were made by blending volumes of 6 wt% poly(ethylene oxide) (PEO) as described previously.19 In this work, we focused on a blending ratio of silk fibroin/PEO of 99/1 (wt%), where the silk fibroin/PEO blends were gently mixed at room temperature using a spatula before sonication for 10 min. They were then left to dry overnight before being cut at each end and placed in a surfactant solution to remove the silk tube from the steel wire. After drying, silk/PEO tubes were immersed in distilled water for 24 h at room temperature, facilitating the extraction of the PEO phase from the silk/PEO tube, leaving a porous silk tube.
Cell viability
To assess cell viability, cell-seeded collagen gels within the bioreactor were washed with PBS and incubated in 2 μM calcein AM and 4 μM ethidium homodimer-1 (Live/Dead assay; Invitrogen) in PBS for 45 min at room temperature. All cell viability images were acquired using a Leica DMIRE2 microscope with a TCS SP2 scanner (Leica Microsystems, Wetzlar, Germany), with image processing done using ImageJ software (National Institutes of Health).
Oxygen measurement
Oxygen tensions throughout the gel were measured using a needle-type oxygen microsensor with the capability of measuring the concentration of oxygen every 50 μm (PreSens, Regensburg, Germany). Before use, a two-point calibration was performed according to manufacturer protocols using an oxygen-free environment (sodium sulfite) and an air-saturated environment (water vapor). A custom-made oxygen probe housing system was used to position the oxygen probe within the cell-seeded collagen hydrogel with precise positioning controlled by a one-axis micromanipulator. For gradient measurement, the probe was inserted into the outer edge of each gel and imaged using a microscope for measurement of starting position. The bioreactor, probe, and housing were then returned to the incubator and the oxygen tension reading was allowed to equilibrate before recording the oxygen tension. The micromanipulator was then used to move the probe toward the silk tube (0.3175 mm/turn). After another equilibration time, measurements were recorded before advancing the probe again. This process was repeated until reaching the silk tube itself, after which the bioreactor was imaged again to determine final positioning of the probe. The start and final position of the probe, as well as the distance per turn of the micromanipulator, was used to determine the distance from the silk tube for each set of oxygen tension data.
Statistical analysis
All assays were repeated with a minimum sample size of n = 3. Experimental groups for the RT-PCR data were compared using a two-sample, two-tailed t-test for pairwise comparisons using Minitab 15 statistical software (State College, PA). Statistically significant values were defined as p < 0.05.
Results and Discussion
Effect of oxygen tension on hMSC differentiation
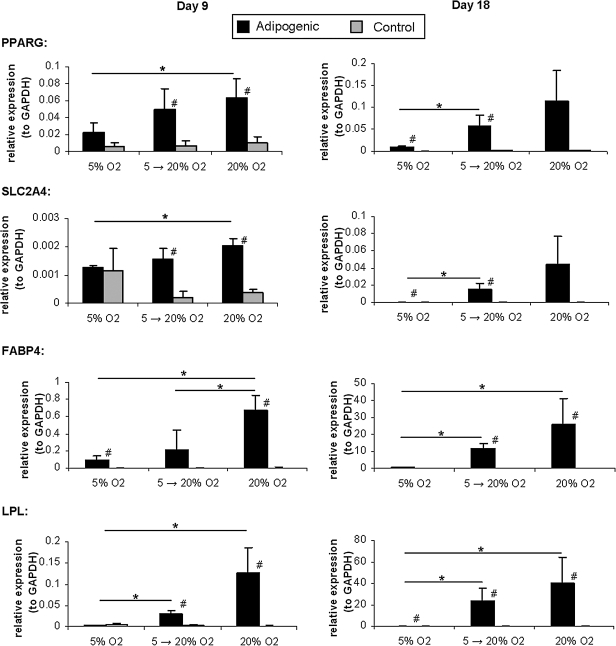
In static collagen gels, hMSCs were evaluated for adipogenic and chondrogenic differentiation using quantitative real-time RT-PCR. These cells were evaluated in the presence of both differentiation and control media and under three different oxygen profiles: two-dimensional (2D) culture and three-dimensional (3D) differentiation at 5% O2, 2D culture at 5% O2 and 3D differentiation at 20% O2 (5%–20% O2), and 2D culture and 3D differentiation at 20% O2. In the presence of adipogenic media, markers of adipogenic differentiation (PPARG, SLC2A4, FABP4, and LPL) were significantly upregulated when compared with controls and exhibited an increase in expression levels from low oxygen tension (5% O2) to higher oxygen tension (20% O2) (Fig. 1). This trend was observed at both days 9 and 18, with the significance of the trend, and relative levels of expression, increasing over time. The adipogenic expression at 5% O2 was nearly undetectable at day 18 for all genes assayed, suggesting that this is a poor oxygen tension for adipogenic differentiation when compared with 20% O2. Although there was a slight increase in the cultures grown at 5% O2 and differentiated within the hydrogels at 20% O2, the best results were achieved with the cultures grown and differentiated at high oxygen tension.
FIG. 1.
Adipogenic differentiation at different oxygen tensions. Quantitative real-time reverse transcriptase–polymerase chain reaction results for adipogenic genes for hMSCs cultured at different oxygen tensions in collagen hydrogels. Results are given for both days 9 and 18 of culture. *p < 0.05 between groups differentiated at different oxygen tensions; #p < 0.05 between differentiated and control groups at the same oxygen tension (two-sample t-test). hMSCs, human mesenchymal stem cells.
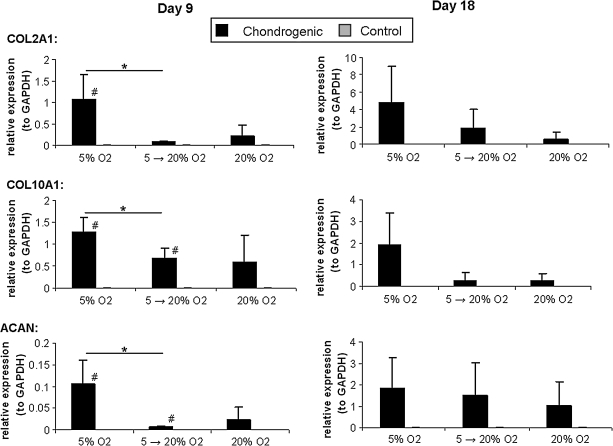
In the case of chondrogenic differentiation, an opposite trend with respect to oxygen was observed, as the expression of chondrogenic differentiation markers (COL2A1, COL10A1, ACAN) was highest at low oxygen tension (5% O2) and was reduced at the high oxygen tension conditions (5%–20% and 20% O2) (Fig. 2). Again this trend was observed over both time points, with increased expression over time. Compared with the results for adipogenic differentiation, however, the correlation between oxygen tension and gene expression was not as pronounced as low levels of expression are seen even at high oxygen tension. This may be a result of reduced oxygen transport throughout the collagen gels, generating discrete areas of low oxygen tension within the gel.
FIG. 2.
Chondrogenic differentiation at different oxygen tensions. Quantitative real-time reverse transcriptase–polymerase chain reaction results for chondrogenic genes for hMSCs cultured at different oxygen tensions in collagen hydrogels. Results are given for both days 9 and 18 of culture. *p < 0.05 between groups differentiated at different oxygen tensions; #p < 0.05 between differentiated and control groups at the same oxygen tension (two-sample t-test).
Cell viability in collagen hydrogels
On the basis of the results in the static well plates, a bioreactor system was developed to differentiate hMSCs seeded within collagen gels at 20% O2 for adipogenic differentiation or 5% O2 for chondrogenic differentiation (Fig. 3). Using this bioreactor system, the hMSC-seeded collagen gels were perfused using porous silk tubes and compared with static hMSC-seeded hydrogels. These gels were supplied with adipogenic, chondrogenic, or control media through the silk tube, which was embedded within the collagen gel. Over the 5–9 days of culture, the perfused gels displayed significant contraction, reducing in size by two- to threefold depending on the type of media and time of culture. Further, the extent of gel contraction varied slightly from sample to sample using the same media and culture conditions. This is observed in the varied dimensions of the adipogenic, chondrogenic, and control gels imaged and measured in Figures 4 and 5. This variability in the amount of contraction may cause local differences in diffusion rates of nutrients and oxygen as well as differences from sample to sample. For all cases, however, the contraction of perfused gels was more significant than that of the static gels, which displayed minimal contraction, indicating a reduction in cell activity and/or differences in nutrient diffusion and oxygen tension.
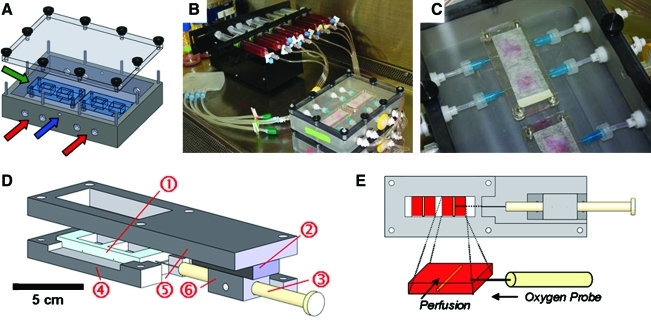
FIG. 3.
Schematic of model system. (A) Schematic of bioreactor culture system indicating bioreactors (green arrow) and ports with Luer fittings for tubing for perfusion (red arrows) and gas exchange (blue arrow). (B) Syringe pump and assembled bioreactor setup for perfusion. (C) Close-up view of bioreactors within culture system. (D) Three-dimensional view of bioreactor and oxygen probe measurement system. The polydimethylsiloxane bioreactor system (1) is housed within an oxygen probe housing setup, which uses a micromanipulator (2) to allow precise positioning of the oxygen probe (3). Custom-made Delrin pieces serve as a bioreactor holder (4), probe mount (5), and probe holder (6), respectively. (E) Bottom view of system schematic showing oxygen probe within well of bioreactor. Blow-up of well (2 × 1.5 cm) demonstrates the silk tube within the collagen gel, which is under either static or perfusion conditions, with the oxygen probe entering from the side and measuring the oxygen gradient as a function of distance from the tube. Color images available online at www.liebertonline.com/ten.
FIG. 4.
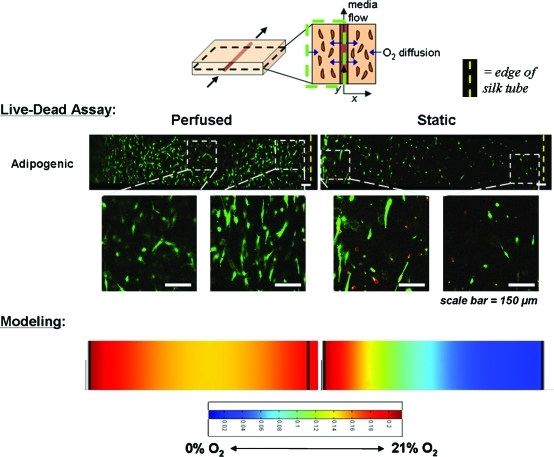
Cell viability as a function of distance from silk tube in the bioreactor system. Live/Dead images of hMSCs seeded within both perfused and static collagen gels in the bioreactor system for adipogenic and chondrogenic differentiation (20% and 5% O2 tension, respectively) as well as cultured in control medium (20% O2 tension). In each case, live cells are indicated by green fluorescence, whereas dead cells are red. Silk tubes are on the right side of each image (indicated by the dotted yellow line) with the edge of the gel to the left. Variations in gel size are due to differences in the amount of gel contraction. Scale bars are 150 μm. Color images available online at www.liebertonline.com/ten.
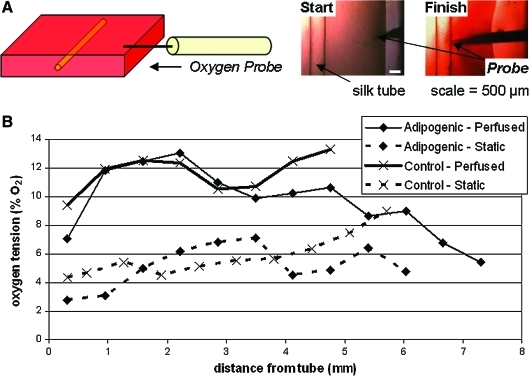
FIG. 5.
Oxygen measurements in perfused and static gels. (A) System schematic. (B) Representative plots of oxygen tension versus distance from silk tube for hMSC-seeded perfused and static adipogenic and control gels at 20% O2 tension on day 7 of culture. The plots span the entire gel, with data from next to the tube to the outer edge of the gel (greatest distance from tube). Variations in the distance from the tube are due to differences in the amount of gel contraction. Color images available online at www.liebertonline.com/ten.
As shown in Figure 4, cell viability within the gels was determined using a Live/Dead assay, with the cells embedded within the perfused chondrogenic, adipogenic, and control gels exhibiting characteristic fibroblast morphology and a greater distribution of viable cells throughout the gel. Cells within the static gels, however, had reduced cell viability throughout the gel, with the viable cells not exhibiting a typical morphology, but were rather small and rounded. In addition to the gross differences between perfused and static gels, there was a spatial dependence on cell viability and morphology as a function of distance from the silk tube in the perfused gels. The perfused adipogenic and chondrogenic gels exhibited the highest cell viability closest to the silk tube, as well as near the outside edge of the gel. This could be a function of diffusion limitations as the access to nutrients and oxygen is not limited for cells adjacent to the perfused tube or at the outside edge of the gel. Higher cell densities are present at the edge of the gels because of contraction. In both cases there is a reduction in cell viability within the gel, perhaps because of mass transport limitations, and these distances may serve as guidelines for tube spacing in future applications. In the static gels, there was no dependence of cell viability on distance from the silk tube, as expected. Edges of the gels displayed slightly improved cell viabilities and morphologies, where diffusion limitations are again reduced.
Oxygen gradients in cell-seeded hydrogels
The distribution of oxygen within the collagen gel was measured using an oxygen microsensor probe (Figs. 3 and 5). The probe was positioned at the outer edge of the gel and moved stepwise toward the silk tube, recording the oxygen tension at each point. On the basis of these data as well as images taken before and after measurement and the distance per turn of the micromanipulator, plots of oxygen tension as a function of distance from the tube were produced for each experimental condition. Representative oxygen profiles are given in Figure 5b for hMSC-seeded perfused and static adipogenic and control hydrogels cultured at 20% O2 on day 7 of culture. As shown in Figure 5, there were clear differences between the overall oxygen tensions of perfused and static gels, with the oxygen tensions for the perfused and static gels being around 7%–13% and 3%–8% O2, respectively. Differences throughout the gel, however, did not exactly match the expected oxygen distribution as oxygen tensions at the edges of both the tube and gel were not higher than in the middle of the gel (Fig. 6). This discrepancy may be due to the heterogenous cell distribution that would be present on the day of measurement (day 7). As demonstrated in Figure 6, there are a greater proportion of viable cells at both the edge of the tube and the edge of the gel in the perfused case, perhaps leading to greater consumption of oxygen locally. This heterogeneity is not accounted for in the model, which assumes a homogenous cell distribution and a steady-state rate of oxygen consumption. Further, because of the invasive nature of inserting a needle probe into the gels to obtain the measurement, the true local oxygen tensions may be compromised and are difficult to repeat. Because of this, future work will focus on noninvasive oxygen-sensitive dyes and imaging-based approaches to determine oxygen tensions in situ. Despite the technical issues, these oxygen measurements provide a starting point for tissue vascularization though further experimentation and modeling approaches are needed to more accurately quantify and correlate these results to cell viability and differentiation.
FIG. 6.
Oxygen modeling and cell viability. Comparison between cell viability results as determined by Live/Dead assay and modeling results. Higher magnification images of the Live/Dead assays highlight differences in cell morphology throughout the gels. Color images available online at www.liebertonline.com/ten.
Modeling of oxygen gradients in cell-seeded hydrogels
Oxygen transport in the hydrogels was modeled based on fluid flow, oxygen diffusion, and oxygen consumption. In the case of this bioreactor system, oxygen diffusion within the media and hydrogel was modeled using Fick's law, oxygen consumption was modeled according to Michaelis-Menten kinetics, and media flow was modeled with the Navier-Stokes equations.9 Appropriate boundary conditions and assumptions were applied to the model, including the assumption of zero-order oxygen consumption, a homogeneous cell distribution, constant oxygen consumption rates, and a fully developed, one-dimensional, laminar incompressible fluid flow. A 2D, steady-state analysis of the system was solved using FemLab multiphysics modeling software and compared with experimental results of cell viability for characterization of minimally sized modules (Fig. 6).
As shown in Figure 6, the distribution of viable cells closely matches the modeled oxygen tensions as a function of distance from the silk tube. In the perfused gels, oxygen is supplied through diffusion from both the porous tube as well as the edges of the gel. This produces an oxygen concentration gradient with the highest oxygen tensions at the interface between the silk tube and the collagen gel as well as at the edges of the gel. A slight decrease in oxygen concentration is modeled in the interior portion of the gel, perhaps explaining the differences in cell morphology between the fibroblast-like cells at the edges of the gel and the rounded cells in the center. In comparison, the static gel is only supplied with oxygen through diffusion from the edges of the gel. This leads to a sharp concentration gradient from these edges toward the silk tube in the middle of the gel. Because of this, fibroblast-like cells are observed at the outer edge of the gel, with a greater proportion of rounded cells in the middle. Taken together, these results demonstrate how oxygen modeling can predict cell behavior and provide insight into the proper spacing of vessels for oxygen and nutrient supply to the engineered tissue.
Conclusions
Perfusion and oxygen transport have direct effects on hMSC viability and differentiation in a silk tube/hMSC-seeded collagen gel composite system. This is especially apparent when considering differentiation into two lineages (adipogenic and chondrogenic) with vastly different oxygen requirements. On the basis of differentiation experiments at both high and low oxygen tension, hMSCs differentiated toward the adipogenic lineage favored high (20%) oxygen tension, whereas those differentiated toward the chondrogenic lineage favored low (5%) oxygen tension. This mirrors in vivo conditions, as fat tissue is highly vascularized, whereas cartilage is avascular. In addition, perfusion in the bioreactor system played a critical role in enhancing cell viability when compared with static cultures. Further studies, however, are required to fully examine the role of perfusion conditions in enhancing hMSC differentiation through the maintenance of higher concentrations of media supplements and waste removal when compared with static cultures.
This bioreactor system, in combination with the ability to quantitatively measure oxygen gradients within the gels, offers great potential for developing models of vessel spacing for vascularizing tissue constructs in vitro. By integrating experimental approaches (cell viability, differentiation) and oxygen transport modeling, it is possible to optimize minimally sized modular designs (size of tube/hydrogel) that may be coupled together to form larger systems with inherent vasculature. This can be done through additional tubes per construct, using single-channel modular systems as building blocks of larger tissues, or combining different cell types or hydrogels in different blocks to build composites. The silk tubes may also be seeded with smooth muscle and/or endothelial cells to help drive functional vessel formation. Overall, the underlying tools and approaches of this quantitative approach to tissue engineering provide design strategies that may be applied to produce a vascularized tissue of any type, provided that specific variables (cell density, metabolic rate, etc.) are known for the cells, biomaterial matrix, and/or target tissue.
Acknowledgments
The authors acknowledge funding from the Tissue Engineering Resource Center through the NIH grant EB002520 from the National Institute of Biomedical Imaging and Bioengineering. They also acknowledge the assistance of Brittany Robbins and Alexander Nectow in the compiling of the RT-PCR data; Jessica Walsh, Andrew Milgroom, and Zachary Fang for aiding in the production of silk tubes; and the Tufts Machine Shop (Larry McMaster, Denis DuPuis, and Scott MacCorkle) for the construction of the bioreactor molds and culture system as well as the oxygen probe holder and mounts.
Disclosure Statement
No competing financial interests exist.
References
- 1.Drury J.L. Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhao F. Pathi P. Grayson W. Xing Q. Locke B.R. Ma T. Effects of oxygen transport on 3-D human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005;21:1269. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- 3.Obradovic B. Carrier R.L. Vunjak-Novakovic G. Freed L.E. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol Bioeng. 1999;63:197. doi: 10.1002/(sici)1097-0290(19990420)63:2<197::aid-bit8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Marolt D. Augst A. Freed L.E. Vepari C. Fajardo R. Patel N. Gray M. Farley M. Kaplan D. Vunjak-Novakovic G. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Augst A. Marolt D. Freed L.E. Vepari C. Meinel L. Farley M. Fajardo R. Patel N. Gray M. Kaplan D.L. Vunjak-Novakovic G. Effects of chondrogenic and osteogenic regulatory factors on composite constructs grown using human mesenchymal stem cells, silk scaffolds and bioreactors. J R Soc Interface. 2008;5:929. doi: 10.1098/rsif.2007.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettinger C.J. Cyr K.M. Matsumoto A. Langer R. Borenstein J.T. Kaplan D.L. Silk fibroin microfluidic devices. Adv Mater. 2007;19:2847. doi: 10.1002/adma.200602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi N.W. Cabodi M. Held B. Gleghorn J.P. Bonassar L.J. Stroock A.D. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 8.Golden A.P. Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 9.Lovett M. Lee K. Edwards A. Kaplan D.L. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis B.H. Schroeder T. Yarmolenko P.S. Guilak F. Dewhirst M.W. Taylor D.A. An in vitro system to evaluate the effects of ischemia on survival of cells used for cell therapy. Ann Biomed Eng. 2007;35:1414. doi: 10.1007/s10439-007-9301-2. [DOI] [PubMed] [Google Scholar]
- 11.Radisic M. Malda J. Epping E. Geng W. Langer R. Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 12.Malda J. Martens D.E. Tramper J. van Blitterswijk C.A. Riesle J. Cartilage tissue engineering: controversy in the effect of oxygen. Crit Rev Biotechnol. 2003;23:175. [PubMed] [Google Scholar]
- 13.Malda J. Rouwkema J. Martens D.E. Le Comte E.P. Kooy F.K. Tramper J. van Blitterswijk C.A. Riesle J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86:9. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 14.Lovett M. Cannizzaro C. Daheron L. Messmer B. Vunjak-Novakovic G. Kaplan D.L. Silk fibroin microtubes for blood vessel engineering. Biomaterials. 2007;28:5271. doi: 10.1016/j.biomaterials.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y. Kim U.J. Blasioli D.J. Kim H.J. Kaplan D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Lewus K.E. Nauman E.A. In vitro characterization of a bone marrow stem cell-seeded collagen gel composite for soft tissue grafts: effects of fiber number and serum concentration. Tissue Eng. 2005;11:1015. doi: 10.1089/ten.2005.11.1015. [DOI] [PubMed] [Google Scholar]
- 17.Kim U.J. Park J. Li C. Jin H.J. Valluzzi R. Kaplan D.L. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 18.Li C. Vepari C. Jin H.J. Kim H.J. Kaplan D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Jin H.J. Park J. Valluzzi R. Cebe P. Kaplan D.L. Biomaterial films of Bombyx mori silk fibroin with poly(ethylene oxide) Biomacromolecules. 2004;5:711. doi: 10.1021/bm0343287. [DOI] [PubMed] [Google Scholar]