Abstract
Purpose
To examine the impact of age and cognitive reserve on cognitive functioning in patients with breast cancer who are receiving adjuvant treatments.
Patients and Methods
Patients with breast cancer exposed to chemotherapy (n = 60; mean age, 51.7 years) were evaluated with a battery of neuropsychological and psychological tests before treatment and at 1, 6, and 18 months after treatment. Patients not exposed to chemotherapy (n = 72; mean age, 56.6 years) and healthy controls (n = 45; mean age, 52.9 years) were assessed at matched intervals.
Results
Mixed-effects modeling revealed significant effects for the Processing Speed and Verbal Ability domains. For Processing Speed, a three-way interaction among treatment group, age, and baseline cognitive reserve (P < .001) revealed that older patients with lower baseline cognitive reserve who were exposed to chemotherapy had lower performance on Processing Speed compared with patients not exposed to chemotherapy (P = .003) and controls (P < .001). A significant group by time interaction for Verbal Ability (P = .01) suggested that the healthy controls and no chemotherapy groups improved over time. The chemotherapy group failed to improve at 1 month after treatment but improved during the last two follow-up assessments. Exploratory analyses suggested a negative effect of tamoxifen on Processing Speed (P = .036) and Verbal Memory (P = .05) in the no-chemotherapy group.
Conclusion
These data demonstrated that age and pretreatment cognitive reserve were related to post-treatment decline in Processing Speed in women exposed to chemotherapy and that chemotherapy had a short-term impact on Verbal Ability. Exploratory analysis of the impact of tamoxifen suggests that this pattern of results may be due to a combination of chemotherapy and tamoxifen.
INTRODUCTION
Cross-sectional and longitudinal studies have found evidence for chemotherapy-induced cognitive changes in a subgroup of patients with breast cancer, although negative studies have been reported.1–3 Studies also have suggested that tamoxifen alone4 or in combination with chemotherapy5 contributes to cognitive decline, with less consistent evidence for the cognitive effects of aromatase inhibitors.4,6–7 In addition, the identification of a subgroup of patients who demonstrate lower than expected neuropsychological performance on the basis of their ages and education levels8,9 before treatment raises the possibility that there are aspects of cancer itself that impact cognitive functioning or that there are common risk factors for the development of cancer and mild cognitive changes.10 Taken together, these findings suggest that there are multiple aspects of breast cancer and its treatment that may impact cognitive functioning in a subgroup of vulnerable individuals,11 which highlights the importance of identifying risk factors for cognitive decline.10
Age is a well-established risk factor for cognitive decline in other disorders,12 and researchers have speculated that older adults may be more vulnerable to cognitive adverse effects of cancer treatments.13 To date, studies of the cognitive effects of adjuvant treatment for breast cancer have controlled for age in the statistical analyses, but none have examined potential interactions of age and cancer treatments on cognitive functioning.
Low cognitive reserve at pretreatment may also be a risk factor for post-treatment cognitive changes. Cognitive reserve represents innate and developed cognitive capacity, which is influenced by various factors, including genetics, education, occupational attainment, and lifestyle.14 Research has demonstrated that people with low cognitive reserve are more vulnerable to the development of neurocognitive disorders (eg, Alzheimer's disease) and to cognitive decline after a variety of insults to the brain.15 In addition, research has demonstrated poorer cognitive outcomes secondary to neurotoxic exposures (eg, lead) in people with low cognitive reserve.16
Therefore, the associations among age, pretreatment cognitive reserve (defined as Wide Range Achievement Test, ed 3 [WRAT-3] Reading score), and post-treatment cognitive performance were evaluated within the context of a longitudinal study, in which patients with early-stage breast cancer exposed to chemotherapy or not exposed to chemotherapy were evaluated with neuropsychological testing before the beginning of adjuvant therapy and at three follow-up assessments, and they were compared to a matched healthy control group.
PATIENTS AND METHODS
Consecutive, newly diagnosed patients with breast cancer scheduled to receive adjuvant chemotherapy (n = 60) or no chemotherapy as part of their adjuvant treatment (n = 72) were recruited from the Breast Cancer Service of the Norris Cotton Cancer Center. Patients were eligible for participation if they were diagnosed with noninvasive (stage 0) or invasive (stage 1, 2, or 3A) breast cancer; undergoing first treatment with systemic chemotherapy or surgery and/or local, non-CNS radiotherapy; between 18 and 70 years of age at time of diagnosis; and fluent in English and able to read English. Patients were excluded on the basis of the following criteria: CNS disease; previous history of cancer (except basal cell carcinoma) or treatment with chemotherapy, CNS radiation, or intrathecal therapy; neurobehavioral risk factors, including history of neurologic disorder (eg, Parkinson's disease, seizure disorder, dementia), alcohol/substance abuse, or moderate to severe head trauma (loss of consciousness > 60 minutes or structural brain changes on imaging); and axis I psychiatric disorder (according to the Diagnostic and Statistical Manual of Mental Disorders, ed 4 [DSM-IV]; eg, schizophrenia, bipolar disorder, depression).
Female healthy controls (n = 45) who met the same inclusion (except for cancer diagnosis) and exclusion criteria were recruited through community advertisements. Healthy controls were frequency matched to patients on age and education. All methods and procedures were approved by the institutional review board of Dartmouth Medical School, and all participants provided written informed consent.
The pretreatment assessment occurred after surgery but before initiation of adjuvant therapy. Follow-up assessments for patients treated with chemotherapy were conducted at 1, 6, and 18 months after treatment. Because the length of chemotherapy varied, the test-retest interval for the first follow-up assessment for patients not exposed to chemotherapy and healthy control participants was frequency matched to the interval for the chemotherapy patients. Analysis of the intervals between neuropsychological assessments by group revealed no differences.
The assessment battery included standardized neuropsychological tests, measures of affective variables, measures of fatigue—which could impact cognitive performance, and a self-report measure of perceived cognitive functioning. Cognitive reserve was defined by pretreatment WRAT-3 Reading score.17 Neuropsychological tests were grouped into domains to reduce the number of statistical comparisons on the basis of expert opinion (A.J.S. and B.C.M.), guided by a factor analysis, as described previously.9 Block Design18 (Wechsler Abbreviated Scale of Intelligence [WASI]) was also administered but did not load on any domain.
Verbal ability.
Vocabulary (WASI,18 Verbal Fluency Test (Delis-Kaplan Executive Function System [D-KEFS])19;
Verbal memory.
California Verbal Learning Test-II,20 Logical Memory I and II (Wechsler Memory Scale-III [WMS-III]21;
Visual memory.
Faces I and II (WMS-III)21;
Working memory.
Paced Auditory Serial Addition Test (PASAT)22;
Processing speed.
Digit Symbol-Coding (Wechsler Adult Intelligence Scale-III [WAIS-III]),21 Trail Making Test (D-KEFS),19 Color-Word Interference Test (D-KEFS),19 and Grooved Pegboard23;
Sorting.
Sorting Test (D-KEFS)19;
Distractibility.
Continuous Performance Test (CPT)24; and
Reaction time.
CPT.24
Self report measures of depression
(Center for Epidemiological Study – Depression),25,26 anxiety (Spielberger State Anxiety Inventory),27 fatigue (Fatigue Symptom Inventory),28 and cognitive ability (Multiple Ability Self-Report Questionnaire)29 were also administered.
Statistical Analysis
All raw neuropsychological test scores were z-transformed on the basis of the mean and standard deviation of the scores of the healthy control group at the first assessment. The longitudinal neuropsychological assessments were analyzed by using a linear mixed-effects modeling approach,30 in which patients exposed to chemotherapy, patients not exposed to chemotherapy, and healthy controls were used as the grouping factor. We chose initially to limit the mixed model to one primary cognitive outcome domain of interest to reduce the probability of chance findings and then to apply the model to the other cognitive domains. The Processing Speed domain was chosen because this domain is among those most sensitive to cancer treatment–related effects in previous research.1 Significance was set at P < .01 to correct for multiple comparisons.
The dependent variable was the longitudinal change of Processing Speed at 1, 6, and 18 months after treatment or the equivalent time periods in the yoked no chemotherapy and control groups. Change scores since baseline have the advantage of yielding clearly interpretable indications of the directions of individual change.31 Several covariates were entered as fixed effects to adjust for baseline individual differences, including age, education, baseline Processing Speed, and baseline WRAT-3 Reading score (ie, measure of cognitive reserve). Additional interaction terms were entered as fixed factors to test specific research hypotheses. The effect of time was entered as a discrete, fixed factor to capture potential differences between waves of neuropsychological outcome assessments. Random terms were fitted to allow for patient-specific intercept. Mixed-effects modeling has several advantages over the more commonly used repeated-measures analysis of variance (ANOVA).32 Casewise deletion of missing observations is not necessary, which allows for the analysis of all available data. It is also a superior approach to handling the correlation structures of repeated measures nested within participants, which circumvents the need to make adjustments for heteroscedascity and sphericity assumption violations.
Hypothesis tests for the fixed effects were carried out by using the Wald F statistics and the Kenward-Roger adjustment as a precaution for inflated error variance in slightly unbalanced group sizes.33,34 Linear combinations of fixed effects were evaluated by t tests.34,35 The mixed models were fitted with the PROC MIXED procedure in SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
A total of 440 patients with breast cancer who were being evaluated and treated at the Norris Cotton Cancer Center or affiliated clinics were identified. After screening, 131 were ineligible on the basis of the exclusion criteria. Of the remaining 309 patients, 152 (49%) declined to participate, primarily because of feeling overwhelmed by the diagnosis and treatment and/or feeling that there were too many appointments between surgery and beginning the next round of treatment to participate. Another 22 patients (14%) agreed to participate, but testing was not able to be scheduled before beginning treatment for logistical reasons, and three patients (1%) signed consents but then withdrew before completing the baseline assessment. A total of 132 patients (n = 22, stage 0; n = 62, stage 1; n = 37, stage 2; and n = 11, stage 3A) were enrolled on the study; however, nine patients completed the pretreatment assessment only. Therefore, the final sample size for the longitudinal study was 123. The overall retention rate over the course of the study was 89%. Examination of the demographic data (Table 1) demonstrated that the groups were well matched on education, ethnicity, and menstrual status; however, patients not treated with chemotherapy were significantly older (P = .003). Patients had significantly higher scores of depression, anxiety, and fatigue compared with healthy controls, although the levels were generally within the normal range.
Table 1.
Baseline Demographic and Clinical Information About Patients With Breast Cancer and Healthy Controls
| Variable | Control (n = 45) |
Chemotherapy (n = 60) |
No Chemotherapy (n = 72) |
Overall P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | .003 | ||||||
| Mean | 52.9 | 51.7 | 56.6 | ||||
| SD | 10 | 7.1 | 8.3 | ||||
| Range | 30-68 | 31-66 | 37-69 | ||||
| WRAT–3 raw score | .414 | ||||||
| Mean | 51.5 | 50.7 | 50.8 | ||||
| SD | 2.9 | 3.3 | 3.9 | ||||
| Range | 44-56 | 42-56 | 39-57 | ||||
| Education | .087 | ||||||
| Mean | 15.2 | 15.7 | 14.8 | ||||
| SD | 2.1 | 2.7 | 2.3 | ||||
| Range | 12-20 | 11-25 | 9-20 | ||||
| Ethnicity | |||||||
| White | 44 | 100 | 58 | 98 | 71 | 99 | .49 |
| Asian | 0 | 0 | 1 | 2 | 0 | 0 | |
| Other | 0 | 0 | 0 | 0 | 1 | 1 | |
| Ethnicity | |||||||
| Hispanic | 1 | 2 | 1 | 2 | 0 | 0 | .48 |
| Not Hispanic | 43 | 98 | 56 | 98 | 71 | 100 | |
| Menstrual status | |||||||
| Regular periods* | 14 | 32 | 16 | 27 | 16 | 24 | .62 |
| Irregular periods | 2 | 5 | 6 | 10 | 5 | 7 | |
| Stopped | 0 | 0 | 0 | 0 | 0 | 0 | |
| Begun to stop | 2 | 5 | 5 | 8 | 2 | 3 | |
| Stopped permanently | 26 | 59 | 32 | 54 | 45 | 66 | |
| CES–Depression | 5.1 | 3.6 | 10.9 | 8 | 8.3 | 7.3 | < .001 |
| STAI State Anxiety | 26.9 | 6.5 | 34.3 | 12.3 | 31.2 | 11.2 | .002 |
| FSI Fatigue | 1.7 | 1.2 | 2.6 | 1.7 | 2.2 | 1.6 | .011 |
Abbreviations: SD, standard deviation; WRAT-3, Wide Range Achievement Test (ed 3); CES-Depression, Center for Epidemiological Study-Depression; STAI State Anxiety, State-Trait Anxiety Inventory; FSI, Fatigue Symptom Inventory.
For longer than 1 year.
Table 2 describes the chemotherapy regimens used. Additionally, 80% of the chemotherapy group (n = 34, tamoxifen; and n = 5, anastrozole) and 66% of the no-chemotherapy group (n = 39, tamoxifen; n = 7, anastrozole; n = 2, raloxifene) were treated with endocrine therapy; 81% of the chemotherapy group and 72% of the no-chemotherapy group were treated with radiation therapy.
Table 2.
Chemotherapy Regimens
| Regimen | No. of Patients |
|---|---|
| Doxorubicin/cyclophosphamide/paclitaxel | 22 |
| Taxotere/doxorubicin/cyclophosphamide | 2 |
| Cyclophosphamide/doxorubicin/fluorouracil | 1 |
| Doxorubicin/cyclophosphamide | 18 |
| Fluorouracil/epirubicin/cyclophosphamide | 10 |
| Cyclophosphamide/methotrexate/fluorouracil | 7 |
Comparison by Treatment Group
Although the initial analysis focused on the Processing Speed domain, Table 3 provides a summary of z-transformed domain scores by group and time. The mixed-effects model for Processing Speed revealed a significant time effect (P < .001) but not a group effect (P = .08) or a time-by-group interaction (P = .40), which indicated that all groups improved during each assessment, consistent with a practice effect (Table 3). Adding the two-way interactions of age and WRAT-3 Reading by group revealed significant group by age (P = .02) and group-by-baseline WRAT-3 Reading (P < .001) interactions. The group by age interaction indicated that older patients who received chemotherapy had lower post-treatment Processing Speed performance (z-score difference, −0.16 per 10 years increase in age; 95% CI, −0.29 to −0.04) compared with healthy controls, as did patients not exposed to chemotherapy (difference, −0.11; 95% CI, −0.21 to −0.001). The two patient groups did not differ from each other. The group-by-baseline WRAT-3 Reading interaction indicated that patients exposed to chemotherapy who had lower baseline WRAT-3 Reading scores had lower post-treatment Processing Speed performance compared with patients not exposed to chemotherapy (P < .005) and healthy controls (P < .001). The comparison between controls and the no-chemotherapy group was not significant (P = .17).
Table 3.
Adjusted Domain Scores by Group and Across Assessment Time Points
| Variable by Time Point | Control |
Chemotherapy |
No Chemotherapy |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Baseline | ||||||
| No. of patients | 44 | 60 | 72 | |||
| Processing speed | −0.05 | 0.59 | −0.18 | 0.56 | −0.26 | 0.66 |
| Verbal ability | −0.03 | 0.70 | −0.21 | 1.00 | −0.23 | 0.85 |
| Verbal memory | −0.04 | 0.75 | −0.25 | 0.93 | −0.20 | 0.91 |
| Visual memory | −0.04 | 0.91 | −0.10 | 0.93 | 0.01 | 0.80 |
| Working memory | −0.01 | 0.92 | −0.29 | 1.40 | −0.45 | 1.26 |
| Sorting | −0.07 | 0.72 | −0.18 | 0.56 | −0.05 | 0.95 |
| Distractibility | −0.01 | 0.90 | −0.01 | 0.91 | −0.04 | 1.00 |
| Reaction time | −0.02 | 0.88 | −0.77 | 1.17 | −0.28 | 0.90 |
| Block design | −0.02 | 0.91 | −0.21 | 0.94 | −0.29 | 0.89 |
| 1 month after treatment | ||||||
| No. of patients | 43 | 55 | 68 | |||
| Processing speed | 0.12 | 0.58 | −0.11 | 0.46 | −0.14 | 0.65 |
| Verbal ability | 0.12 | 0.58 | −0.23 | 0.53 | −0.15 | 0.73 |
| Verbal memory | 0.45 | 0.72 | 0.15 | 0.86 | 0.09 | 0.87 |
| Visual memory | 0.67 | 0.96 | 0.77 | 0.68 | 0.63 | 0.85 |
| Working memory | 0.33 | 0.96 | 0.24 | 0.86 | 0.03 | 0.99 |
| Sorting | 0.26 | 0.68 | 0.12 | 0.91 | −0.05 | 0.95 |
| Distractibility | 0.28 | 0.47 | 0.12 | 0.47 | −0.04 | 1.00 |
| Reaction time | 0.07 | 0.97 | −0.47 | 1.07 | −0.28 | 0.90 |
| Block design | 0.14 | 0.75 | −0.11 | 0.89 | −0.13 | 0.91 |
| 6 months after treatment | ||||||
| No. of patients | 42 | 49 | 67 | |||
| Processing speed | 0.17 | 0.61 | −0.08 | 0.45 | −0.17 | 0.64 |
| Verbal ability | 0.14 | 0.59 | 0.03 | 0.92 | −0.06 | 0.78 |
| Verbal memory | 0.67 | 0.73 | 0.53 | 0.80 | 0.33 | 0.91 |
| Visual memory | 0.98 | 0.71 | 0.97 | 0.65 | 0.83 | 0.82 |
| Working memory | 0.65 | 0.82 | 0.39 | 0.76 | 0.28 | 0.96 |
| Sorting | 0.39 | 0.75 | 0.40 | 0.90 | 0.26 | 0.89 |
| Distractibility | 0.38 | 0.29 | 0.13 | 0.49 | −0.05 | 1.09 |
| Reaction time | 0.15 | 0.82 | −0.39 | 0.94 | −0.26 | 0.89 |
| Block design | 0.14 | 0.79 | 0.01 | 0.79 | −0.20 | 0.99 |
| 18 months after treatment | ||||||
| No. of patients | 39 | 46 | 64 | |||
| Processing speed | 0.25 | 0.52 | −0.01 | 0.45 | −0.09 | 0.65 |
| Verbal ability | 0.17 | 0.71 | 0.17 | 0.87 | −0.04 | 0.73 |
| Verbal memory | 0.69 | 0.69 | 0.68 | 0.80 | 0.38 | 0.93 |
| Visual memory | 1.05 | 0.80 | 1.04 | 0.69 | 1.02 | 0.71 |
| Working memory | 0.64 | 0.92 | 0.69 | 0.65 | 0.44 | 0.95 |
| Sorting | 0.55 | 0.73 | 0.52 | 0.91 | 0.21 | 0.86 |
| Distractibility | 0.16 | 0.81 | 0.20 | 0.45 | −0.02 | 1.05 |
| Reaction time | 0.16 | 0.88 | −0.57 | 1.14 | −0.28 | 0.95 |
| Block design | 0.18 | 0.76 | 0.11 | 0.84 | −0.07 | 0.82 |
NOTE. Analyses adjusted for age, education, and baseline score.
Abbreviation: SD, standard deviation.
Finally, the three-way interaction (group by age by baseline WRAT-3 Reading score) was also significant (P < .001), which indicated that older patients with lower baseline WRAT-3 Reading scores who were exposed to chemotherapy had lower performance on Processing Speed compared with patients not exposed to chemotherapy (difference, −0.15 per 10 years increase in age and one standard deviation lower in WRAT-3 Reading; 95% CI, −0.25 to −0.05; P = .003). A greater difference was seen when comparing patients exposed to chemotherapy and controls (difference, −0.23; 95% CI, −0.34 to −0.13; P < .001). The comparison of patients not exposed to chemotherapy and healthy controls was in similar direction but was not significant (P = .08).
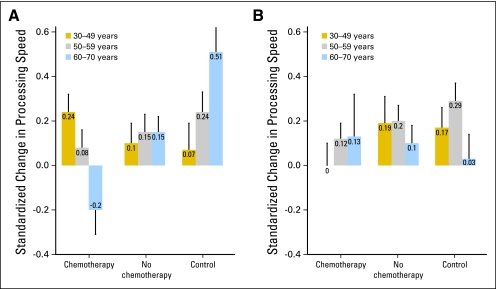
Figure 1 provides an illustration of the three-way interaction by displaying change scores averaged over the three post-treatment assessments for the three groups divided by age tertiles (ie, 30 to 49, 50 to 59, and 60 to 70 years) and a median split on baseline WRAT-3 Reading score. Consistent with the analysis, the older patients with lower baseline WRAT-3 Reading scores who were exposed to chemotherapy demonstrated a decline in adjusted post-treatment scores, whereas most other groups improved.
Fig 1.
Pre- to post-treatment change in processing speed by treatment, age groups, and level of cognitive reserve. (A) Wide Range Achievement Test (WRAT-3) below median; (B) WRAT-3 above median. High and low pretreatment cognitive reserve were defined by a median split on the WRAT-3 reading score of less than 0.0 or of greater than or equal to 0.0. The bar heights represent the observed post-treatment averages pooled across three assessment time points. The error bars represent the estimated standard errors of the averages accounting for repeated assessments from the same individuals.
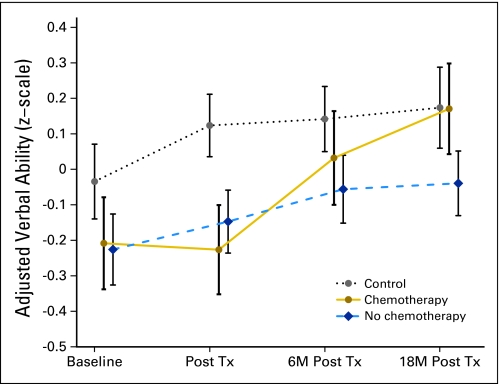
Applying the mixed-effects model to the other cognitive domains revealed a significant group-by-time interaction for the Verbal Ability domain (P = .01) as well as a significant age-by–WRAT-3 Reading interaction (P = .039) but no three-way interaction with treatment group. Examination of Figure 2 reveals that patients not exposed to chemotherapy and healthy controls demonstrated improved performance over time, whereas patients exposed to chemotherapy demonstrated no improvement from baseline to the first post-treatment assessment, which was followed by improvement over the next two assessments.
Fig 2.
Adjusted verbal ability scores (z-scale) by group across time. Tx, treatment; M, months.
Finally, age was significantly related to performance on the Verbal Memory, Visual Memory, Working Memory, and Sorting domains, and WRAT-3 Reading was significantly related to performance in the Distractibility domain. However, no significant main effects for group or interactions between time, age and/or WRAT-3 Reading and treatment group emerged.
Because the majority of patients were treated with tamoxifen, we conducted an exploratory analysis comparing patients in the no- chemotherapy group treated with tamoxifen (n = 39) with those not receiving endocrine therapy (n = 20) and healthy controls. Main effects for treatment were found for Processing Speed (P = .036) and Verbal Memory (P = .05), with a trend for the Verbal Ability (P = .067). In all instances, patients treated with tamoxifen performed worse than healthy controls (Processing Speed, P = .016; Verbal Memory, P = .018; Verbal Ability Domain, P = .023), whereas the performance of patients not treated with tamoxifen did not differ significantly from controls. In no instance did the patients treated and not treated with tamoxifen differ.
Menopausal status at baseline or change in menopausal status with treatment (eg, chemotherapy-induced menopause) has been proposed as important in moderating post-treatment cognitive change. However, no main effects or interactions were found when baseline menopausal status or change in menopausal status was added to the model. Similarly, adding depression, anxiety, and fatigue scores as time-varying covariates did not yield any main effects or interactions.
Self-Report of Cognitive Function
Inclusion of the Multiple Ability Self-Report Questionnaire (MASQ) total score in the model revealed a significant main effect for time (P < .02) and a significant group-by-time interaction (P = .04), but no significant interactions with age or pretreatment cognitive reserve. Table 4 displays the MASQ total score means and standard deviations by group. Tukey Honestly Significant Difference tests revealed that the increase in cognitive symptoms from pretreatment was significantly greater (P < .05) for the chemotherapy group compared with the no-chemotherapy group and healthy controls, which did not differ from each other.
Table 4.
Average MASQ Total Score by Group
| Variable by Time Point | Control* |
Chemotherapy |
No Chemotherapy |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Baseline | 85.5 | 17.8 | 83.9 | 24.3 | 86.8 | 20.8 |
| 1 month after treatment | 88.7 | 20.6 | 98.8 | 25.3 | 91.8 | 24.2 |
| 6 months after treatment | 86.2 | 21.3 | 96.2 | 25.2 | 92.9 | 23.4 |
| 18 months after treatment | 83.1 | 20.6 | 99.7 | 26.2 | 90.8 | 26.3 |
Abbreviations: MASQ, Multiple Ability Self-Report Questionnaire; SD, standard deviation.
Sample size for the control group was 44 at baseline, and 43, 42 and 39 at 1, 6, and 18 months after treatment, respectively. For the chemotherapy group, the sample sizes were 60, 55, 49 and 46, respectively. For the no chemotherapy group, the sample sizes were 72, 68, 67 and 64, respectively.
DISCUSSION
There is general consensus in the field that only a subgroup of patients with breast cancer experience long-term cognitive changes after adjuvant treatment.3 Consequently, identification of factors that increase risk for cognitive changes is critical. Data from this study demonstrated that patients who were older, had lower pretreatment cognitive reserve, and were exposed to chemotherapy performed worse on measures of Processing Speed compared with patients not exposed to chemotherapy and healthy controls. This finding is consistent with studies examining interactions of age and cognitive reserve and changes in cognition associated with environmental exposures16 and age-related neurocognitive disorders.15
The data also suggest that chemotherapy has an acute effect on Verbal Ability, which resolved over time. This pattern is consistent with the results of another longitudinal study that examined this domain shortly after chemotherapy or radiation therapy36 and data from an imaging study that demonstrated decreased gray matter density in bilateral frontal, temporal, and cerebellar regions at 1 month after chemotherapy, with partial recovery over 1 year.37
Exploratory analyses of the impact of tamoxifen in the no-chemotherapy group produced results consistent with data from the cognitive substudy of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial that demonstrated a negative impact on cognitive performance across various domains.4 This study was not powered to address the effects of hormonal therapy specifically, and we did not have sufficient numbers of patients treated with chemotherapy but not tamoxifen to make this comparison; therefore, this result must be interpreted with caution. However, because the majority of patients in the chemotherapy group were also treated with tamoxifen, our results may reflect the combined effects of chemotherapy and tamoxifen. Given the pattern of results of this study, future studies designed to specifically evaluate the cognitive impact of endocrine therapies should examine interactions between endocrine therapy alone and cognitive reserve and age.
Examination of self-reported cognitive symptoms revealed that the chemotherapy-exposed group as a whole reported more cognitive symptoms compared with the other two groups, but no interactions with age or cognitive reserve were seen. Clinically, many young, highly educated women report persistent cognitive changes after chemotherapy.1–3 A potential explanation is that younger women with higher levels of cognitive reserve may perceive changes in cognitive capacity but are able to maintain performance on neuropsychological tests by recruiting alternate brain circuitry. Preliminary functional magnetic resonance imaging and positron emission tomography studies support this hypothesis.38–40
The lack of significant findings for domains reported by others (eg, working memory) may be related to the measures chosen or our assessment approach. In an attempt to reduce patient burden, some domains were based on data from one measure. We examined standard error over time for each domain and found that the Processing Speed domain demonstrated the lowest amount of variance. Additionally, analyses of the individual tests that comprised the Processing Speed domain revealed less robust results (analyses not shown). An assessment strategy designed to optimize the sensitivity of measurement by including multiple tests for each domain may have produced significant results in other domains.
Strengths of this study include the longitudinal design, inclusion of patients with breast cancer exposed to chemotherapy and those not exposed to chemotherapy as well as healthy controls, and the use of sophisticated modeling approaches that allowed us to examine important main effects and interactions. However, there are limitations as well. We chose to focus on interactions with age and cognitive reserve; however, it is possible that models that included other factors/interactions may have produced significant results in other domains. Additionally, Figure 1 is a useful representation of the interactions identified in the model. However, the cut points used and the details of the pattern of results require replication, because dividing each study group into six subgroups produced relatively small sample sizes per group. Finally, our sample consisted primarily of well-educated, white women; therefore, the generalizability of these results to other populations with more diversity in terms of ethnicity and education/cognitive reserve is limited.
The results of this study suggest that age and pretreatment cognitive reserve are important predictors of post-treatment cognitive functioning in the Processing Speed domain. Future studies examining potential factors associated with other domains of cognitive functioning and independent and combined effects of chemotherapy and endocrine therapy will be important.
Footnotes
Supported by Grants No. R01 CA87845, R01 CA101318, and R01 CA129769 from the Office of Cancer Survivorship, National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Tim A. Ahles, Andrew J. Saykin, Brenna C. McDonald, Gary N. Schwartz, Peter A. Kaufman
Financial support: Tim A. Ahles, Andrew J. Saykin
Administrative support: Tim A. Ahles, Andrew J. Saykin, Charlotte T. Furstenberg, Tamsin J. Mulrooney
Provision of study materials or patients: Tamsin J. Mulrooney, Gary N. Schwartz, Peter A. Kaufman
Collection and assembly of data: Brenna C. McDonald, Charlotte T. Furstenberg, Brett S. Hanscom, Tamsin J. Mulrooney
Data analysis and interpretation: Tim A. Ahles, Andrew J. Saykin, Brenna C. McDonald, Yuelin Li, Charlotte T. Furstenberg, Brett S. Hanscom
Manuscript writing: Tim A. Ahles, Andrew J. Saykin, Brenna C. McDonald, Yuelin Li
Final approval of manuscript: Tim A. Ahles, Andrew J. Saykin, Brenna C. McDonald, Yuelin Li, Charlotte T. Furstenberg, Brett S. Hanscom, Tamsin J. Mulrooney, Gary N. Schwartz, Peter A. Kaufman
REFERENCES
- 1.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. The Cancer Journal. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 2.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 3.Vardy J, Wefel JS, Ahles T, et al. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 4.Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive function of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. J Clin Oncol. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 5.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 6.Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psycho-Oncology. 2009;18:811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 7.Schilder CMT, Schagen SB. Effects of hormonal therapy on cognitive functioning in breast cancer patients: A review of the literature. Minerava Ginecologica. 2007;59:387–401. [PubMed] [Google Scholar]
- 8.Wefel JS, Lenzi R, Theriault R. Chemobrain in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 9.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Invest. 2007;25:373–377. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 12.Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits, in WS Sossin, J-C Lacaille, VF Castellucci, et al (eds): Progress in Brain Research. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 15.Whalley LJ, Deary IJ, Appleton CL, et al. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Bleecker ML, Ford DP, Celio MA, et al. Impact of cognitive reserve on the relationship of lead exposure and neurobehavioral performance. Neurology. 2007;69:470–476. doi: 10.1212/01.wnl.0000266628.43760.8c. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson GS. WRAT3 Administration Manual. Wilmington DE: Wide Range; 1993. [Google Scholar]
- 18.The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 19.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test-Second Edition: Adult Version Manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 21.The Psychological Corporation. WAIS-III Wechsler Adult Intelligence Scale (ed 3) and WMS-III Wechsler Memory Scale (ed 3) Updated Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 22.Fischer JS, Jak AJ, Kniker JE, et al. Multiple Sclerosis Functional Composite Administration and Scoring Manual. New York, NY: National Multiple Sclerosis Society; 2001. [Google Scholar]
- 23.Lafayette Instrument. Grooved Pegboard: Instruction/Owner's Manual. Lafayette, IN: Lafayette Instrument; 2001. [Google Scholar]
- 24.Gordon M, McClure FD, Aylward GP. Dewitt, NY: Gordon Systems; 1986. The Gordon Diagnostic System Instruction Manual and Interpretive Guide. [Google Scholar]
- 25.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Measure. 1977;1:385–392. [Google Scholar]
- 26.Plutchick R, Conte HR. Self-report scales for the measurement of depression. Psych Ann. 1989;19:367–371. [Google Scholar]
- 27.Spielberger CD, Gorsuch RL, Lushene RG. Manual for the State-Trait Anxiety Inventory for Adults. Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- 28.Hann DM, Jacobsen PB, Martin SC, et al. Fatigue in women treated with bone marrow transplantation for breast cancer: A comparison with women with no history of cancer. Support Care Cancer. 1997;5:44–52. doi: 10.1007/BF01681961. [DOI] [PubMed] [Google Scholar]
- 29.Seidenberg M, Haltiner A, Taylor MA, et al. Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 30.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 31.Flay BR, Miller TQ, Hedeker D, et al. The television, school, and family smoking prevention and cessation project. VIII: Student outcomes and mediating variables. Prev Med. 1995;24:29–40. doi: 10.1006/pmed.1995.1005. [DOI] [PubMed] [Google Scholar]
- 32.Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- 33.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 34.Brown H, Prescott R. Applied Mixed Models in Medicine. New York, NY: John Wiley & Sons; 2006. [Google Scholar]
- 35.Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 36.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 37.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. doi: 10.1007/s10549-010-1088-4. [epub ahead of print on August 6, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 39.McDonald BC, Saykin AJ, Ahles TA. Brain imaging investigation of chemotherapy-induced neurocognitive changes. In: Meyers CA, Perry JR, editors. Cognition and Cancer. Cambridge, United Kingdom: Cambridge University Press; 2008. pp. 19–32. [Google Scholar]
- 40.Ferguson RJ, McDonald BC, Saykin AJ, et al. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]