Abstract
Purpose
To determine whether the place of death for patients with cancer is associated with patients' quality of life (QoL) at the end of life (EOL) and psychiatric disorders in bereaved caregivers.
Patients and Methods
Prospective, longitudinal, multisite study of patients with advanced cancer and their caregivers (n = 342 dyads). Patients were followed from enrollment to death, a median of 4.5 months later. Patients' QoL at the EOL was assessed by caregiver report within 2 weeks of death. Bereaved caregivers' mental health was assessed at baseline and 6 months after loss with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and the Prolonged Grief Disorder interview.
Results
In adjusted analyses, patients with cancer who died in an intensive care unit (ICU) or hospital experienced more physical and emotional distress and worse QoL at the EOL (all P ≤ .03), compared with patients who died at home with hospice. ICU deaths were associated with a heightened risk for posttraumatic stress disorder, compared with home hospice deaths (21.1% [four of 19] v 4.4% [six of 137]; adjusted odds ratio [AOR], 5.00; 95% CI, 1.26 to 19.91; P = .02), after adjustment for caregivers' preexisting psychiatric illnesses. Similarly, hospital deaths were associated with a heightened risk for prolonged grief disorder (21.6% [eight of 37] v 5.2% [four of 77], AOR, 8.83; 95% CI, 1.51 to 51.77; P = .02), compared with home hospice deaths.
Conclusion
Patients with cancer who die in a hospital or ICU have worse QoL compared with those who die at home, and their bereaved caregivers are at increased risk for developing psychiatric illness. Interventions aimed at decreasing terminal hospitalizations or increasing hospice utilization may enhance patients' QoL at the EOL and minimize bereavement-related distress.
INTRODUCTION
Patients with advanced cancer are receiving increasingly aggressive care at the end of life (EOL).1–5 Although most patients with cancer prefer to die at home,6 36% die in a hospital and 8% die in an intensive care unit (ICU).7,8 Nearly 25% of Medicare expenditures are spent on intensive care in the final month of life,9–11 despite limited evidence of improved patient outcomes.12 A few studies have found that hospice care is associated with better patient quality of life (QoL) at the EOL13,14 and lower rates of major depressive disorder among bereaved caregivers.15 Research is needed, however, to examine prospectively whether patients' place of death is associated with QoL at the EOL and caregivers' bereavement adjustment.
In 1995, SUPPORT (the Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment) found that 50% of hospitalized patients experienced moderate to severe pain at the EOL.16 Another study found that 55% to 75% of patients with cancer in an ICU reported moderate to severe pain, discomfort, or anxiety, despite the routine integration of palliative care services.17 Other research suggests that family members of critically ill patients experience greater psychological distress relative to the general population, including anxiety, depression, posttraumatic stress, and prolonged grief.18–20
To date, only one study has used the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID), a sensitive and specific tool for diagnosing psychiatric illness, to examine bereaved family members' mental health.21 This study did not measure posttraumatic stress disorder (PTSD), however, and could not account for caregivers' preexisting psychiatric illnesses as a result of its cross-sectional design.
The first aim of this prospective, longitudinal, cohort study was to examine associations between the place of death of patients with cancer and their QoL at the EOL. We hypothesized that ICU deaths would be associated with worse QoL compared with hospital or home deaths. Our second aim was to examine associations between patients' place of death and their bereaved caregivers' risk of developing psychiatric illnesses. We hypothesized that caregivers of patients who die in an ICU would witness more trauma (ie, events “involving death, injury, or a threat to the personal integrity of another person” evoking feelings of “intense fear, helplessness or horror,” Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criterion A1 and A2 for PTSD) than caregivers of patients who died elsewhere as a result of the often frightening nature of aggressive, life-prolonging care, which would heighten the risk for onset of PTSD after an ICU death.22
PATIENTS AND METHODS
Coping with Cancer was a prospective, longitudinal, multisite psychiatric epidemiologic study of terminally ill patients with cancer and their informal caregivers designed to determine the rates, causes, and consequences of psychiatric illness in terminally ill patients with cancer and their bereaved caregivers.13
Patients were recruited from September 2002 to August 2008 from seven outpatient sites: Yale Cancer Center, the West Haven Veterans' Affairs Connecticut Comprehensive Cancer Clinic, Parkland Hospital, Simmons Comprehensive Cancer Center, Memorial Sloan-Kettering Cancer Center, Dana-Farber/Partners Cancer Center, and New Hampshire Oncology-Hematology. The institutional review boards of participating sites approved all study procedures.
Eligibility criteria included diagnosis of advanced cancer (distant metastases and disease refractory to first-line chemotherapy), age at least 20 years, presence of an informal caregiver, and clinic staff/interviewer assessment that the patient had adequate stamina to complete the interview. Patient–caregiver dyads in which either the patient or caregiver refused to participate, met criteria for dementia or delirium (by neuro-behavioral cognitive status examination), or did not speak English or Spanish were excluded.
Of 993 eligible patients, 718 (72.3%) enrolled. Sociodemographic characteristics of participants and nonparticipants did not differ, except that participants were more likely to be Hispanic (12.1% v 5.8%; P = .005). For this analysis assessing place of death, we restricted our sample to patients who died by August 2008 (n = 414). We excluded patients who died in miscellaneous other settings (ie, nursing homes, inpatient hospices, in transit, other, or unknown; total n = 72) because numbers were too small for analyses, or who had missing data on more than seven variables (n = 9). As expected, deceased patients had worse performance status and were more often younger, nonwhite, unmarried, less well educated, and uninsured than the full cohort (all P ≤ .05). At follow-up 6 months after loss, 93 bereaved caregivers could not be reached; these subjects did not differ significantly from assessed bereaved caregivers on baseline mental health measures or sociodemographic characteristics, except they were more likely to be nonwhite (35.8% v 21.4%; P = .004). All participants provided written informed consent.
Patients and caregivers were interviewed at baseline in English or Spanish and received a $25 payment. A chart review was performed at enrollment and after death. Within 2 weeks of death, a postmortem questionnaire was administered to the caregiver most closely involved in the patient's care during the last week of life. The caregiver assessed at baseline was interviewed again 6 months after loss. This time point was chosen so caregivers would likely be beyond a state of acute grief,23 but close enough to the death to avoid recall bias.
Outcomes
Patient QoL at the EOL.
During the postmortem interview, caregivers were asked about patients' QoL at the EOL: “In your opinion, how would you rate the overall quality of the patients' last week of life?”13; caregivers responded to a Likert scale from 0 (“worst possible”) to 10 (“best possible”). Caregivers also rated the level of physical and psychological distress separately. Patients' QoL has been previously correlated with the validated Quality of Dying and Death scale24 and bereaved caregivers' QoL, self-reported health, and physical functioning 6 months after loss.13
Caregivers' mental health.
The SCID, a sensitive and specific tool for diagnosing psychiatric illness, was used at baseline and 6 months after loss to assess whether caregivers met established criteria for psychiatric disorders.22,25 The validated Prolonged Grief Disorder (PGD) scale assessed caregivers' preloss grief and identified caregivers with intense, disabling grief 6 months after loss.26
Primary Predictor
Place of death.
The patients' place of death (ICU, hospital, home with hospice, or home without hospice) was determined through chart review and caregiver interviews. Patients who died at home with hospice services were analyzed separately from patients who died at home without hospice because hospice is associated with better patient QoL at EOL and less depression in bereaved caregivers.13–15
Additional Covariates
Patient and caregiver factors found in the literature to be associated with site of death, EOL medical care, and caregivers' bereavement adjustment13–21,26–44 were examined as potential confounders. These include the following:
Sociodemographic characteristics.
Patients and caregivers reported their sex, age, race/ethnicity, marital status, household size, insurance status, and education.
Patient health.
Patients' cancer type, Karnofsky score,30 Charlson Comorbidity Index,31 and medical care was documented at baseline with input from the treating physician. Patient QoL was assessed with the McGill Quality of Life Index.32
Terminal illness acknowledgment.
Patients were asked to “describe your current health status” with response options of “relatively healthy,” “seriously ill but not terminally ill,” or “seriously and terminally ill.” Patients responding “seriously and terminally ill” were coded as “understands illness is terminal.” This measure is associated with higher rates of do-not-resuscitate orders and hospice use.33
Treatment preferences.
Patients' preferences for life-extending care were examined with a previously validated measure34–36: “If you could choose, would you prefer: (1) treatment that focused on extending life as much as possible, even if it meant more pain and discomfort, or (2) care that focused on relieving pain and discomfort as much as possible, even if that meant not living as long?”
Positive religious coping.
The validated Brief Religious Coping Scale (RCOPE) was used to identify patients who use positive religious coping (eg, “seeking God's love and care”) to cope with their cancer diagnosis because this is associated with more intensive care near death.37
EOL discussions, doctor-patient relationships, and advance care planning.
At baseline, patients were asked (yes/no), “Have you and your doctor discussed any particular wishes you have about the care you would want to receive if you were dying?” A close doctor–patient relationship was defined as one where patients trusted and respected their doctor, felt respected and “seen as a whole person,” and were very comfortable asking questions about their care.38 Patients were also asked if they had a do-not-resuscitate order.
Caregiver health and functional status.
Caregivers' baseline QoL was assessed with a single-item summary measure of the Medical Outcomes Study Short-Form Health Survey, including all eight subscales.39 The Index of Activities of Daily Living measured caregivers' functional status.40
Caregiver burden and support.
The Caregiving Burden Scale and Covinsky Family Impact Survey assessed caregivers' burden and satisfaction.41,42 The Stressful Caregiving Response to Experiences of Dying scale examined caregivers' exposure to traumatic experiences (eg, witnessing severe pain/discomfort).43 The Interpersonal Support Evaluation List assessed caregivers' social support.44
Statistical Analysis
t tests, analysis of variance, χ2, and Fisher's exact test statistics were used, as appropriate, to identify confounders (ie, patient/caregiver characteristics associated with both the predictor [place of death] and outcomes [patients' QoL at the EOL or caregivers' mental health]). A log-rank test was used to determine whether the groups differed significantly in survival.
Analysis of covariance models were used to examine relationships between patients' place of death and (1) QoL at the EOL and (2) physical/emotional distress. For each model, every variable associated (P < .20) with patients' place of death was entered and retained if significant (P < .05) while controlling for other confounders.
Multivariable logistic regression models examined associations between caregivers' psychiatric illnesses at baseline and 6 months after loss. Next, multivariable logistic regression models estimated the effect of patients' place of death on bereaved caregivers' mental health, adjusting for caregivers' baseline psychiatric illnesses and significant confounders, using home death with hospice services as a reference. Firth's penalized maximum likelihood estimation method was used to minimize bias in parameter estimates because the outcomes were rare in some instances. Statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient and Caregiver Characteristics
The cohort consisted of 333 patients with advanced cancer who died a median of 4.5 months after enrollment. Patients' baseline sociodemographic, clinical, and psychosocial characteristics are presented in Table 1. In this study, 58.6% of patients died at home with hospice services, 7.5% died at home without hospice services, 25.5% died in a hospital, and 8.4% died in an ICU.
Table 1.
Patients' Baseline Characteristics by Place of Death
| Characteristic | Total (N = 333) |
Place of Death |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Home Without Hospice (n = 25) |
Home With Hospice (n = 195) |
Hospital (n = 85) |
Intensive Care Unit (n = 28) |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age, years | .005 | ||||||||||
| Mean | 58.0 | 63.4 | 58.6 | 57.0 | 51.5 | ||||||
| SD | 12.8 | 16.2 | 11.8 | 12.9 | 13.1 | ||||||
| Sex, male | 185 | 55.6 | 13 | 52.0 | 103 | 52.8 | 48 | 56.5 | 21 | 75.0 | .17 |
| Race/ethnicity | .01 | ||||||||||
| White, non-Hispanic | 216 | 64.9 | 14 | 56.0 | 129 | 66.2 | 62 | 72.9 | 11 | 39.3 | |
| Black, non-Hispanic | 56 | 16.8 | 4 | 16.0 | 31 | 15.9 | 9 | 10.6 | 12 | 42.9 | |
| Hispanic | 54 | 15.9 | 7 | 28.0 | 32 | 16.4 | 11 | 12.9 | 4 | 14.3 | |
| Other | 8 | 2.4 | 0 | 0.0 | 4 | 2.1 | 3 | 3.5 | 1 | 3.6 | |
| Married | 197 | 59.2 | 12 | 48.0 | 116 | 59.5 | 51 | 60.0 | 18 | 64.3 | .65 |
| Education, years | .07 | ||||||||||
| Mean | 12.6 | 10.8 | 12.6 | 13.2 | 12.5 | ||||||
| SD | 4.0 | 5.1 | 4.1 | 3.4 | 2.8 | ||||||
| Health insurance | 194 | 60.0 | 14 | 56.0 | 107 | 56.4 | 59 | 71.1 | 15 | 53.6 | .12 |
| Site | .0009 | ||||||||||
| Yale Cancer Center | 58 | 17.4 | 4 | 16.0 | 20 | 10.6 | 26 | 30.6 | 8 | 28.6 | |
| West Haven VA Cancer Center | 14 | 4.2 | 0 | 0.0 | 6 | 3.1 | 8 | 9.4 | 0 | 0.0 | |
| Memorial Sloan-Kettering Cancer Center | 26 | 7.8 | 2 | 8.0 | 14 | 7.2 | 9 | 10.6 | 1 | 3.6 | |
| Simmons Comprehensive Cancer Center | 31 | 9.3 | 3 | 12.0 | 18 | 9.2 | 8 | 9.4 | 2 | 7.1 | |
| Parkland Hospital | 140 | 42.0 | 10 | 40.0 | 91 | 46.7 | 24 | 28.2 | 15 | 53.6 | |
| Dana-Farber/Partners Cancer Care | 8 | 2.4 | 0 | 0.0 | 6 | 3.1 | 1 | 1.2 | 1 | 3.6 | |
| New Hampshire Oncology-Hematology | 56 | 16.8 | 6 | 24.0 | 41 | 20.5 | 9 | 10.6 | 1 | 3.6 | |
| Positive religious coping† | 161 | 56.3 | 14 | 63.6 | 93 | 54.4 | 34 | 50.8 | 20 | 76.9 | .11 |
| Cancer type | .47 | ||||||||||
| Breast | 38 | 11.64 | 0 | 0.0 | 26 | 13.3 | 10 | 11.8 | 2 | 7.1 | |
| GI | 126 | 37.8 | 9 | 36.0 | 78 | 40.0 | 30 | 35.3 | 9 | 32.1 | |
| Lung | 69 | 20.7 | 7 | 28.0 | 34 | 17.4 | 19 | 22.4 | 9 | 32.1 | |
| Other cancers‡ | 100 | 30.0 | 9 | 36.0 | 57 | 29.2 | 26 | 30.6 | 8 | 28.6 | |
| Treatment | .0001 | ||||||||||
| Chemotherapy or clinical trial | 184 | 57.3 | 10 | 40.0 | 95 | 50.5 | 55 | 68.8 | 24 | 85.7 | |
| Radiation | 23 | 7.3 | 4 | 16.0 | 13 | 7.1 | 4 | 5.1 | 2 | 7.1 | |
| Pain control exclusively | 102 | 32.5 | 11 | 44.0 | 72 | 39.6 | 17 | 21.5 | 2 | 7.1 | |
| Health status | |||||||||||
| Karnofsky score§ | .65 | ||||||||||
| Mean | 63.5 | 66.8 | 62.6 | 64.0 | 65.4 | ||||||
| SD | 18.2 | 17.7 | 16.6 | 23.0 | 12.5 | ||||||
| Charlson comorbidity‖ | .03 | ||||||||||
| Mean | 8.3 | 9.2 | 8.4 | 8.0 | 7.3 | ||||||
| SD | 2.7 | 2.6 | 2.7 | 2.5 | 2.9 | ||||||
| Quality of life¶ | .07 | ||||||||||
| Mean | 6.9 | 6.8 | 6.7 | 7.1 | 7.3 | ||||||
| SD | 1.5 | 1.6 | 1.5 | 1.6 | 1.4 | ||||||
| Preferences and communication | |||||||||||
| Understands illness is terminal | 114 | 38.4 | 5 | 21.7 | 79 | 45.1 | 23 | 31.5 | 7 | 26.9 | .03 |
| Preference for life-extending therapy | 76 | 28.7 | 7 | 35.0 | 32 | 20.3 | 22 | 33.9 | 15 | 68.2 | < .0001 |
| EOL discussion with physician | 108 | 36.0 | 4 | 17.4 | 81 | 46.0 | 19 | 25.7 | 4 | 14.8 | .0002 |
| Close relationship with physician# | 212 | 69.7 | 18 | 78.3 | 121 | 67.6 | 55 | 73.3 | 18 | 66.7 | .62 |
| Do-not-resuscitate order | 116 | 38.9 | 8 | 34.8 | 78 | 44.6 | 25 | 34.3 | 5 | 18.5 | .05 |
| Survival, months | .18 | ||||||||||
| Median | 4.5 | 3.4 | 4.5 | 5.8 | 4.0 | ||||||
| Interquartile range | 2.0-10.1 | 1.9-7.4 | 1.8-8.7 | 2.4-12.1 | 1.9-13.3 | ||||||
NOTE. Missing data: health insurance (n = 9), positive religious coping (n = 47), treatment (n = 19), Karnofsky (n = 9), Charlson comorbidity (n = 11), understands illness is terminal (n = 36), preference for life-extending therapy (n = 68), EOL discussion with physician (n = 33), close relationship with physician (n = 29), do-not-resuscitate order (n = 35), survival (n = 5).
Abbreviations: SD, standard deviation; VA, Veterans Affairs; EOL, end of life.
Using χ2 tests for categorical variables, Fisher's exact test for categorical variables with small sample sizes, analysis of variance for continuous variables, and the log-rank test for survival.
Assessed with the Brief Religious Coping Scale (RCOPE), a validated questionnaire about religious coping (scale 0 to 21), where 0 is low and 21 is high. The sample was dichotomized at the median (12).
The remaining patients had cancer types representing < 5% of the sample.
Karnofsky score is a measure of functional status that is predictive of survival, where 0 is dead and 100 is perfect health.
Charlson comorbidity index is an age-adjusted measure of comorbid illness, where higher numbers signify greater burden.
The McGill Quality of Life Questionnaire measured patients' overall quality of life (scale 0 to 10), where 0 is undesirable and 10 is desirable.
Close doctor–patient relationship defined: Patients trusted and respected their physician, felt respected, “seen as a whole person,” and were very comfortable asking questions about their care.
In unadjusted analyses, patients who died in an ICU were often younger with fewer comorbidities, compared with patients who died elsewhere, and more likely to prefer life-extending therapies (all P ≤ .03). They were also less likely to have had an EOL discussion with a physician compared with patients who died elsewhere (P = .0002). There was no difference in patients' survival by location of death.
As shown in Table 2, 55.4% of caregivers were spouses, 23.3% were adult children, and 21.2% were other relatives/friends. At baseline, caregivers of patients who died in an ICU reported fewer traumatic experiences compared with caregivers (P ≤ .02) of patients who died elsewhere.
Table 2.
Caregivers' Characteristics by Patients' Place of Death
| Characteristic | Total (N = 333) |
Place of Death |
P* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home Without Hospice (n = 25) |
Home With Hospice (n = 195) |
Hospital (n = 85) |
Intensive Care Unit (n = 28) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Age, years | .10 | |||||||||||
| Mean | 51.3 | 45.1 | 52.0 | 52.1 | 49.3 | |||||||
| SD | 14.1 | 15.9 | 14.0 | 13.5 | 13.8 | |||||||
| Sex, male | 81 | 24.8 | 5 | 20.0 | 45 | 23.4 | 25 | 30.1 | 6 | 22.2 | .60 | |
| Relationship | .07 | |||||||||||
| Spouse | 159 | 55.4 | 5 | 25.0 | 100 | 56.8 | 43 | 61.4 | 11 | 52.4 | ||
| Adult child | 67 | 23.3 | 10 | 50.0 | 39 | 22.2 | 14 | 20.0 | 4 | 19.1 | ||
| Other relative/friend | 61 | 21.2 | 5 | 25.0 | 37 | 21.0 | 13 | 18.6 | 6 | 28.6 | ||
| Household size | .01 | |||||||||||
| Mean | 3.1 | 3.9 | 3.1 | 2.8 | 3.4 | |||||||
| SD | 1.5 | 1.8 | 1.5 | 1.3 | 1.3 | |||||||
| Education, years | .49 | |||||||||||
| Mean | 13.3 | 13.6 | 13.3 | 13.6 | 12.4 | |||||||
| SD | 3.6 | 3.6 | 3.8 | 3.2 | 3.1 | |||||||
| Health status | ||||||||||||
| ADL impairments† | .13 | |||||||||||
| Mean | 1.4 | 1.5 | 1.5 | 1.0 | 2.2 | |||||||
| SD | 2.5 | 2.9 | 2.4 | 2.1 | 3.8 | |||||||
| Quality of life‡ | .52 | |||||||||||
| Mean | 27.4 | 26.8 | 27.3 | 28.1 | 26.3 | |||||||
| SD | 6.3 | 7.2 | 6.1 | 5.8 | 7.5 | |||||||
| Mental disorders§ | ||||||||||||
| Major depressive disorder | 12 | 3.9 | 1 | 4.2 | 7 | 3.9 | 3 | 3.9 | 1 | 4.0 | 1.00 | |
| Generalized anxiety disorder | 16 | 5.3 | 0 | 0.0 | 10 | 5.6 | 4 | 5.3 | 2 | 8.0 | .69 | |
| Panic disorder | 13 | 4.3 | 2 | 8.3 | 4 | 2.2 | 5 | 6.6 | 2 | 8.0 | .09 | |
| Posttraumatic stress disorder | 12 | 3.9 | 3 | 12.5 | 5 | 2.8 | 2 | 2.6 | 2 | 8.0 | .06 | |
| Preloss grief | 22 | 9.9 | 0 | 0.0 | 15 | 10.6 | 5 | 10.6 | 2 | 11.8 | .59 | |
| Caregiver burden and support | ||||||||||||
| Help required since diagnosis‖ | .01 | |||||||||||
| Mean | 2.2 | 2.2 | 2.3 | 1.9 | 2.3 | |||||||
| SD | 0.8 | 0.9 | 0.8 | 0.9 | 0.8 | |||||||
| Stressful caregiving experiences¶ | .02 | |||||||||||
| Mean | 7.3 | 6.0 | 7.9 | 6.4 | 5.4 | |||||||
| SD | 5.0 | 4.4 | 5.2 | 4.2 | 4.3 | |||||||
| Positive caregiving experiences | .62 | |||||||||||
| Mean | 3.2 | 3.1 | 3.1 | 3.2 | 3.3 | |||||||
| SD | 0.7 | 0.6 | 0.7 | 0.6 | 0.7 | |||||||
| Interpersonal support# | .76 | |||||||||||
| Mean | 11.3 | 11.4 | 11.4 | 11.3 | 10.8 | |||||||
| SD | 2.5 | 2.4 | 2.4 | 2.3 | 3.2 | |||||||
NOTE. Missing data: age (n = 6), sex (n = 6), relationship (n = 46), household size (n = 6), education (n = 7), ADL impairments (n = 6), quality of life (n = 14), major depressive disorder (n = 25), generalized anxiety disorder (n = 29), panic disorder (n = 28), posttraumatic stress disorder (n = 25), preloss grief (n = 111), help required since diagnosis (n = 20), stressful caregiving experiences n = (49), positive caregiving experiences (n = 42), interpersonal support (n = 24).
Abbreviations: SD, standard deviation; ADL, activities of daily living.
Using χ2 tests for categorical variables, Fisher's exact test for categorical variables with small sample sizes, and analysis of variance for continuous variables.
The Functional Disability scale measured caregivers' impairments in ADL (scale 0 to 14), where 0 is no impairments and 14 is severely impaired.
Quality of life measured with the 36-item Medical Outcomes Study Short-Form Health Survey (scale 0 to 36), where 0 is worst and 36 is best.
Diagnosed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and Prolonged Grief Disorder interview.
Measured with the Covinsky Family Impact Survey question: “How much help has s/he needed from someone in the family?” (scale 0 to 3), where 0 is “none” and 3 is “a great deal.”
The SCARED scale is a validated tool that measures the frequency of caregivers' exposure to patient distress (scale 0 to 24), where 0 is never and 24 is witnessed eight potential traumatic events (eg, pain, delirium).
The Interpersonal Support Evaluation List measured caregivers' social support (scale 0 to 16), where 0 is least support and 16 is most.
Patients' QoL at the EOL
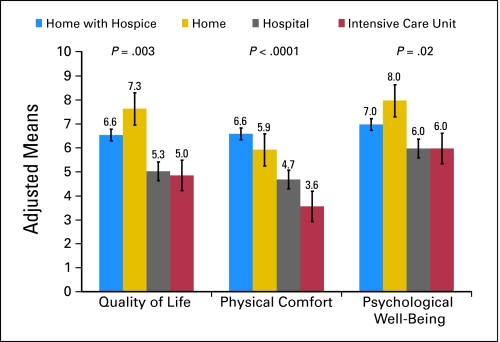
In adjusted analyses, patients who died in an ICU or hospital had worse QoL and more physical and emotional distress at the EOL compared with patients who died at home with hospice services (Fig 1). Mean QoL scores were lowest among patients who died in an ICU (5.0) or hospital (5.3) and highest among patients who died at home with hospice services (6.6) or without hospice (7.3; overall F = 4.87; df = 3; P = .003), after adjusting for significant confounds.
Fig 1.
Patients' end-of-life experiences by place of death. Measures scored (0 to 10) where 0 is the worst possible and 10 is the best possible. Every patient/caregiver variable associated (P < .20) with patients' location of death in univariate analyses was entered into each analysis of covariance model and retained if it remained significant at a level of P < .05 while controlling for other confounders. The P values displayed represent the significance of the effect of the place of death on patients' quality of life (QoL; F = 4.87; df = 3; P = .003), physical comfort (F = 8.86; df = 3; P < .0001), and psychological well-being (F = 3.27; df = 3; P = .02) from the analysis of covariance models. The QoL model was adjusted for patients' age, baseline QoL, treatment site, panic disorder in caregivers, and the source of report (ie, formal/informal caregiver). The physical comfort model was adjusted for treatment site and the source of report. The psychological well-being model was adjusted for survival, do-not-resuscitate order, and the source of report.
In adjusted analyses, patients who died in ICUs also had lower mean physical comfort scores (3.6) than patients who died in hospitals (4.7), home without hospice (5.9), or home with hospice (6.6; overall F = 8.86; df = 3; P < .0001). Similarly, patients dying in ICUs and hospitals had lower psychological well-being scores (both 6.0) than patients dying at home with (7.0) or without (8.0) hospice (overall F = 3.27; df = 3; P = .02).
Bereavement Outcomes
As shown in Table 3, caregivers' preexisting psychiatric morbidity was a significant predictor of psychiatric illness during bereavement. For example, caregivers with panic disorder at baseline had higher odds of panic disorder (odds ratio, 15.31; 95% CI, 3.24 to 72.33) during bereavement than caregivers without this disorder at baseline.
Table 3.
Bereaved Caregivers' Mental Health Outcomes by Caregivers' Baseline Mental Health
| Bereaved Caregivers' Mental Health Outcome | Caregivers' Baseline Mental Health* |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Posttraumatic Stress Disorder |
Generalized Anxiety Disorder |
Panic Disorder |
Major Depressive Disorder |
Preloss Grief |
|||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| PTSD | 6.30 | 1.26 to 31.52 | 0.03 | 8.10 | 1.92 to 34.11 | .004 | 6.24 | 1.25 to 31.20 | .03 | 5.01 | 0.67 to 37.32 | .12 | 4.15 | 0.82 to 21.24 | .09 |
| GAD | 2.00 | 0.09 to 44.37 | 0.66 | 6.98 | 0.90 to 54.31 | .06 | 8.39 | 1.05 to 66.95 | .04 | 3.61 | 0.14 to 92.77 | .44 | 9.42 | 0.88 to 100.83 | .06 |
| PD | 3.90 | 0.57 to 26.85 | 0.17 | 7.33 | 1.42 to 37.86 | .02 | 15.31 | 3.24 to 72.33 | .0006 | 6.42 | 0.84 to 49.03 | .07 | 1.96 | 0.29 to 13.09 | .49 |
| MDD | 3.35 | 0.72 to 15.72 | 0.12 | 2.48 | 0.56 to 11.11 | .23 | 3.32 | 0.71 to 15.56 | .13 | 2.81 | 0.39 to 19.98 | .30 | 6.31 | 1.66 to 23.96 | .007 |
| PGD | 1.47 | 0.22 to 9.99 | 0.69 | 2.10 | 0.29 to 15.27 | .47 | 1.59 | 0.23 to 10.85 | .64 | 0.72 | 0.03 to 18.10 | .84 | 7.47 | 1.81 to 30.83 | .005 |
NOTE. Missing data at baseline: PTSD (n = 25), GAD (n = 29), PD (n = 28), MDD (n = 25), and preloss grief (n = 111). Missing data at follow-up (6 months after loss): PTSD (n = 95), GAD (n = 97), PD (n = 95), MDD (n = 94), and PGD (n = 191). Number of observations used for analyses examining associations between caregivers' psychiatric illness at follow-up and baseline mental health: PTSD (PTSD, n = 222 of 333; GAD and PD, n = 220 of 333; MDD, n = 223 of 333; and preloss grief, n = 153 of 333); GAD (PTSD, n = 221 of 333; GAD and PD, n = 219 of 333; MDD, n = 222 of 333; and preloss grief, n = 152 of 333); PD (PTSD, n = 222 of 333; GAD n = 220 of 333; PD, n = 220 of 333; MDD, n = 222 of 333; and preloss grief, n = 153 of 333); and MDD (PTSD, n = 223 of 333; GAD and PD, n = 221 of 333; MDD, n = 223 of 333; and preloss grief, n = 154 of 333), and PGD (PTSD and MDD, n = 134 of 333; GAD, n = 132 of 333; PD, n = 133 of 333; and preloss grief, n = 81 of 333).
Abbreviations: OR, odds ratio; PTSD, posttraumatic stress disorder; GAD, generalized anxiety disorder; PD, panic disorder; MDD, major depressive disorder; PGD, prolonged grief disorder.
Using logistic regression.
ICU and hospital deaths were associated with more psychiatric illness in bereaved caregivers compared with home hospice deaths, even after adjusting for caregivers' baseline mental health (Table 4). Bereaved caregivers of patients who died in ICUs had a heightened risk of developing PTSD compared with caregivers of patients who died at home with hospice (21.1% [four of 19] v 4.4% [six of 137]; adjusted odds ratio, 5.00; 95% CI, 1.26 to 19.91; P = .02, adjusted for preloss PTSD). Caregivers of patients who died in the hospital had higher odds of meeting criteria for PGD (21.6% [eight of 37] v 5.2% [four of 77]; adjusted odds ratio, 8.83; 95% CI, 1.51 to 51.77; P = .02, adjusted for preloss grief) compared with caregivers of patients who died with home hospice.
Table 4.
Bereaved Caregivers' Mental Health Outcomes by Patients' Place of Death
| Bereaved Caregivers' Mental Health Outcomes | n | N | % | Patients' Place of Death* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensive Care Unit |
Hospital |
Home Without Hospice |
Home With Hospice |
||||||||||||
| AOR | 95% CI | P† | AOR | 95% CI | P† | AOR | 95% CI | P† | AOR | 95% CI | P† | ||||
| PTSD‡ | 10 | 238 | 4.2 | 5.00 | 1.26 to 19.91 | .02 | 0.16 | 0.009 to 2.94 | .22 | 0.35 | 0.02 to 7.19 | .49 | – | Ref | – |
| GAD§ | 4 | 236 | 1.7 | 5.35 | 0.69 to 41.51 | .11 | 0.47 | 0.03 to 8.31 | .61 | 0.69 | 0.03 to 15.68 | .81 | – | Ref | – |
| PD‖ | 9 | 238 | 3.8 | 0.60 | 0.04 to 9.27 | .71 | 0.95 | 0.18 to 4.96 | .95 | 0.39 | 0.02 to 6.75 | .52 | – | Ref | – |
| MDD¶ | 17 | 239 | 7.1 | 3.49 | 0.86 to 14.22 | .08 | 1.89 | 0.63 to 5.69 | .26 | 1.34 | 0.21 to 8.55 | .92 | – | Ref | – |
| PGD# | 15 | 142 | 10.6 | 5.24 | 0.62 to 44.36 | .13 | 8.83 | 1.51 to 51.77 | .02 | 1.98 | 0.07 to 60.11 | .69 | – | Ref | – |
NOTE. Missing data at follow-up: PGD (n = 191), PTSD (n = 95), GAD (n = 97), PD (n = 95), MDD (n = 94). Number of observations used for analyses examining associations between caregivers' psychiatric illness at follow-up and patients' place of death: PTSD (n = 222 of 333), GAD (n = 217 of 333), PD (n = 218 of 333), MDD (223 of 333), and PGD (142 of 333).
Abbreviations: AOR, adjusted odds ratio; PTSD, posttraumatic stress disorder; GAD, generalized anxiety disorder; PD, panic disorder; MDD, major depressive disorder; PGD, prolonged grief disorder; ANCOVA, two-way analysis of covariance.
The reference group for these models is patients who died at home with hospice services.
Using logistic regression to control for significant confounders and baseline mental health. Every patient/caregiver variable associated (P < .20) with patients' location of death in univariate analyses was entered into each ANCOVA model, and retained if it remained significant at a level of P < .05 while controlling for other confounders.
Model adjusted for baseline PTSD.
Model adjusted for baseline GAD, caregiver age, and functional status.
Model adjusted for baseline PD, caregiver age, and functional status.
Model adjusted for baseline MDD.
Model adjusted for preloss grief.
DISCUSSION
Our results suggest that patients with advanced cancer who die in a hospital or ICU have worse QOL and their bereaved caregivers are at increased risk for developing psychiatric illness compared with those who died at home with hospice services. Specifically, bereaved caregivers of patients who die in the ICU had higher odds of developing PTSD and caregivers of patients who died in a hospital were at heightened risk of developing PGD compared with caregivers of patients who died at home with hospice, even after adjustment for caregivers' preexisting psychiatric illnesses.
Few studies have prospectively examined whether patients' EOL experiences differ by their place of death. In a retrospective, cross-sectional study of bereaved family members, patients who died at home with hospice services had fewer unmet needs at the EOL.14 Others have questioned the importance of dying at home, suggesting that physicians may romanticize the experience.45
This study provides evidence that patients with cancer who die at home have better QoL at the EOL than patients who die in hospitals. Surprisingly, patients who received hospice services did not have significantly better QoL than patients who died at home without hospice. This may be because patients who died at home without hospice were a particularly well-adjusted group who either did not need additional services, had more family support, and/or received services we did not assess. Future research is needed to determine why home deaths result in better QoL for patients, but we expect that it may be due to differences in the focus of care provided. Hospital—and especially ICU—care often focuses on keeping patients alive at all costs, whereas home deaths may emphasize patients' QoL and symptom management.
To our knowledge, this is the first study to show that caregivers of patients who die in ICUs are at a heightened risk for developing PTSD. Prior studies have measured caregivers' psychiatric symptoms18–20 or examined other diagnosable psychiatric illnesses cross-sectionally.21 In this study we used the well-validated SCID to determine whether caregivers met clinical criteria for psychiatric illness at baseline and 6 months after loss to better isolate the caregiver's risk of developing psychiatric illness as a result of the patients' place of death. Our finding that bereaved caregivers of critically ill patients have higher odds of developing PTSD suggests that caregivers' ICU experiences may prove traumatic for them. Future research is needed to specifically identify which experiences lead to caregiver PTSD and interventions to mitigate these traumatic exposures.
Our finding that caregivers of patients who died in the hospital had increased odds of developing PGD was initially surprising because emotional dependency is the best established risk factor for this disorder.46–50 It may be that attached caregivers cannot accept the patient's impending death and attempt to care for them at home until a medical crisis precipitates a terminal hospitalization.
There are several limitations to this study. This observational study could not randomly assign terminally ill patients with cancer to die in different locations for ethical and logistical reasons. Many patient and caregiver characteristics that influence patients' location of death or caregivers' bereavement were assessed, but other confounding influences may not have been measured. For example, we had insufficient information about patients' clinical status near death and could not directly examine how patients' clinical course (eg, gradual v precipitous decline) influenced their place of death. Future research is needed to disentangle how patients' disease trajectory influences their QoL at the EOL, place of death, and caregivers' bereavement adjustment. In addition, some bereaved caregivers could not be reached for interviews 6 months after loss, and preloss grief and PGD were only assessed in a subset of caregivers because the PG-13 scale underwent revision during the study to match evolving diagnostic criteria. In addition, we may have been underpowered to detect some associations because the sample sizes were small and the rates of psychiatric disorders in bereaved caregivers were low. Finally, our measures of patients' QoL were obtained from caregivers and therefore may not accurately capture patients' true levels of distress at the EOL. In the baseline interview, however, patient/caregiver assessments of patients' QoL were significantly correlated (P < .0001), and more than 35% of patients were confused or unconscious at the EOL, suggesting that a patient measure might have been biased because only 65% of the sample could have participated.51
Despite these limitations, our study has many strengths. First, we assessed caregivers most closely involved in the patients' care within 2 weeks of death, whereas other studies have relied on bereaved caregivers' recall of patients' experiences 3 to 15 months after loss, which may result in more accurate recall. Second, we assessed caregivers' mental health with the SCID and PGD scale both at baseline and 6 months after loss, thereby enabling us to better isolate the effects of place of death on changes in mental health.
Our study demonstrates that patients with advanced cancer who die in a hospital or ICU have worse QoL at the EOL and their caregivers have higher odds of developing bereavement-related psychiatric illnesses compared with those who die at home with hospice. These findings are important because patients with advanced cancer are receiving increasingly aggressive care at the EOL.1–5 Interventions aimed at reducing terminal hospitalizations or increasing the utilization of hospice services may improve the QoL of patients with advanced cancer at the EOL and reduce the risk of psychiatric illness in bereaved caregivers.
Footnotes
Supported in part by the following grants to H.G.P.: Grant No. MH63892 from the National Institute of Mental Health and Grant No. CA 106370 from the National Cancer Institute. Support for A.A.W. was also provided by 5R25CA092203 from the National Cancer Institute, the ASCO Cancer Foundation Young Investigator Award, funded by Susan G. Komen for the Cure, the National Palliative Care Research Center, and the Dana-Farber Cancer Institute Center for Psycho-Oncology and Palliative Care Research.
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology (ASCO) and The ASCO Cancer Foundation. The funding institutions did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Alexi A. Wright, Holly G. Prigerson
Financial support: Holly G. Prigerson
Administrative support: Holly G. Prigerson
Provision of study materials or patients: Holly G. Prigerson
Collection and assembly of data: Holly G. Prigerson
Data analysis and interpretation: Alexi A. Wright, Nancy L. Keating, Tracy A. Balboni, Ursula A. Matulonis, Susan D. Block, Holly G. Prigerson
Manuscript writing: Alexi A. Wright, Nancy L. Keating, Tracy A. Balboni, Ursula A. Matulonis, Susan D. Block, Holly G. Prigerson
Final approval of manuscript: Alexi A. Wright, Nancy L. Keating, Tracy A. Balboni, Ursula A. Matulonis, Susan D. Block, Holly G. Prigerson
REFERENCES
- 1.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 2.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138:639–643. doi: 10.7326/0003-4819-138-8-200304150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Temel JS, McCanon J, Greer JA, et al. Aggressiveness of care in a prospective cohort of patients with advanced NSCLC. Cancer. 2008;113:826–833. doi: 10.1002/cncr.23620. [DOI] [PubMed] [Google Scholar]
- 5.Sharma G, Freeman J, Zhang D, et al. Trends in end-of-life ICU use among older adults with advanced lung cancer. Chest. 2008;133:72–78. doi: 10.1378/chest.07-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruera E, Russell N, Sweeney C, et al. Place of death and its predictors for local patients registered at a comprehensive cancer center. J Clin Oncol. 2002;20:2127–2133. doi: 10.1200/JCO.2002.08.138. [DOI] [PubMed] [Google Scholar]
- 7.Brown University Center on Gerontology and Health Care Research: Facts on dying: Policy relevant data on care at the end of life. http://www.chcr.brown.edu/dying/cancersod.htm.
- 8.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 9.Hogan C, Lunney J, Gabel J, et al. Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood) 2001;20:188–195. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 10.Lubitz J, Greenberg LG, Gorina Y, et al. Three decades of health care use by the elderly, 1965-1998. Health Aff (Millwood) 2001;20:19–32. doi: 10.1377/hlthaff.20.2.19. [DOI] [PubMed] [Google Scholar]
- 11.Yu W. Health Services Research & Development Service, United States Department of Veterans Affairs; End of life care: Medical treatments and costs by age, race, and region. HSR&D study IIR 02-189. http://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141693100. [Google Scholar]
- 12.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patients' mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 15.Kris AE, Cherlin EJ, Prigerson HG, et al. Length of hospice enrollment and subsequent depression in family caregivers: 13-month follow-up study. Am J Geriatr Psychiatry. 2006;14:264–269. doi: 10.1097/01.JGP.0000194642.86116.ce. [DOI] [PubMed] [Google Scholar]
- 16.The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 17.Nelson JE, Meier DE, Oei EJ, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–282. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Pochard F, Darmon M, Fassier T, et al. Symptoms of anxiety and depression in family members of ICU patients before discharge or death: A prospective multicenter study. J Crit Care. 2005;20:90–96. doi: 10.1016/j.jcrc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 20.Anderson WG, Arnold RM, Angus DC, et al. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23:1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel MD, Hayes E, Vanderwerker LC, et al. Psychiatric illness in the next of kin of patients who die in the intensive care unit. Crit Care Med. 2008;36:1722–1728. doi: 10.1097/CCM.0b013e318174da72. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, et al. New York, NY: New York State Psychiatric Institute; 1995. Structured Clinical Interview for the DSM-IV Axis I Disorders: Patient Edition (SCID-I/P) (ed 2) [Google Scholar]
- 23.Maciejewski PK, Zhang B, Block SD, et al. An empirical examination of the stage theory of grief. JAMA. 2007;297:716–723. doi: 10.1001/jama.297.7.716. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JR, Patrick DL, Engelberg RA, et al. A measure of the quality of dying and death: Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24:17–31. doi: 10.1016/s0885-3924(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 25.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID): Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 26.Prigerson HG, Horowitz MJ, Jacobs SC, et al. Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Med. 2009;6:e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitzen S, Teno JM, Fennell M, et al. Factors associated with site of death: A national study of where people die. Med Care. 2003;41:323–335. doi: 10.1097/01.MLR.0000044913.37084.27. [DOI] [PubMed] [Google Scholar]
- 28.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: Systematic review. BMJ. 2006;332:515–521. doi: 10.1136/bmj.38740.614954.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruneir A, More V, Weitzen S, et al. Where people die: A multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64:351–378. doi: 10.1177/1077558707301810. [DOI] [PubMed] [Google Scholar]
- 30.Karnofsky D, Abelmann W, Craver L, et al. The use of nitrogen mustard in the palliative treatment of cancer. Cancer. 1948;1:634–656. [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SR, Mount BM, Bruera E, et al. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: A multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11:3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 33.Prigerson HG. Socialization to dying: Social determinants of death acknowledgment and treatment among terminally ill geriatric patients. J Health Soc Behav. 1992;33:378–395. [PubMed] [Google Scholar]
- 34.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 35.Phillips RS, Wenger NS, Teno J, et al. Choices of seriously ill patients about cardiopulmonary resuscitation: Correlates and outcomes—SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Am J Med. 1996;100:128–137. doi: 10.1016/s0002-9343(97)89450-8. [DOI] [PubMed] [Google Scholar]
- 36.Wright AA, Mack JW, Kritek PA, et al. Influence of patients' preferences and treatment site on cancer patients end-of-life care. Cancer. doi: 10.1002/cncr.25217. [epub ahead of print on June 22, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelps AC, Maciejewski PK, Nilsson M, et al. Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA. 2009;301:1140–1147. doi: 10.1001/jama.2009.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: The Human Connection Scale. Cancer. 2009;115:3302–3311. doi: 10.1002/cncr.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short Form Health Survey (SF-36), II: Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Katz S, Downs TD, Cash HR, et al. Progress in the development of an index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 41.Carey PJ, Oberst MT, McCubbin MA, et al. Appraisal and caregiving burden in family members caring for patients receiving chemotherapy. Oncol Nurs Forum. 1991;18:1341–1348. [PubMed] [Google Scholar]
- 42.Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients' families: SUPPORT Investigators—Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. JAMA. 1994;272:1839–1844. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 43.Prigerson HG, Cherlin E, Chen JH, et al. The Stressful Caregiving Adult Reactions to Experiences of Dying (SCARED) Scale: A measure for assessing caregiver exposure to distress in terminal care. Am J Geriatr Psychiatry. 2003;11:309–319. [PubMed] [Google Scholar]
- 44.Cohen S, Hoberman H. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;58:304–309. [Google Scholar]
- 45.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 46.Cleiren M, Diekstra RF, Kerkhof AJ, et al. Mode of death and kinship in bereavement: Focusing on “who” rather than “how.”. Crisis. 1994;15:22–36. [PubMed] [Google Scholar]
- 47.Johnson JG, Zhang B, Greer JA, et al. Parental control, partner dependency and complicated grief among widowed adults in the community. J Nerv Ment Dis. 2007;195:26–30. doi: 10.1097/01.nmd.0000252009.45915.b2. [DOI] [PubMed] [Google Scholar]
- 48.Barry LC, Kasl SV, Prigerson HG. Psychiatric disorders among bereaved persons: The role of perceived circumstances of death and preparedness for death. Am J Geriatr Psychiatry. 2002;10:447–457. [PubMed] [Google Scholar]
- 49.Barry LC, Prigerson HG. Perspectives on preparedness for a death among bereaved persons. Conn Med. 2002;66:691–696. [PubMed] [Google Scholar]
- 50.Zhang B, El-Jawahri A, Prigerson HG. Update on bereavement research: Evidence-based guidelines for the diagnosis and treatment of complicated bereavement. J Palliat Med. 2006;9:1188–1203. doi: 10.1089/jpm.2006.9.1188. [DOI] [PubMed] [Google Scholar]
- 51.McPherson C, Addington-Hall J. Judging the quality of care at the end of life: Can proxies provide reliable information? Soc Sci Med. 2003;56:95–109. doi: 10.1016/s0277-9536(02)00011-4. [DOI] [PubMed] [Google Scholar]