Abstract
Purpose
The role of adjuvant chemoradiotherapy (CRT) in resectable pancreatic cancer is still debated. This randomized phase II intergroup study explores the feasibility and tolerability of a gemcitabine-based CRT regimen after R0 resection of pancreatic head cancer.
Patients and Methods
Within 8 weeks after surgery, patients were randomly assigned to receive either four cycles of gemcitabine (control arm) or gemcitabine for two cycles followed by weekly gemcitabine with concurrent radiation (50.4 Gy; CRT arm). The primary objective was to exclude a < 60% treatment completion and a > 40% rate of grade 4 hematologic or GI toxicity in the CRT arm with type I and II errors of 10%. Secondary end points were late toxicity, disease-free survival (DFS), and overall survival (OS).
Results
Between September 2004 and January 2007, 90 patients were randomly assigned (45:45). Patient characteristics were similar in both arms. Treatment was completed per protocol by 86.7% and 73.3% (80% CI, 63.1% to 81.9%; 95% CI, 58.1% to 85.4%) in the control and CRT arms, respectively, and grade 4 toxicity was 0% and 4.7% (two of 43; 80% CI, 1.2% to 11.9%), respectively. In the CRT arm, three patients experienced grade 3–related late toxicity. Median DFS was 12 months in the CRT arm and 11 months in the control arm. Median OS was 24 months in both arms. First local recurrence was less frequent in the CRT arm (11% v 24%).
Conclusion
Adjuvant gemcitabine-based CRT is feasible, well-tolerated, and not deleterious; adding this treatment to full-dose adjuvant gemcitabine after resection of pancreatic cancer should be evaluated in a phase III trial.
INTRODUCTION
Pancreatic cancer remains a dismal disease and is the fourth leading cause of death from cancer in the United States.1 At this time, surgery is the only path to cure, but only a small number of patients present with resectable disease at the time of diagnosis. After resection, median survival is limited to around 20 months, strongly indicating that a multimodal approach is needed to decrease the high incidence of both locoregional and distant recurrence.2–4
The role of adjuvant therapy in resectable tumors is still debated, particularly the impact of postoperative chemoradiotherapy (CRT). CRT using fluorouracil (FU) is considered standard of care in the United States, based on the small Gastrointestinal Tumor Study Group (GITSG) trial and large case series analysis from Johns Hopkins and the Mayo Clinic.5–7 By contrast, chemotherapy alone is now widely recommended in Europe in the adjuvant setting, on the basis of the European Study Group for Pancreatic Cancer 1 (ESPAC-1) and Charité Onkologie 001 (CONKO-001) trials, both showing survival benefit using FU or gemcitabine, respectively.8–10 Data derived from the ESPAC-1 and the previous European Organisation for Research and Treatment of Cancer 40891 (EORTC-40891) study11 do not support the use of FU-based CRT, but these results cannot be considered definitively conclusive, mainly because of the small numbers of patients underpowering the EORTC trial,11 the radiation regimen used (2 × 20 Gy split course) for both studies, the complex design, and the lack of quality control of radiation treatment of the ESPAC-1 trial.8
Both FU and gemcitabine given in the adjuvant setting have shown a substantial benefit and were recently reported to have equivalent efficacy after resection of the primary tumor.12 Gemcitabine has shown less toxicity than FU bolus in the adjuvant setting and a clinical benefit in advanced stages; it is also a good radiosensitizer.13–15 Preliminary data have shown promising results in locally advanced and neoadjuvant settings using gemcitabine-based CRT by combining gemcitabine at a weekly dose of 300 to 500 mg/m2 with 30 to 54 Gy of radiation.16–21
Optimizing adjuvant strategies should therefore be addressed. This study was initiated to assess a modern gemcitabine-based CRT regimen in the adjuvant setting of pancreatic cancer. This randomized phase II trial primarily aimed to assess the feasibility and toxicity of the CRT treatment compared with standard gemcitabine alone. Secondary end points were late toxicity, disease-free survival (DFS), and overall survival (OS).
PATIENTS AND METHODS
Study Design
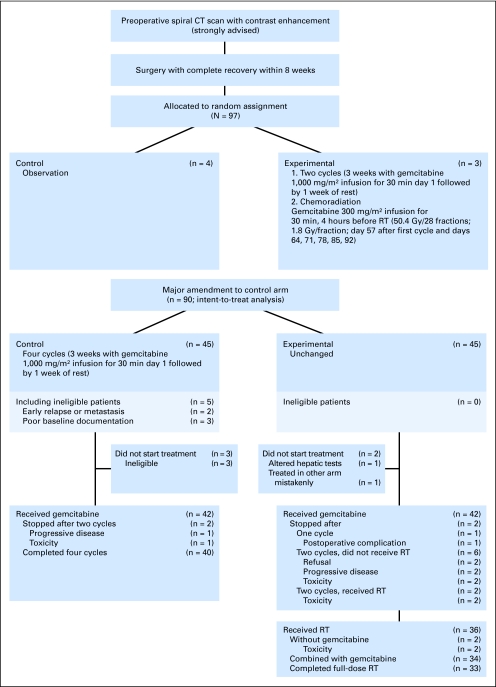
This is an open, multicenter, randomized, controlled phase II study, promoted by the EORTC Gastrointestinal Group and Radiation Oncology Group (ROG). The study was performed in Europe with the collaboration of the Federation Francophone de Cancérologie Digestive (FFCD) and the Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR), both in France. Initially, within 8 weeks after curative microscopically complete (R0) resection of pancreatic head adenocarcinoma, patients had to be randomly assigned (1:1) between observation (control arm) and CRT (experimental arm). There was a major amendment to the trial protocol on September 7, 2004, when the control arm was changed to gemcitabine alone instead of observation. After September 7, 2004, patients were randomly assigned (1:1) to gemcitabine alone for four cycles of 4 weeks (control arm) and gemcitabine for two cycles followed by gemcitabine weekly and concurrent radiation therapy (experimental arm). Patients were stratified by institution, WHO performance status (PS), and nodal status. Figure 1 depicts the study flow chart.
Fig 1.
Study flowchart. CT, computed tomography; RT radiation therapy.
Eligibility
Patients with histologically confirmed pancreatic head adenocarcinoma with R0 duodenopancreatectomy (Whipple procedure or pylorus-preserving procedure), documented histologic examination of surgical margins (including retroperitoneal margins), and documented lymph node examination (< 10 v ≥ 10; International Union Against Cancer [UICC] TNM classification, 2006) were eligible. Patients had to be recovered completely from surgery within 8 weeks. An abdominal spiral computed tomography (CT) scan had to be performed 8 weeks maximum before random assignment to exclude manifest distant metastases. Other inclusion criteria were age > 18 years; WHO PS 0 to 2; adequate bone marrow, liver, and renal functions; and written informed consent. Exclusion criteria were previous chemotherapy or radiotherapy; previous or coexistent malignant disease (except basal cell carcinoma or carcinoma in situ of the cervix); periampullary, neuroendocrine, intraductal papillary, or mucinous tumors; and incomplete resection. The protocol was approved by appropriate ethics committees at each participating institution.
Treatment
Treatment started within 8 weeks after surgery. In the control arm, treatment consisted of four cycles of gemcitabine 1,000 mg/m2 by 30-minute infusion during 3 consecutive weeks followed by 1 week of rest. In the experimental arm, treatment consisted of two cycles of gemcitabine 1,000 mg/m2 by 30-minute infusion during 3 consecutive weeks followed by 1 week of rest. In the experimental arm, cycle 1 treatment was given on days 1, 8, and 15; cycle 2 treatment was given on days 29, 36, and 43.
After the 1-week rest, CRT was started on day 57: gemcitabine 300 mg/m2 by 30-minute infusion once per week, given 4 hours before radiation (50.4 Gy in 28 fractions, 1.8 Gy per fraction) for 5 to 6 weeks.
Radiotherapy was delivered according to the guidelines of the International Commission on Radiation Units and Measurements Report 50. Patients were treated in the supine position. A CT scan in treatment position was obtained before the start of treatment. The clinical target volume was delineated on this CT scan on the basis of the preoperative radiologic examinations and the pathology report. The clinical target volume included the former pancreatic tumor site and lymph node areas. The retroperitoneal para-aortic lymphatics between the celiac trunk and the upper mesenteric artery to the anterior level of the vertebral bodies had to be included. To reduce toxicity, the inclusion of the pancreatic tail was not mandatory. A safety margin of 5 mm in all directions had to be shaped for subclinical extension of tumor cells.
Participating radiation oncology departments had to fulfill the EORTC ROG Quality Assurance requirements that consisted of a regularly updated facility questionnaire and an external radiation dosimetry audit of their treatment units. Additional details on radiation delivery are provided in the Appendix (online only).
Evaluation and Follow-Up
Before patients were randomly assigned, a complete medical history was taken and a complete physical examination was performed that included routine laboratory studies, carcinoembryonic antigen and carbohydrate antigen 19-9 tumor markers, vital signs, body weight, height, and evaluation of the PS using the WHO scale. Tumor assessment included abdominal CT or magnetic resonance imaging to rule out distant metastases and plan radiation therapy, if any. Before CRT, imaging was repeated only in case of clinical suggestion of early recurrence. All pathologic reports were centrally reviewed to definitively determine the status of the resection (R0, R1, or undetermined).
During the treatment period, all patients were evaluated weekly for clinical and laboratory findings. All adverse events and toxicities were recorded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 2.0).
After treatment completion, all patients included in the study were followed up clinically, biologically, and radiologically every 3 months until death by evaluating blood count, liver tests, tumor markers, PS, weight, late adverse events (Radiation Therapy Oncology Group [RTOG] scale), and disease status by using imaging. Only radiologic findings were considered to determine recurrence.
Statistical Considerations
The coprimary end points of this trial were feasibility and tolerability of the experimental treatment. The primary aim was that the experimental treatment could be completed in 80% (60% being an unacceptable rate) of the enrolled patients and that treatment-related grade 4 hematologic toxicity or GI toxicity occurring up to 30 days after the completion of the treatment be approximately 15% (40% being an unacceptable rate).
Using a one-step Bryant and Day design,22 39 patients were required in the experimental arm to reject a < 60% treatment completion and a > 40% rate of grade 4 hematologic or GI toxicity with one-sided type I error rate of 10% and type II error rate of 10% under the alternative of an 80% completion rate with ≤ 15% toxicity. The experimental arm was to be considered as feasible if the upper bound of the two-sided 80% CI for grade 4 toxicity excluded 40% and if the lower bound of the two-sided 80% CI for the treatment completion rate excluded 60%. Secondary end points were late toxicity, DFS, and OS.
Statistical Analyses
A total of 97 patients were randomly assigned in this trial, seven patients before the amendment. These seven patients were excluded from the main analysis. The statistical analysis is presented for the 90 eligible patients randomly assigned after amendment (intent-to-treat population; Fig 1).
The rate of full completion administration of the experimental treatment was computed as the percentage of patients who received the full dose of radiation and chemotherapy prescribed according to the protocol in the intent-to-treat population. Dose delays were not considered as treatment failures.
DFS was defined as the time from random assignment to disease recurrence or death, whichever came first. OS was defined as the time from random assignment to death. The analyses of DFS and OS were done in the intent-to-treat population, and a sensitivity analysis excluded the patients who did not start the allocated treatment or had no radiation therapy in the experimental arm (treated population was 42 in the control arm and 36 in the experimental arm). Kaplan-Meier curves were used to estimate DFS and OS in both arms. Patients without events were censored at the date of last contact. CIs are presented at the two-sided 80% (primary end points) and 95% level. Toxicity data are presented per treatment allocated at random assignment in patients randomly assigned after the amendment who had started their allocated treatment. Statistical analysis was conducted at the EORTC Headquarters by two statisticians (L.C. and M.M.).
RESULTS
Patient Characteristics
Between September 2004 and January 2007, 90 patients were enrolled by 29 centers, 45 in each arm. Five patients, all in the control arm, were considered to be ineligible (two because of early discovery of recurrence or metastases and three because they lacked data from one center).
Patients' baseline characteristics were similar in both treatment arms (Table 1). Most of the tumors were pT3N1 with perineural invasion. R0 resection was performed in 43 patients (96%) in the experimental arm and in 44 patients (98%) in the control arm. Follow-up was also similar in both arms, with a median follow-up of 30.7 months in the experimental arm and 33.3 months in the control arm (P = .44).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Gemcitabine + Radiotherapy(n = 45) |
Gemcitabine Alone(n = 45) |
Total(N = 90) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 61 | 58 | 59 | |||
| Range | 44-75 | 32-77 | 32-77 | |||
| Sex | ||||||
| Male | 24 | 53 | 27 | 60 | 51 | 57 |
| Female | 21 | 47 | 18 | 40 | 39 | 43 |
| WHO PS | ||||||
| 0 | 22 | 49 | 22 | 49 | 44 | 49 |
| 1 | 22 | 49 | 20 | 44 | 42 | 47 |
| 2 | 1 | 2 | 3 | 7 | 4 | 4 |
| Type of surgery | ||||||
| Whipple | 28 | 62 | 23 | 51 | 51 | 57 |
| PPPD | 15 | 34 | 20 | 45 | 35 | 39 |
| Unknown | 2 | 4 | 2 | 4 | 4 | 4 |
| Mesentericoportal resection | ||||||
| No | 34 | 75 | 31 | 69 | 65 | 72 |
| Yes | 8 | 18 | 13 | 29 | 21 | 23 |
| Unknown | 3 | 7 | 1 | 2 | 4 | 5 |
| CA 19-9 (baseline) | ||||||
| Normal | 29 | 64 | 23 | 51 | 52 | 58 |
| Above normal | 14 | 31 | 19 | 42 | 33 | 37 |
| Unknown | 2 | 5 | 3 | 7 | 5 | 5 |
| Number of lymph nodes examined | ||||||
| Median | 12 | 12 | 12 | |||
| Range | 5-36 | 3-28 | 3-36 | |||
| pT status | ||||||
| 1 | 3 | 7 | 2 | 4 | 5 | 6 |
| 2 | 9 | 20 | 8 | 18 | 17 | 19 |
| 3 | 32 | 71 | 30 | 67 | 62 | 69 |
| 4 | 0 | 4 | 9 | 4 | 4 | |
| Unknown | 1 | 2 | 1 | 2 | 2 | 2 |
| pN status | ||||||
| pN0 | 13 | 29 | 14 | 30 | 27 | 30 |
| pN1a | 12 | 27 | 9 | 20 | 21 | 23 |
| pN1b | 19 | 42 | 22 | 50 | 41 | 46 |
| pNx | 1 | 2 | 0 | 0 | 1 | 1 |
| pM status | ||||||
| pM0 | 44 | 98 | 44 | 98 | 88 | 98 |
| pM1 | 0 | 1 | 2 | 1 | 1 | |
| Unknown | 1 | 2 | 0 | 1 | 1 | |
| Vascular invasion + | 15 | 33 | 18 | 40 | 33 | 37 |
| Lymphatic invasion + | 15 | 33 | 17 | 38 | 32 | 36 |
| Perineural invasion + | 33 | 73 | 34 | 76 | 67 | 74 |
| Grade | ||||||
| 1 | 10 | 22 | 12 | 27 | 22 | 24 |
| 2 | 20 | 44 | 26 | 58 | 46 | 51 |
| 3 | 10 | 22 | 6 | 13 | 16 | 18 |
| Unknown | 5 | 12 | 1 | 2 | 6 | 7 |
| Margin of resection | ||||||
| R0 | 43 | 96 | 44 | 98 | 87 | 97 |
| Rx | 2 | 4 | 1 | 2 | 3 | 3 |
| Baseline CA 19-9 levels | ||||||
| Normal | 29 | 64,4 | 23 | 51 | 52 | 58 |
| Above normal | 14 | 31 | 19 | 42 | 33 | 37 |
| Unknown | 2 | 4§ | 3 | 7 | 5 | 5 |
Abbreviations: PS, performance status; PPPD pylorus-preserving pancreaticoduodenectomy; CA 19-9, carbohydrate antigen 19-9; +, presence of.
Treatment Delivery
In the control arm, two patients did not start treatment, and data are missing for a third patient. In the experimental arm, one patient did not start treatment, and one was allocated to the wrong treatment arm by mistake. For patients who started the allocated treatment (42:43), the gemcitabine median relative dose intensity was 88.6% in the control arm and 87.3% in the experimental arm. The median dose intensity was 664.6 mg/m2 × week × cycle for the control arm and 480.6 mg/m2 × week × cycle for the experimental arm. Thirty-two (76%) and 34 (79%) patients in the control and experimental arms, respectively, received > 70% of the planned dose.
Radiation therapy was started in 36 (80%) of 45 patients, nine of them being not irradiated for several reasons, mainly the patient's refusal (two), rapid progression (two), early postoperative death (one), gemcitabine-alone toxicity (two), and altered liver tests (one; Fig 1 and Table 2).
Table 2.
Radiation Therapy
| Total Dose (Gy) | Gemcitabine + Radiotherapy(n = 45) |
|
|---|---|---|
| No. of Patients | % | |
| 25.2 in 14 fractions (postoperative occlusion) | 1 | 2.2 |
| 48.7 in 27 fractions (scaphogia) | 1 | 2.2 |
| 50.4 in 28 fractions | 28 | 62.2 |
| 54 in 30 fractions (protocol mistake) | 5 | 11.1 |
| Unknown (radiation therapy received) | 1 | 2.2 |
| Did not receive radiation therapy | 9 | 20.0 |
| Refusal | 2 | |
| Rapid progression | 2 | |
| Postoperative death | 1 | |
| Gemcitabine toxicity | 2 | |
| Wrong arm | 1 | |
| Altered liver tests | 1 | |
NOTE. Temporary treatment interruption (≤ 4 days) was observed in 24 patients (68.6%).
Toxicity
Globally, the experimental treatment was well tolerated and no deaths due to toxicity were reported. Main acute toxicities were hematologic, fatigue, nausea/vomiting, diarrhea, and gastritis and were slightly more frequent in the experimental arm (Table 3).
Table 3.
No. of Patients With Relevant Acute Toxicities (CTC version 2.0)
| Toxicity | Grade | Treatment |
Total(N = 85) |
||||
|---|---|---|---|---|---|---|---|
| Gemcitabine + Radiotherapy(n = 43) |
Gemcitabine Alone(n = 42) |
||||||
| No. | % | No. | % | No. | % | ||
| WBC | All | 39 | 38 | 77 | |||
| 3 | 7 | 16 | 6 | 14 | 13 | 15 | |
| 4 | 0 | 0 | 0 | ||||
| Neutrophils | All | 32 | 33 | 65 | |||
| 3 | 12 | 28 | 15 | 36 | 27 | 32 | |
| 4 | 2 | 5 | 3 | 7 | 5 | 6 | |
| Platelets | All | 20 | 18 | 38 | |||
| 3 | 1 | 2 | 0 | 1 | 1 | ||
| 4 | 0 | 0 | 0 | ||||
| Hemoglobin | All | 42 | 40 | 83 | |||
| 3 | 2 | 5 | 0 | 2 | 2 | ||
| 4 | 1 | 2 | 0 | 1 | 1 | ||
| SGPT | All | 30 | 29 | 59 | |||
| 3 | 5 | 12 | 5 | 12 | 10 | 12 | |
| 4 | 0 | 0 | 0 | ||||
| Fatigue | All | 31 | 28 | 59 | |||
| 3 | 3 | 7 | 2 | 5 | 5 | 6 | |
| 4 | 0 | 0 | 0 | ||||
| Fever | All | 15 | 12 | 27 | |||
| 3 | 3 | 7 | 0 | 0 | 3 | 4 | |
| 4 | 0 | 0 | 0 | ||||
| Weight loss | All | 10 | 6 | 16 | |||
| 3 | 1 | 2 | 0 | 0 | 1 | 1 | |
| 4 | 0 | 0 | 0 | ||||
| Anorexia | All | 21 | 8 | 29 | |||
| 3 | 1 | 2 | 0 | 1 | 1 | ||
| 4 | 1 | 2 | 0 | 0 | 1 | 1 | |
| Nausea | All | 27 | 24 | 51 | |||
| 3 | 1 | 2 | 0 | 1 | 1 | ||
| 4 | 0 | 0 | 0 | ||||
| Vomiting | All | 20 | 8 | 28 | |||
| 3 | 0 | 0 | 0 | ||||
| 4 | 1 | 2 | 0 | 1 | 1 | ||
| Gastritis | All | 2 | 0 | 2 | |||
| 3 | 1 | 2 | 0 | 1 | 1 | ||
| 4 | 1 | 2 | 0 | 1 | 1 | ||
| Diarrhea | All | 26 | 18 | 44 | |||
| 3 | 0 | 0 | 0 | ||||
| 4 | 1 | 2 | 0 | 1 | 1 | ||
| Hemorrhage | All | 1 | 2 | 3 | |||
| 3 | 0 | 0 | 1 | 2 | 1 | 1 | |
| 4 | 1 | 2 | 0 | 0 | 1 | 1 | |
| Other GI toxicity | All | 17 | 14 | 31 | |||
| 3 | 0 | 0 | 0 | ||||
| 4 | 1 | 2 | 0 | 0 | 1 | 1 | |
| Other toxicity | All | 30 | 22 | 52 | |||
| 3 | 7 | 16 | 2 | 5 | 9 | 11 | |
| 4 | 1 | 2 | 1 | 2 | 2 | 2 | |
Abbreviations: CTC, Common Toxicity Criteria; SGPT, serum glutamic- pyruvic transaminase.
For the predefined coprimary toxicity end point (grade 4 WBC, platelet, hemoglobin, vomiting, or diarrhea toxicities), the rate of occurrences was zero (0%) of 42 in the control arm and two (4.7%) of 43 in the experimental arm (80% CI, 1.2% to 11.9%; 95% CI, 0.5% to 15.8%). The upper bound of the 80% CI is below the protocol-specified threshold of 15% for the experimental arm.
In the experimental arm, three patients who received CRT, experienced grade 3–related late toxicities consisting of anorexia and gastritis (one), epigastric pain (one), and insulin requirement (one). There were no related late toxicities in the control arm.
Completion of the Treatment According to Protocol (coprimary end point)
Of the 90 randomly assigned patients, 33 (73.3%) of 45 completed treatment according to protocol in the experimental arm (80% CI, 63.1% to 81.9%; 95% CI, 58.1% to 85.4%) and 39 (86.7%) of 45 completed treatment according to protocol in the control arm (95% CI, 73.2% to 95.0%). The lower bound of the 80% CI excludes the protocol-specified threshold of 60%.
Secondary End Points
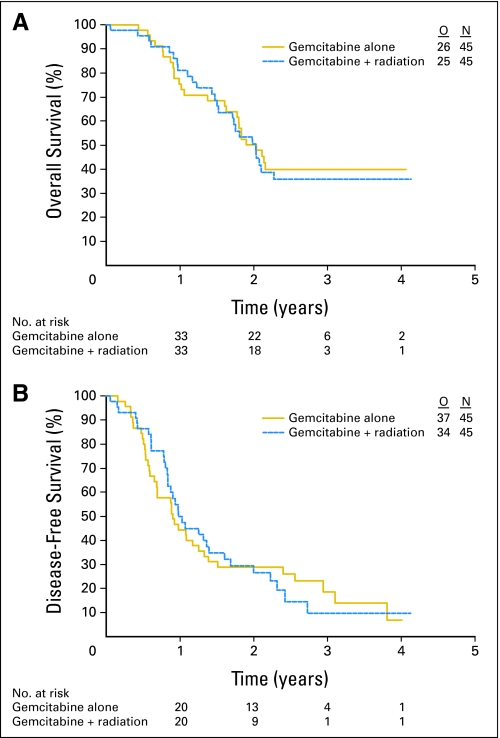
Median OS was 24.4 months (95% CI, 21.5 to ∞ months) in the control arm and 24.3 months (20.5 to ∞ months) in the experimental arm (Fig 2A); the 2-year survival rate was 50.2% (95% CI, 34.8% to 63.8%) and 50.6% (95% CI, 34.3% to 64.8%), respectively. When restricted to the treated population, results were similar, with 2-year survival rates of 53.8% (95% CI, 37.6% to 67.6%) in the control arm and 53.8% (95% CI, 35.4% to 69.1%) in the experimental arm.
Fig 2.
Kaplan-Meier curves for overall survival (A) and disease-free survival (B). O, No. of observed events; N, No. of patients.
Median DFS was 10.9 months (95% CI, 8.3 to 16.0 months) in the control arm and 11.8 months (95% CI, 10.1 to 19.3 months) in the experimental arm (Fig 2B). In the treated population, the medians were 10.9 months (95% CI, 8.3 to 16.7 months) and 12.4 months (95% CI, 10.1 to 19.3 months), respectively.
The rate of local recurrence alone as first progression was notably lower in the experimental arm (11% v 24%). The rate of simultaneous local and distant progression as first progression was 13% in the control arm versus 20% in the experimental arm, and the rate of distant progression only was quite similar in both arms (40% in the control arm and 42% in the experimental arm).
DISCUSSION
Postoperative adjuvant therapy remains a challenge in pancreatic cancer for several reasons. Long-term survival data after surgical resection are disappointing and only slightly improved by adjuvant therapy.8–12 Both local and distant recurrences are frequent and difficult to control.3 Combining chemotherapy and radiation therapy in adjuvant therapy has shown conflicting results over the last two decades, and the true impact of postoperative CRT remains questionable. Chemotherapy based on FU (plus folinic acid) or gemcitabine is now advocated as standard adjuvant therapy in Europe.8–10 In the United States, FU-based CRT is still widely proposed on the basis of the nonrandomized experience of high-volume expertise centers6,7 and the small randomized GITSG trial.5 Subsequent trials from European organizations8,11 could not confirm the GITSG results. These trials can be criticized for lack of quality control of radiotherapy and suboptimal FU-based CRT schedules according to current standards.
Our multicenter study aimed to evaluate a modern regimen of radiation therapy, applied in the framework of expert multicenters, combined with the currently most active drug in advanced pancreatic cancer—gemcitabine. Gemcitabine is also known to be a good radiosensitizer and was reported to be easily combined with radiation in pancreatic cancer, both in neoadjuvant and locally advanced disease16–19; we also generated phase II data showing a good feasibility and toxicity profile for combining radiation with weekly gemcitabine.20,21
This multicenter phase II trial was designed to investigate feasibility and toxicity of this new regimen in the postoperative adjuvant setting before continuing with a large phase III trial. Before knowing the results from the CONKO-001 trial, we chose to treat our patients with 4 months of gemcitabine to provide similar periods of postoperative therapy in both arms. The results show that the combination of gemcitabine and CRT is feasible and only slightly more toxic than gemcitabine alone. By contrast to the ESPAC-1–derived results, well-conducted CRT was not shown to be deleterious here; poor results from the ESPAC-1 trial could be explained by the low total dose regimen that was used and the poor quality control for radiation delivery in many centers, possibly leading to treatment deviation.8 Moreover, the good tolerability we observed is probably due to the sequence of starting initially with gemcitabine alone and then proceeding with CRT when the patient had shown good postoperative recovery and absence of early disease progression. This sequential concept has been suggested as clinically appropriate in the locally advanced setting.23 In view of this experience, the sequence with CRT in the end portion of the adjuvant treatment will be evaluated in the joint RTOG-0848/EORTC-40084-22084 phase III study.
Our randomized phase II study did not reveal DFS and survival benefits. Obviously, the current phase II design is not appropriate to detect such differences, but the DFS seems disappointing even if most of the tumors were pT3N1. Of note, we did find a lower rate of local recurrence as first progression in the CRT arm, and the survival data we observed in the experimental arm, although not methodologically comparable to that in the control arm, may suggest that CRT, without any deleterious effects, could lead to a similar effect, as reported with only adjuvant chemotherapy in other studies.8–10,12 The potential effect of CRT on local recurrence of pancreatic cancer could possibly be underestimated in our study because we included only patients with an R0 resection. Yet R1 resections are quite common and often underestimated, and they pose an important prognostic factor in pancreatic cancer.24–26 It is likely that achieving local control is relatively more important in patients with an R1 resection and that the impact of CRT should thus be evaluated after R1 resection. Therefore, in the above-mentioned joint RTOG-EORTC phase III study, patients with R0 or R1 disease are both eligible. Again, we can hypothesize that adding CRT to full-dose adjuvant gemcitabine therapy could offer a more beneficial multimodal approach after resection by optimizing local control.
Finally, selection of patients who will benefit from gemcitabine-based adjuvant therapy may be improved by the use of specific biomarkers, as recently shown.27,28 These markers need to be prospectively incorporated in future adjuvant trials. Similarly, patterns of therapy failure for treatment of pancreatic cancer can be represented and therefore predicted by distinct genetic subtypes, notably DPC4 status, that can be used to stratify patients for local control versus systemic therapy.29
In conclusion, our randomized phase II trial shows that adjuvant gemcitabine, followed by gemcitabine-based modern CRT is feasible and only slightly more toxic than gemcitabine alone. In view of the remaining uncertainty about the role of CRT as a complement to systemic therapy, in particular after R1 resection, such multimodal approach should be further investigated in a phase III trial.
Acknowledgment
We thank all the patients who kindly agreed to participate into this study and the local investigators from all participating institutions for their involvement. Apart from the coauthors, they are as follows: Dr R. Weytjens, Allgemeine Ziekenhuis Sint-Augustinus, Wilrijk; Dr Th. Conroy, Centre Alexis Vautrin, Vandoeuvre lez Nancy; Dr J.F. Seitz, Centre Hospitalier Universitaire de La Timone, Marseille; Dr F. Desseigne, Centre Leon Berard, Lyon; Dr J. Taieb, Centre Hospitalier Universitaire Pitie-Salpetrière, Paris; Dr I. Borbath, Cliniques Universitaires Saint-Luc, Brussels; Dr L. Mineur, Hôpital Sainte Catherine, Avignon; Dr E.A. Pariente, Centre Hospitalier de Pau; Dr N. Stremsdoerfer, Centre Hospitalier Pierre Oudot, Bourgoin Jailleu; Dr R. Faroux, Centre Hospitalier de La Roche-sur-Yon; Dr J. Balosso, Centre Hospitalier Régional Universitaire de Grenoble; Dr D. Arsene, Centre Hospitalier Régional Universitaire de Caen; Dr O. Dupuis, Clinique Victor Hugo, Le Mans; Dr B. Duclos, Hôpitaux Universitaires de Strasbourg; Dr A. Roth, Hôpital Cantonal Universitaire de Genève; Dr T. Aparicio, Hôpital Bichat, Paris; Dr A. Confente, Hôpital De Jolimont, Haine-Saint-Paul; Dr B. Lecaille, Polycliniques de Bordeaux; and Dr A. Kuten, Rambam Medical Center, Haïfa. We also thank our data managers at European Organisation for Research and Treatment of Cancer (EORTC), Marie-Ange Lentz and Larissa Polders, and our Pharmacovigilance manager, Sara Meloen.
Appendix
Radiotherapy Procedure
The planning target volume (PTV) was defined as clinical target volume plus a safety margin of 1 cm in all transverse directions and 2.0 cm cranio-caudally to allow for breathing motion (PTV1). A dose of 50.4 Gy in fractions of 1.8 Gy five times per week was prescibed at the isocenter of the PTV. At 45 Gy, the PTV margins were reduced ventrally, bilaterally, and posteriorly to 5 mm and cranio-caudally to 1 cm. A dose heterogeneity of ± 5% of the prescribed dose within the PTV was allowed. The 95% isodose had to completely encompass the PTV. The minimum photon energy required was 6 MV.
The following organs at risk had to be delineated: spinal cord, liver, and kidneys. A maximum dose equivalent to 45 Gy in 1.8-Gy fractions was allowed to the spinal cord. The maximum allowed liver dose was defined as equivalent to 30 Gy in 2-Gy fractions for the whole liver, or 50 Gy in 2-Gy fractions for one third of the liver. One whole kidney should not receive more than 23.4 Gy in 1.8-Gy fractions or the equivalent. One-third of one kidney was allowed to receive the full protocol dose provided the contralateral kidney was outside the radiation fields. No specific radiation technique was prescribed as long as the target volume definitions were fulfilled. Generally, multiple conformal beams (three to five beams) were used to apply the prescribed doses to the PTV. Customized blocks or multileaf settings were used to minimize the radiation dose to the normal tissues and organs at risk. Dose volume histograms of PTV, kidneys, liver, and spinal cord were mandatory to select the optimal dose distribution.
Dose Modifications
In both arms, chemotherapy dose reductions and interruptions were implemented on the basis of weekly assessments of blood count before administration of chemotherapy and grading of nonhematologic toxicities (Appendix Table A1).
Omitted doses of gemcitabine were not replaced and no re-increasing of doses was permitted. In case of febrile neutropenia or Common Toxicity Criteria grade 4 thrombocytopenia with bleeding, the treatment was discontinued.
In the experimental arm, in case of grade 4 toxicity, both gemcitabine and radiotherapy were discontinued. If toxicity was severe enough to require a treatment break from radiotherapy, both radiotherapy and gemcitabine were held until toxicity had declined to grade 2 or lower. If the combination therapy had to be delayed by more than 2 weeks, the treatment was stopped completely.
Table A1.
Dose Modifications
| Adjustment | Hematologic Toxicities |
Nonhematologic Toxicities CTC Grade | % Dose of Chemotherapy | |
|---|---|---|---|---|
| ANC ×109/L | Platelets ×109/L | |||
| Gemcitabine in the control arm and before combination therapy in the experimental arm | 1-< 1.5 | And/or 75-99 | And/or 3 | 75 |
| < 1 | And/or < 50 | And/or 4 | Hold; restart at full recovery with 50% dose | |
| Gemcitabine and radiation during combination therapy | 0.5-< 1 | And/or 50-99 | And/or 3 | Hold; restart at recovery (grade ≤ 2 toxicity) with 50% dose* |
Abbreviations: ANC, absolute neutrophil count; CTC, Common Toxicity Criteria.
The decision to hold both radiotherapy and gemcitabine instead of just gemcitabine was left to the investigator's discretion.
Footnotes
Supported in part by an educational grant from Eli Lilly, by Grants No. 5U10 CA11488-30 through 5U10CA011488-40 from the National Cancer Institute, and by a donation from the Federation Belge Contre le Cancer through the European Organisation for Research and Treatment of Cancer Charitable Trust. Study drugs were provided by Eli Lilly.
Presented in part as poster discussion at the 44th Annual Meeting of the American Society of Clinical Oncology (ASCO), May 30-June 3, 2008, Chicago, IL, and the 45th Annual Meeting of ASCO, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00064207.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jean-Luc Van Laethem, Pascal Hammel, Geertjan Van Tienhoven, Laurence Collette, Volker Budach, Manfred Lutz, Eric Van Cutsem, Karin Haustermans
Administrative support: Michel Praet
Provision of study materials or patients: Jean-Luc Van Laethem, Pascal Hammel, Françoise Mornex, David Azria, Geertjan Van Tienhoven, Philippe Vergauwe, Marc Peeters, Marc Polus, Volker Budach, Manfred Lutz, Karin Haustermans
Collection and assembly of data: Jean-Luc Van Laethem, Michel Praet, Eric Van Cutsem
Data analysis and interpretation: Jean-Luc Van Laethem, Pascal Hammel, Murielle Mauer, Laurence Collette, Eric Van Cutsem, Karin Haustermans
Manuscript writing: Jean-Luc Van Laethem, Geertjan Van Tienhoven, Marc Peeters, Murielle Mauer, Eric Van Cutsem, Karin Haustermans
Final approval of manuscript: Jean-Luc Van Laethem, Pascal Hammel, Françoise Mornex, David Azria, Geertjan Van Tienhoven, Philippe Vergauwe, Marc Peeters, Marc Polus, Michel Praet, Murielle Mauer, Laurence Collette, Volker Budach, Manfred Lutz, Eric Van Cutsem, Karin Haustermans
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: Is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 3.Kayahara M, Nagakawa T, Ueno K, et al. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::aid-cncr2820720710>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Kalser MH, Ellenberg SS. Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 6.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: The Mayo clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 7.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the John Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus P, Riess H, Post S, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC) J Clin Oncol. 2008;26(suppl; abstr LBA4504):214s. [Google Scholar]
- 11.Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: Long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos J, Büchler M, Stocken DD, et al. ESPAC-3(v2): A multicenter, international, open-label, randomized controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(suppl; abstr LBA4505):203s. [Google Scholar]
- 13.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence TS, Chang EY, Hahn TM, et al. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 15.Milas L, Fujii T, Hunter N, et al. Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer Res. 1999;59:107–114. [PubMed] [Google Scholar]
- 16.Mason KA, Milas L, Hunter NR, et al. Maximizing therapeutic gain with gemcitabine and fractionated radiation. Int J Radiat Oncol Biol Phys. 1999;44:1125–1135. doi: 10.1016/s0360-3016(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 17.Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–2212. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 19.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 20.Van Laethem JL, Demols A, Gay F, et al. Postoperative adjuvant gemcitabine and concurrent radiation after curative resection of pancreatic head carcinoma: A phase II study. Int J Radiat Oncol Biol Phys. 2003;56:974–980. doi: 10.1016/s0360-3016(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 21.Demols A, Peeters M, Polus M, et al. Adjuvant gemcitabine and concurrent continuous radiation (45 Gy) for resected pancreatic head carcinoma: A multicenter Belgian Phase II study. Int J Radiat Oncol Biol Phys. 2005;62:1351–1356. doi: 10.1016/j.ijrobp.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51:1372–1383. [PubMed] [Google Scholar]
- 23.Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 24.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 25.Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 26.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 28.Maréchal R, Mackey JR, Lai R, et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913–2919. doi: 10.1158/1078-0432.CCR-08-2080. [DOI] [PubMed] [Google Scholar]
- 29.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]