Abstract
Purpose
This open-label, phase I, dose-escalation study assessed the maximum-tolerated dose (MTD), safety, pharmacokinetics, and antitumor activity of sunitinib in combination with capecitabine in patients with advanced solid tumors.
Patients and Methods
Sunitinib (25, 37.5, or 50 mg) was administered orally once daily on three dosing schedules: 4 weeks on treatment, 2 weeks off treatment (Schedule 4/2); 2 weeks on treatment, 1 week off treatment (Schedule 2/1); and continuous daily dosing (CDD schedule). Capecitabine (825, 1,000, or 1,250 mg/m2) was administered orally twice daily on days 1 to 14 every 3 weeks for all patients. Sunitinib and capecitabine doses were escalated in serial patient cohorts.
Results
Seventy-three patients were treated. Grade 3 adverse events included abdominal pain, mucosal inflammation, fatigue, neutropenia, and hand-foot syndrome. The MTD for Schedule 4/2 and the CDD schedule was sunitinib 37.5 mg/d plus capecitabine 1,000 mg/m2 twice per day; the MTD for Schedule 2/1 was sunitinib 50 mg/d plus capecitabine 1,000 mg/m2 twice per day. There were no clinically significant pharmacokinetic drug-drug interactions. Nine partial responses were confirmed in patients with pancreatic cancer (n = 3) and breast, thyroid, neuroendocrine, bladder, and colorectal cancer, and cholangiocarcinoma (each n = 1).
Conclusion
The combination of sunitinib and capecitabine resulted in an acceptable safety profile in patients with advanced solid tumors. Further evaluation of sunitinib in combination with capecitabine may be undertaken using the MTD for any of the three treatment schedules.
INTRODUCTION
Antiangiogenic agents improve overall survival in colorectal and non–small-cell lung cancer1,2 and increase disease-free survival in breast cancer3 when combined with chemotherapy. Postulated mechanisms for these improvements include direct inhibition of tumor neovascularization, normalization of intratumoral perfusion thus improving chemotherapy delivery, and/or prevention of tumor growth between chemotherapy cycles, thereby reducing tumor burden.4
Sunitinib malate (SUTENT) is an oral inhibitor of multiple receptor tyrosine kinases, including vascular endothelial growth factor receptors and platelet-derived growth factor receptors, stem-cell factor receptor (KIT), and colony-stimulating factor 1 receptor.5–7 It is currently approved for the treatment of advanced renal cell carcinoma and for imatinib-resistant/imatinib-intolerant GI stromal tumors. Capecitabine, an oral prodrug of fluorouracil (FU), is approved for metastatic breast and colorectal cancer and for adjuvant therapy for Dukes' stage III colon cancer.8 Sunitinib plus FU significantly inhibited tumor growth and conferred a survival benefit compared with either agent alone in preclinical studies of mice with established human breast (MX-1) tumors.9 The synergistic antitumor effect with combined therapy also conferred a survival benefit in animal models.
Sunitinib and capecitabine have manageable safety profiles when administered as single agents. Grade 3 to 4 adverse events (AEs) following treatment with single-agent sunitinib include hand-foot syndrome (HFS) reported in 4% to 9% of patients, nausea in 1% to 8%, diarrhea in 3% to 6%, and fatigue in 5% to 14%.10–12 Similarly, few severe AEs have been reported with capecitabine monotherapy: grade 3 to 4 HFS in 6% to 13% of patients, nausea in ≤ 3%, diarrhea in 2% to 11%, and fatigue in ≤ 1%.13–15 The incidence of grade 3 to 4 AEs is low in patients treated with either agent, with some AEs common to both drugs (namely, HFS and diarrhea).
The different mechanisms of action of sunitinib and capecitabine, synergistic antitumor activity in animal models, and manageable single-agent safety profiles provide a strong rationale for combining these agents in the clinical setting. The primary objective of this phase I dose-escalation study was to determine the maximum-tolerated doses (MTDs) of sunitinib and capecitabine when administered to patients with advanced treatment-refractory solid tumors. Three different dosing schedules of sunitinib were used: 4 weeks on treatment followed by 2 weeks off (Schedule 4/2); 2 weeks on treatment followed by 1 week off (Schedule 2/1); and the continuous daily dosing (CDD) schedule. These schedules were studied to define the optimal treatment regimen for future drug evaluation.
PATIENTS AND METHODS
Patient Eligibility
Patients age ≥ 18 years with histologically proven advanced solid malignancies for which curative treatment was not available were enrolled. All patients were to have received two or fewer prior systemic chemotherapy regimens (excluding capecitabine), while any number of prior biologic (excluding antiangiogenic agents) or immunotherapeutic agents were permitted if completed > 4 weeks before study entry. Given the possible effect of sunitinib and capecitabine on hematopoiesis, previous chemotherapy regimens were limited to two or fewer to exclude patients with impaired bone marrow reserve. Biologic/immunotherapeutic agents are less likely to cause long-term impairment of bone marrow reserve; their prior use was not excluded. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, life expectancy of ≥ 12 weeks, and adequate organ function as defined by blood tests (criteria included AST and ALT ≤ 2.5 × upper limit of normal [ULN], total serum bilirubin ≤ 1.5 × ULN, absolute neutrophil count ≥ 1,500/mL without growth factor support, and serum creatinine ≤ 1.5 × ULN). Patients with previously treated stable brain metastases were eligible.
Patients were excluded if they had unstable angina, congestive heart failure, cardiac dysrhythmias of grade ≥ 2, atrial fibrillation, or QTc interval prolongations; had a grade 3 hemorrhage within 4 weeks of starting study treatment; used coumarin-derivative anticoagulants; or had hypertension (blood pressure > 150/100 mmHg) uncontrolled with standard antihypertensive agents.
All patients provided written informed consent. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and all applicable local regulatory requirements and laws.
Study Design and Treatments
This phase I, dose-escalation study (protocol A6181044; NCT 00618124) was conducted at three centers in the United States. The primary objective was to determine the MTDs of oral sunitinib (25, 37.5, or 50 mg once daily) and oral capecitabine (825, 1,000, or 1,250 mg/m2 twice daily) on three different sunitinib schedules (Schedule 4/2, Schedule 2/1, and the CDD schedule) and a standard capecitabine schedule (2 weeks on treatment, 1 week off; Appendix Table A1, online only). Secondary objectives included safety, antitumor activity, and pharmacokinetics of sunitinib, capecitabine, and key metabolites when these agents were given alone or in combination.
Enrollment was sequential beginning with Schedule 4/2. Once the MTD was determined for Schedule 4/2, enrollment for Schedule 2/1 and then the CDD schedule commenced, starting at the MTD defined on Schedule 4/2. Sunitinib and capecitabine doses were escalated in serial patient cohorts using a standard 3 + 3 design. Dose escalation was allowed if no dose-limiting toxicities (DLTs) were observed during cycle 1 of Schedule 4/2 (6 weeks) or Schedule 2/1 (3 weeks) or the first two cycles of the CDD schedule (6 weeks [each cycle being 3 weeks]). DLTs were defined as grade 3 to 4 nonhematologic toxicities lasting ≥ 7 days (to qualify as DLTs, nausea, vomiting, and diarrhea were required to persist at grade 3 to 4 despite maximal supportive care), grade 4 neutropenia or thrombocytopenia ≥ 7 days, grade 4 febrile neutropenia ≥ 24 hours, and grade 3 to 4 neutropenic infection. Once the MTD was reached, that cohort was expanded by an additional six patients to further characterize safety, pharmacokinetics, and antitumor effects. Patients who tolerated treatment without evidence of disease progression were permitted to receive study treatment for ≤ 1 year. Patients who showed evidence of clinical benefit could continue treatment with sunitinib and capecitabine by enrolling in a separate extension study. Depending on the type and severity of any AEs, dose adjustments were permitted for either or both drugs. Together with clinical interview, patients were asked to return medication bottles at the beginning of each cycle for compliance monitoring.
Study Assessments
Clinical status was assessed at baseline; safety was assessed at regular intervals throughout the study and for 28 days post-treatment. AEs were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Radiographic tumor assessments were also performed for Schedule 4/2 at the end of the dosing period of cycles 1, 2, and 4 and even cycles thereafter, and for Schedule 2/1 and the CDD schedule at the end of the dosing period of cycles 2, 4, and 8 and alternate even cycles thereafter. Assessments were also made whenever disease progression was suspected. Tumor response was assessed in patients with measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST).16
Pharmacokinetic Analyses
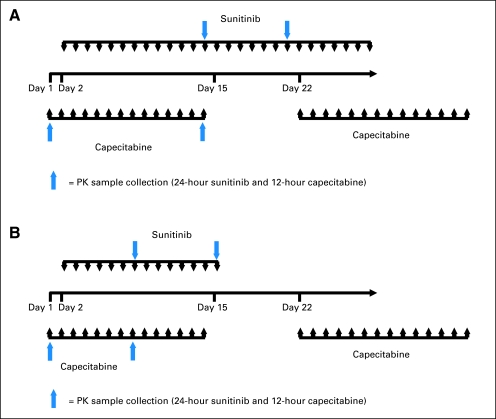
Plasma pharmacokinetic parameters for sunitinib, its active metabolite (SU12662), capecitabine and its metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, and cytotoxic FU) were determined using validated analytic methods (Appendix, online only). Full pharmacokinetic-profile blood samples were collected for Schedules 4/2 and 2/1. Only predose trough samples were collected for the CDD schedule (cycles 1 through 6). For capecitabine, blood samples were obtained predose, and at 0.25, 0.5, 1, 2, 3, 4, 9, and 12 hours postdose on days 1 and 14 for Schedule 4/2 and on days 1 and 8 for Schedule 2/1 during cycle 1. For sunitinib, blood samples were obtained predose and at 3, 4, 7, 9, 12, and 24 hours postdose on days 14/15 (in combination with capecitabine) and 21/22 (alone) for Schedule 4/2 and days 8 and 15 for Schedule 2/1 during cycle 1 (Appendix Fig A1, online only). Where possible, additional blood samples for determination of predose trough plasma concentrations (Ctrough) were collected for all sunitinib dosing schedules through cycle 5 for Schedule 4/2 and through cycle 10 for Schedule 2/1.
The maximum plasma concentration (Cmax), Ctrough, time to Cmax (Tmax), and area under the plasma [concentration-time] curve from 0 to 24 hours (AUC0-24) were determined for sunitinib, SU12662, and total drug (sunitinib plus SU12662), while AUC from 0 to 12 hours (AUC0-12) was determined for capecitabine and its metabolites. Apparent oral clearance (Cl/F) was determined for sunitinib and capecitabine only. Pharmacokinetic parameters were determined by noncompartmental methods17 using a validated proprietary software system.
Statistical Analysis
The study population for all analyses included patients who received at least one dose of study medication. Descriptive statistics were used to summarize patient characteristics, treatment administration, efficacy, safety, and pharmacokinetic data.
RESULTS
Patient Demographics and Clinical Characteristics
Between May 2005 and January 2008, 73 patients received at least one dose of study treatment. Twenty-eight patients were treated on Schedule 4/2, 19 patients on Schedule 2/1, and 26 patients on the CDD schedule. Patient characteristics are summarized in Table 1. Thirteen patients enrolled in the separate extension study; at the time of rollover, two patients were receiving sunitinib and capecitabine in combination and 11 were receiving sunitinib alone. These 13 patients received treatment for 28 to 882 days.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Schedule 4/2 (n = 28) |
Schedule 2/1 (n = 19) |
Continuous Daily Dosing Schedule (n = 26) |
Total (N = 73) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| < 65 | 26 | 93 | 9 | 47 | 16 | 62 | 51 | 70 |
| ≥ 65 | 2 | 7 | 10 | 53 | 10 | 38 | 22 | 30 |
| Median | 51 | 65 | 60 | 58 | ||||
| Range | 24-69 | 50-77 | 46-80 | 24-80 | ||||
| Sex | ||||||||
| Male | 18 | 64 | 9 | 47 | 11 | 42 | 38 | 52 |
| Female | 10 | 36 | 10 | 53 | 15 | 58 | 35 | 48 |
| ECOG PS | ||||||||
| 0 | 19 | 68 | 15 | 79 | 21 | 81 | 55 | 75 |
| 1 | 9 | 32 | 4 | 21 | 5 | 19 | 18 | 25 |
| Tumor type | ||||||||
| Pancreas | 2 | 7 | 7 | 37 | 9 | 35 | 18 | 25 |
| Esophageal | 4 | 14 | 1 | 5 | 0 | 0 | 5 | 7 |
| Breast | 2 | 7 | 1 | 5 | 1 | 4 | 4 | 5 |
| Gastric | 1 | 4 | 0 | 0 | 4 | 15 | 5 | 7 |
| Renal | 2 | 7 | 0 | 0 | 2 | 8 | 4 | 5 |
| Other | 17 | 61 | 10 | 53 | 10 | 38 | 37 | 51 |
| Prior therapy | ||||||||
| Surgery | 27 | 96 | 18 | 95 | 24 | 92 | 69 | 95 |
| Radiotherapy | 8 | 29 | 6 | 32 | 7 | 27 | 21 | 29 |
| Systemic therapy | 21 | 75 | 15 | 79 | 18 | 69 | 54 | 74 |
| One prior treatment | 12 | 43 | 12 | 63 | 9 | 35 | 33 | 45 |
| At least two prior treatments | 9 | 32 | 3 | 16 | 9 | 35 | 21 | 29 |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Safety and Tolerability
DLTs were similar on all sunitinib dosing schedules and included grade 3 HFS, myalgia, fatigue, neutropenia, and mucosal inflammation (none were grade 4). All DLTs are summarized in Table 2; MTDs are presented in Table 3.
Table 2.
DLTs
| Sunitinib(mg/d) | Capecitabine (mg/m2) | No. of DLT-Evaluable Patients | No. of Patients With DLT | Primary Diagnosis of Patients With DLT | DLT | No. of Days on Treatment |
||
|---|---|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | ||||||
| Schedule 4/2 | ||||||||
| 37.5 | 825 | 6 | 1 | Renal cell (n = 1) | Grade 3 myalgia (n = 1) | 162 | 41 | 216 |
| 50 | 825 | 3 | 0 | N/A | None | 178 | 90 | 209 |
| 37.5* | 1,000* | 9 | 0 | N/A | None | 69 | 29 | 249 |
| 50 | 1,000 | 5 | 2 | Urothelial (n = 1) | Grade 3 fatigue (n = 2) | 81 | 34 | 454 |
| Tongue (n = 1) | ||||||||
| 37.5 | 1,250 | 2 | 2 | Breast (n = 1) | Grade 3 hand-foot syndrome (n = 2) | 84 | 13 | 201 |
| Renal cell (n = 1) | ||||||||
| Schedule 2/1 | ||||||||
| 37.5 | 1,000 | 3 | 0 | N/A | None | 175 | 174 | 280 |
| 50* | 1,000* | 12 | 2 | Esophageal cancer (n = 1) | Grade 3 neutropenia (n = 1)† | 90 | 21 | 275 |
| Mesothelioma (n = 1) | Grade 3 hand-foot syndrome (n = 1) | |||||||
| 50‡ | 1,250‡ | 3 | 1 | Cholangiocarcinoma (n = 1) | Grade 3 fatigue (n = 1) | 184 | 160 | 231 |
| CDD schedule | ||||||||
| 37.5*§ | 1,000* | 21 | 5 | Hepatic (n = 1) | Grade 3 mucosal inflammation (n = 1) | 50 | 1 | 438 |
| Gastric (n = 2) | Grade 3 hand-foot syndrome (n = 4) | |||||||
| Cholangiosarcoma (n = 1) | ||||||||
| Pancreatic (n = 1) | ||||||||
| 25 | 1,250 | 2 | 2 | Renal cell (n = 1) | Grade 3 hand-foot syndrome (n = 2) | 81 | 15 | 156 |
| Pancreatic (n = 1) | ||||||||
NOTE. If a dose-limiting toxicity (DLT) was experienced by one of the three patients at any dose level, the cohort was expanded to six patients. If none of the additional three patients experienced a DLT, the dose was escalated to the next level. If DLTs occurred in two or more patients at any dose level, the dose level was deemed to have exceeded the maximum-tolerated dose (MTD), and the prior, lower dose level was further expanded (if only three patients were previously treated at that dose level). The MTD was defined as the dose level at which no more than one patient in a cohort of six experienced a DLT during the first treatment cycle of each schedule. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Abbreviations: N/A, not applicable; CDD, continuous daily dosing.
MTD; DLT.
Considered a DLT because of inability to restart study therapy within 5 days.
Further expansion of this cohort was not considered on the basis of observed toxicity and consequential dose reductions for all three patients after cycle 1; therefore, the MTD was declared to be sunitinib 50 mg/capecitabine 1,000 mg/m2.
Twenty-one patients were enrolled at the CDD schedule MTD to better characterize the feasibility of continuous daily dosing with sunitinib.
Table 3.
Treatment Exposure and Dose Reductions at the Maximum-Tolerated Doses
| Variable | Schedule 4/2 (n = 9) |
Schedule 2/1 (n = 13) |
Continuous Daily Dosing Schedule (n = 22) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Cycles started* | ||||||
| Median | 3 | 5 | 2 | |||
| Range | 1-9 | 1-12 | 1-18 | |||
| Dose reductions | ||||||
| Sunitinib | 0 | 6 | 46 | 9 | 41 | |
| Capecitabine | 5 | 56† | 10 | 77 | 11 | 50 |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Across all doses and treatment schedules.
These dose reductions occurred after the dose-limiting toxicity observation period (three patients received a reduced dose of 875 mg/m2, while two patients either missed days of dosing or had slightly lower doses administered).
For all schedules, nonhematologic AEs experienced at the MTDs (all cycles) were generally mild to moderate in severity (Table 4): grade 3 to 4 nonhematologic AEs were infrequent and were managed with dose reductions. The most frequent grade 3 AE was HFS (range, 22% to 39%; Table 4). Medians for number of weeks to onset of any-grade HFS at the MTDs were 4.4 weeks (range, 1.6 to 7.9 weeks; Schedule 4/2), 2.9 weeks (range, 0.9 to 11.4 weeks; Schedule 2/1), and 2.0 weeks (range, 0.3 to 6.9 weeks; CDD schedule). In terms of HFS management at the MTDs, no action was required for the majority of patients (87 of 133, any grade). Patients with HFS received 6, 14, and 22 dose delays or reductions on Schedule 4/2, Schedule 2/1, and CDD-schedule MTDs, respectively. The number of patients with HFS who experienced dose delays or reductions by drug were as follows: sunitinib: zero, two, and zero; capecitabine: three, six, and 15 on Schedule 4/2, Schedule 2/1, and CDD-schedule MTDs, respectively. HFS resulted in permanent withdrawal of sunitinib in three patients (two on Schedule 2/1; one on the CDD schedule).
Table 4.
Nonhematologic AEs Experienced by > 25% of Patients Treated at the MTDs (all cycles)
| AE | Schedule 4/2 (n = 9) |
Schedule 2/1 (n = 13) |
Continuous Daily Dosing Schedule (n = 22) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3* |
Total |
Grade 3* |
Total |
Grade 3* |
Total |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Fatigue/asthenia† | 1 | 11 | 4 | 44 | 2 | 15 | 7 | 54 | 3 | 14 | 16 | 73 |
| Nausea | 0 | 0 | 6 | 67 | 1 | 8 | 8 | 62 | 1 | 5 | 10 | 46 |
| Pain in extremity | 0 | 0 | 1 | 11 | 0 | 0 | 2 | 15 | 1 | 5 | 6 | 27 |
| Diarrhea | 0 | 0 | 2 | 22 | 1 | 8 | 8 | 62 | 0 | 0 | 10 | 46 |
| Peripheral sensory neuropathy | 0 | 0 | 1 | 11 | 0 | 0 | 4 | 31 | 0 | 0 | 4 | 18 |
| Hand-foot syndrome | 2 | 22 | 5 | 56 | 5 | 39 | 10 | 77 | 7 | 32 | 12 | 55 |
| Mucosal inflammation | 1 | 11 | 4 | 44 | 1 | 8 | 5 | 39 | 2 | 9 | 10 | 46 |
| Vomiting | 0 | 0 | 4 | 44 | 1 | 8 | 5 | 39 | 0 | 0 | 9 | 41 |
| Anorexia | 0 | 0 | 4 | 44 | 0 | 0 | 5 | 39 | 1 | 5 | 8 | 36 |
| Dyspepsia | 0 | 0 | 4 | 44 | 0 | 0 | 4 | 31 | 0 | 0 | 2 | 9 |
| Dysgeusia | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 31 | 0 | 0 | 4 | 18 |
| Abdominal pain†‡ | 2 | 22 | 6 | 67 | 1 | 8 | 2 | 15 | 2 | 9 | 4 | 18 |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment. There were six deaths on study; no deaths occurred at the maximum-tolerated dose (MTD) on Schedule 4/2, but there were two grade 5 adverse events (AEs) related to disease progression on the other dose levels. On Schedule 2/1, there was one death at the MTD (acute renal failure) and one death on sunitinib 50 mg + capecitabine 1,250 mg/m2 (gastric perforation). Two patients died on the continuous daily dosing schedule (both patients were at the MTD, and both deaths were attributed to disease progression).
No grade 4 AEs occurred on Schedule 4/2 or the continuous daily dosing schedule; two grade 4 AEs occurred on Schedule 2/1: hyperuricemia and hypomagnesemia.
Combined terms used; therefore, a patient may be counted more than once.
Includes AE terms “abdominal pain” and “abdominal pain upper”.
Other grade 3 AEs included abdominal pain, fatigue/asthenia, nausea, vomiting, diarrhea, anorexia, mucosal inflammation, and pain in extremities. No grade 4 hematologic AEs occurred on Schedule 4/2. Three grade 4 hematologic AEs occurred on the CDD schedule: one patient experienced grade 4 neutropenia (sunitinib 37.5 mg with capecitabine 1,000 mg/m2), and two patients experienced grade 4 thrombocytopenia (one patient received sunitinib 37.5 mg with capecitabine 1,000 mg/m2; the other received sunitinib 25 mg with capecitabine 1,250 mg/m2). Four grade 4 hematologic AEs occurred on Schedule 2/1: one case of neutropenia and one case of leukopenia (both on sunitinib 50 mg with capecitabine 1,250 mg/m2) and two cases of lymphopenia (both on sunitinib 50 mg with capecitabine 1,000 mg/m2). Most grade 3 to 4 AEs requiring intervention were easily managed with dose modification and/or standard medical or supportive therapy.
There were six deaths on study: on Schedule 4/2 there were two deaths related to disease progression below the MTD level; on Schedule 2/1 one death occurred at the MTD (acute renal failure) attributed to the patient's primary malignancy, and one treatment-related death occurred at sunitinib 50 mg and capecitabine 1,250 mg/m2 (gastric perforation). On the CDD schedule, two patients died at the MTD, both because of disease progression. All patients who died had a baseline ECOG performance status of 0 or 1 but had primary malignancies with poor prognoses (Appendix Table A2, online only): gastric (n = 2), pancreatic (n = 1), esophageal (n = 1), and unknown primary cancer (n = 2). Most deaths were the result of progressive disease (n = 4) or were attributed to the patient's primary malignancy (n = 1).
Some patients remained on treatment for several months: the median number of days (range) on treatment at the MTDs were 69 (29 to 249; Schedule 4/2), 90 (21 to 275; Schedule 2/1), and 50 (1 to 438; CDD schedule; Table 2).
Pharmacokinetics
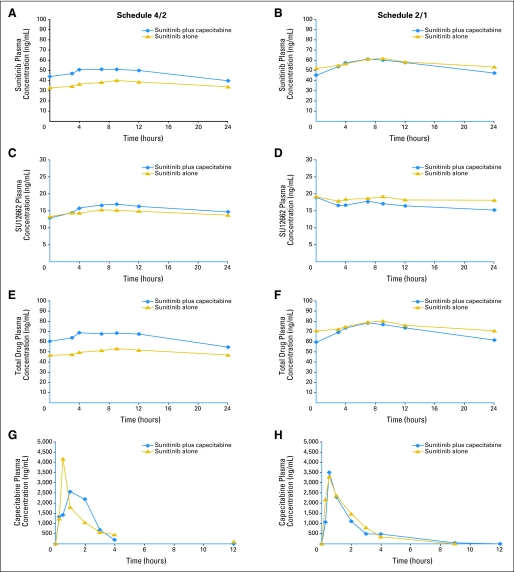
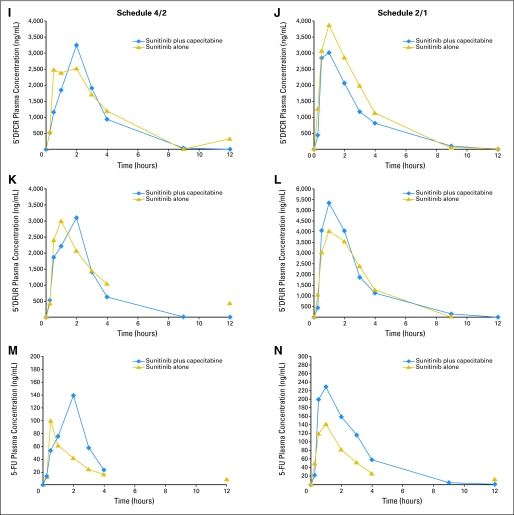
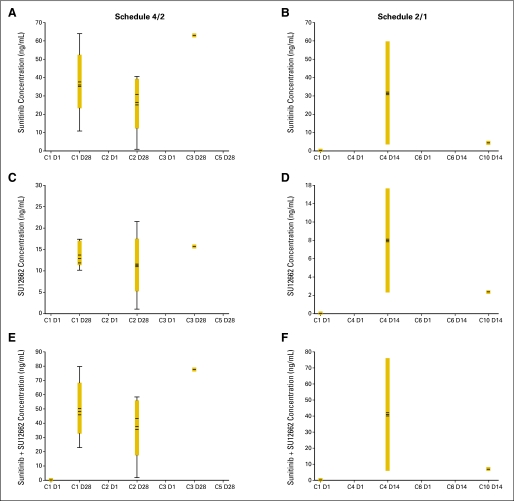
Dose-normalized pharmacokinetic parameters for Schedules 4/2 and 2/1 are presented in Table 5 (statistical analyses are shown in Appendix Table A3, online only). Overall, pharmacokinetic parameters for sunitinib, SU12662, capecitabine, 5′-deoxy-5-fluorocytidine, and 5′-deoxy-5-fluorouridine were similar when sunitinib and capecitabine were administered alone or in combination. In the case of FU, systemic exposure (Cmax and AUC) was somewhat higher and Cl/F somewhat lower when both drugs were coadministered compared with capecitabine given alone. Plasma concentration versus time curves for sunitinib, capecitabine, and all metabolites at the MTDs are shown in Appendix Figure A2 (online only). Steady-state Ctrough at the MTDs are shown in Appendix Table A4 and Appendix Figure A3 (both online only). The findings with dose-normalized data indicate that the pharmacokinetics of capecitabine and sunitinib were linear and dose proportional.
Table 5.
Dose-Corrected PK Parameters for All Doses on Schedules 4/2 and 2/1
| PK Parameter | Sunitinib (n = 24) |
SU12662 (n = 24) |
Sunitinib + SU12662 (n = 24) |
Capecitabine (n = 21) |
5′-DFCR (n = 21) |
5′-DFUR (n = 21) |
FU (n = 21) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alone | Combined | Alone | Combined | Alone | Combined | Alone | Combined | Alone | Combined | Alone | Combined | Alone | Combined | |
| Schedule 4/2 | ||||||||||||||
| Cmax, ng/mL | 47 | 52 | 20 | 21 | 66 | 73 | 4,194 | 4,861 | 4,503 | 4,231 | 5,077 | 5,893 | 140 | 249 |
| %CV | 36 | 30 | 42 | 40 | 34 | 28 | 76 | 84 | 46 | 75 | 47 | 85 | 81 | 122 |
| AUC, ng · h/mL | 998 | 1,081 | 425 | 437 | 1,423 | 1,518 | 5,346 | 6,241 | 10,071 | 9,215 | 11,291 | 10,380 | 240 | 391 |
| %CV | 34 | 31 | 40 | 39 | 32 | 29 | 43 | 41 | 43 | 41 | 43 | 39 | 51 | 45 |
| Tmax, hours | 9 | 7 | 8 | 9 | 7 | 7 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Range | 0-24 | 0-12 | 0-24 | 0-24 | 0-24 | 0-12 | 0.5-3.0 | 0.3-4.0 | 0.5-3.0 | 0.5-4.0 | 0.5-4.0 | 0.5-4.0 | 0.5-3.0 | 0.5-4.0 |
| Cl/F, L/h | 42.9 | 38.5 | — | — | — | — | 456.6 | 386.1 | — | — | — | — | — | — |
| %CV | 41 | 34 | — | — | — | — | 41 | 49 | — | — | — | — | — | — |
| Schedule 2/1 | ||||||||||||||
| Cmax, ng/mL | 73 | 67 | 27 | 21 | 100 | 88 | 4,508 | 4,927 | 4,871 | 4,192 | 6,055 | 6,865 | 186 | 329 |
| %CV | 43 | 39 | 51 | 51 | 42 | 37 | 50 | 65 | 52 | 71 | 43 | 64 | 70 | 76 |
| AUC, ng · h/mL | 1,570 | 1,390 | 591 | 448 | 2,161 | 1,839 | 6,459 | 6,370 | 11,485 | 9,405 | 13,222 | 13,627 | 331 | 596 |
| %CV | 46 | 42 | 54 | 53 | 45 | 41 | 39 | 46 | 46 | 43 | 41 | 43 | 38 | 56 |
| Tmax, hours | 7 | 7 | 4 | 7 | 9 | 7 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
| Range | 0-24 | 3.0-12 | 0-24 | 3.0-24 | 0-24 | 3.0-12 | 0.5-3 | 0.3-4 | 0.5-4.0 | 0.5-4.0 | 0.5-4.0 | 0.5-4.0 | 0.5-4.0 | 0.5-4.0 |
| Cl/F, L/h | 38.1 | 41.1 | — | — | — | — | 327 | 754 | — | — | — | — | — | — |
| %CV | 45 | 36 | — | — | — | — | 35 | 217 | — | — | — | — | — | — |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Abbreviations: PK, pharmacokinetics; SU12662, active metabolite of sunitinib; 5′-DFCR, 5′-deoxy-5-fluorocytidine; 5′-DFUR, 5′-deoxy-5-fluorouridine; FU, fluorouracil; Cmax, maximum plasma concentration; %CV, percent coefficient of variation; AUC, area under the [concentration-time] curve (0 to 24 hours for sunitinib and SU12662 and 0 to 12 hours for capecitabine and its metabolites); Tmax, time to Cmax (median values); Cl/F, apparent oral clearance.
Antitumor Activity
Measurable disease at baseline was reported for 27 patients on Schedule 4/2, 17 patients on Schedule 2/1, and 24 patients on the CDD schedule, all of whom were included in the efficacy analyses. Stable disease for ≥ 6 weeks was observed in 52% of patients on Schedule 4/2, 59% of patients on Schedule 2/1, and 29% of patients on the CDD schedule. The median duration of stable disease was 25 weeks (range, 6 to 55 weeks) on Schedule 4/2, 20 weeks (range, 7 to 40 weeks) on Schedule 2/1, and 26 weeks (range, 11 to 63 weeks) on the CDD schedule. In addition, partial responses were confirmed in nine patients: one patient each with breast cancer, thyroid cancer, and neuroendocrine tumor on Schedule 4/2; one patient each with bladder cancer, colorectal cancer, pancreatic cancer, and cholangiocarcinoma on Schedule 2/1; and two patients with pancreatic cancer on the CDD schedule (Table 6).
Table 6.
Patients With Partial Response by Tumor Type (all schedules)
| Primary Diagnosis | Sex | Age (years) | Schedule | Dose | Duration of Response (weeks) |
|---|---|---|---|---|---|
| Breast cancer | F | 51 | 4/2 | 37.5 mg sunitinib + 1,000 mg/m2 capecitabine | 22.0 |
| Thyroid cancer | M | 69 | 4/2 | 37.5 mg sunitinib + 825 mg/m2 capecitabine | 8.3+ |
| Neuroendocrine cancer | M | 47 | 4/2 | 37.5 mg sunitinib + 1,250 mg/m2 capecitabine | 19.7+ |
| Pancreatic cancer | M | 63 | 2/1 | 50 mg sunitinib + 1,000 mg/m2 capecitabine | 33.0+ |
| F | 59 | Continuous daily dosing | 37.5 mg sunitinib + 1,000 mg/m2 capecitabine | 25.9 | |
| F | 46 | Continuous daily dosing | 37.5 mg sunitinib + 1,000 mg/m2 capecitabine | 8.3+ | |
| Bladder cancer | F | 65 | 2/1 | 50 mg sunitinib + 1,000 mg/m2 capecitabine | 8.0+ |
| Colorectal cancer | F | 68 | 2/1 | 50 mg sunitinib + 1,250 mg/m2 capecitabine | 25.3+ |
| Cholangiocarcinoma | M | 67 | 2/1 | 50 mg sunitinib + 1,250 mg/m2 capecitabine | 20.9 |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Abbreviations: F, female; M, male.
DISCUSSION
This phase I dose-escalation study demonstrates that sunitinib and capecitabine can be safely combined. No clinically relevant pharmacokinetic drug-drug interactions (DDIs) were apparent, and AEs were manageable. There was evidence of antitumor activity, with responses reported on each treatment schedule and across multiple tumor types.
Angiogenesis plays an important role in tumor development. Thus, numerous targeted drugs have been developed that inhibit angiogenesis. Sunitinib is a small-molecule tyrosine kinase inhibitor of vascular endothelial growth factor receptors and platelet-derived growth factor receptors (as well as other receptors related to tumor growth) and therefore directly inhibits tumor neovascularization. Capecitabine is a pro-drug that is activated in target tumor tissue by thymidine phosphorylase, an angiogenic factor that is frequently overexpressed in tumor cells.8 It is a particularly attractive strategy to combine two oral agents such as sunitinib and capecitabine, since they may have synergistic tumor-specific activity. Sunitinib may normalize tumor vasculature and improve delivery of capecitabine to the tumor,18 while capecitabine may then be specifically activated in tumor tissue expressing thymidine phosphorylase.
In this phase I study, the MTDs for Schedule 4/2 and the CDD schedule were sunitinib 37.5 mg/d plus capecitabine 1,000 mg/m2 twice a day, while the MTD for Schedule 2/1 was sunitinib 50 mg/d plus capecitabine 1,000 mg/m2 twice a day. The MTD for capecitabine in this study (1,000 mg/m2) is the dose most frequently administered to patients in the United States, whereas 1,250 mg/m2 is the dose more commonly administered in Europe. This difference in dosing is due to a higher incidence of grade 3 to 4 AEs in US patients with capecitabine at 1,250 mg/m2. The precise reason for this geographic variation in treatment tolerability is unknown; however, a study conducted by Haller et al19 in the United States has suggested that cultural/ethnic differences or differences in levels of dietary folate may explain this variation. This difference may have affected the determination of the MTD of the combination, because all participating sites were located in the United States.
Most AEs were mild to moderate in severity, were manageable with dose reduction, and rarely led to study withdrawal. DLTs included HFS, myalgia, and fatigue and were reported on each treatment schedule. Importantly, although the AEs reported in this study are shared by both sunitinib and capecitabine as single agents, the frequency of most of these events appears similar to that reported for each agent alone.11,15,20–24
Of the AEs reported in this study, HFS is a commonly reported (but generally manageable) toxicity in trials of sunitinib and capecitabine.25 The overall incidence of grade 3 HFS across all treatment schedules and doses was 33%, a value greater than that observed with either sunitinib or capecitabine as single agents; however, HFS could be managed through treatment breaks and supportive measures.26 Similarly, diarrhea is also reported in trials of both sunitinib and capecitabine. For sunitinib, diarrhea of any grade has been reported in 20% to 58% of patients10,11,23 compared with 23% to 30% of patients receiving capecitabine.13–15 The rate of diarrhea did not appear to be additive, and the incidence (any grade) in this study was 59%.
Pharmacokinetic results do not indicate that there are clinically relevant DDIs between sunitinib and capecitabine on either Schedule 4/2 or 2/1. Pharmacokinetic parameters for both drugs and their metabolites were consistent with those from previous monotherapy studies of sunitinib and capecitabine.27,28 Steady-state sunitinib, SU12662, and sunitinib-plus-SU12662 Ctrough (Schedules 4/2 and 2/1 and the CDD schedule) were similar to those seen historically with single-agent sunitinib29 and indicated that coadministration of capecitabine did not affect sunitinib pharmacokinetics. Increases in FU systemic exposure (63% to 80%) with sunitinib coadministration were similar to or moderately higher than increases observed when capecitabine is dosed as a single agent.30 The high variability and absence of increases for the other capecitabine metabolites do not suggest a pharmacokinetic-mediated DDI. The doses achieved were either at, or slightly below, the single-agent MTDs of both agents, suggesting at most a modest pharmacodynamic interaction.
Polymorphisms in the genes for several enzymes involved in the metabolism of capecitabine, including thymidylate synthase and dihydropyrimidate, have been shown to influence the tolerability of this agent.31,32 Similarly, polymorphisms in genes encoding metabolizing enzymes and drug targets have been postulated to increase the risk of some AEs in patients taking sunitinib.33 Whether there is a correlation between polymorphisms and AEs associated with sunitinib and capecitabine in this study is unknown, although additional pharmacogenomic analyses may identify patients at greatest risk with this treatment combination.
In conclusion, sunitinib and capecitabine may be safely administered together to patients with advanced, treatment-refractory solid tumors, and preliminary data show antitumor activity in several tumor types.
Acknowledgment
We thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff; and Cherie Tanski of Pfizer (Connecticut) and Janessa Mount of Pfizer (California) for data collection and assembly. Medical writing support was provided by Nicola Crofts, PhD, at ACUMED (Tytherington, United Kingdom) and was funded by Pfizer.
Appendix
Pharmacokinetic Analysis
Human potassium EDTA plasma samples were assayed for the determination of sunitinib and SU12662 concentrations using a validated, sensitive, and specific liquid chromatographic tandem mass spectrometric (LC/MS/MS) method in the positive ionization mode. In brief, the plasma samples (50 μL) were extracted by liquid/liquid extraction at alkaline pH with ethyl acetate. Before the extraction, a deuterated internal standard (IS) of sunitinib was added. The organic layer was collected and evaporated to dryness. The residue was reconstituted with an ammonium formate/acetonitrile mixture and injected into an LC/MS/MS using a Betasil C18 column (MD Scientific ApS, Aarhus, Denmark) with an ammonium formate/acetonitrile mobile phase. The analytes were detected by positive ionization using Turbo-Ionspray (Artisan Scientific Corp, Champaign, IL) in multiple reaction monitoring mode. The quasi-molecular ions were m/z 399 (sunitinib), m/z 371 (SU12662) and m/z 409 (IS). The assay was calibrated over the assay range of 1.00 to 100 ng/mL for sunitinib and 1.00 to 100 ng/mL for SU12662 using a weighted linear regression of the standard curve [1/(concentration2)].
Human sodium heparin plasma samples were assayed for the determination of capecitabine, 5′-deoxy-5-fluorocytidine (5′DFCR), 5′-deoxy-5-fluorouridine (5′DFUR), and fluorouracil (FU) concentrations using validated, sensitive, and specific LC/MS/MS methods in the positive ion mode for capecitabine and 5′DFCR and in the negative ion mode for 5′DFUR and FU. In brief, the plasma samples (100 μL for capecitabine, 5′DFCR, and 5′DFUR, and 100 μL for FU) were extracted using a 96-well plate solid-phase extraction method for capecitabine, 5′DFCR, and 5′DFUR, and a liquid-liquid extraction method was used for FU. The assays were calibrated over the range of 30.0 to 10,000 ng/mL for capecitabine and 75.0 to 10,000 ng/mL for 5′DFCR and 5′DFUR, and 10.0 to 600 ng/mL for FU, using a weighted quadratic regression of the standard curve [1/(concentration2)].
Fig A1.
Pharmacokinetic sampling schema. (A) Schedule 4/2, cycle 1. (B) Schedule 2/1, cycle 1. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; schedule 2/1, 2 weeks on treatment, 1 week off treatment; continuous daily dosing schedule. PK, pharmacokinetic.
Fig A2.
Plasma concentration versus time curves for sunitinib, capecitabine, and all metabolites at the maximum-tolerated doses. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment (A), (C), (E), (G), Figure A2A; (I), (K), and (M), Figure A2B. Schedule 2/1, 2 weeks on treatment, 1 week off treatment (B), (D), (F), (H), Figure A2A; (J), (L), and (N), Figure A2B.
Fig A3.
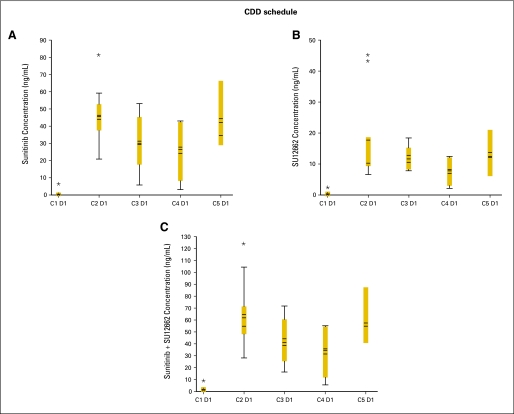
Dose-corrected trough plasma concentration versus cycle box plots at the maximum-tolerated doses. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment, panels (A), (C), and (E), Fig A3A; schedule 2/1, 2 weeks on treatment, 1 week off treatment, panels (B), (D), and (F), Fig A3A. Continuous daily dosing (CDD) schedule, panels (A), (B), and (C), Fig A3B. Boxes indicate mean data; horizontal bands within boxes indicate median data; vertical lines (“whiskers”) represent the range between the smallest and largest observations within 1.5 interquartile ranges. C, cycle; D, day. (*) indicates outliers.
Table A1.
Sunitinib and Capecitabine Dose Levels
| Dose Level | Sunitinib once daily (mg) | Capecitabine twice daily (mg/m2)* |
|---|---|---|
| Schedules 2/1 and 4/2 | ||
| –1 | 25 | 825 |
| 1 (starting dose) | 37.5 | 825 |
| 2 | 50 | 825 |
| 3a | 37.5 | 1,000 |
| 3 | 50 | 1,000 |
| Continuous daily dosing | ||
| A | 25 | 825 |
| B | 37.5 | 825 |
| C | 25 | 1,000 |
| D | 37.5 | 1,000 |
NOTE. Schedule 2/1, 2 weeks on treatment followed by 1 week off treatment (3-week cycles); Schedule 4/2, 4 weeks on treatment followed by 2 weeks off treatment (6-week cycles); continuous daily dosing (3-week cycles).
Capecitabine was administered on Schedule 2/1 on all three sunitinib dosing schedules (ie, days 1 to 14 of all schedules as well as days 22 to 35 of Schedule 4/2).
Table A2.
Deaths on Study
| Baseline ECOG PS | Primary Malignancy | Cause of Death | Time Since First Dose of Study Drug (weeks) | Time Since Initial Diagnosis (weeks) |
|---|---|---|---|---|
| 0 | Esophageal | PD | 14.1 | 54.2 |
| 0 | Gastric | PD | 12.6 | 14.3 |
| 0 | Gastric | PD | 14.7 | 33.7 |
| 0 | Pancreatic | GI perforation (attributed to capecitabine and sunitinib) | 23.0 | 91.4 |
| 0 | Unknown primary | Acute renal failure (attributed to disease under study) | 5.6 | 21.7 |
| 1 | Unknown primary | PD | 6.6 | 46.5 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; PD, progressive disease.
Table A3.
Statistical Analysis of Pharmacokinetics for Sunitinib and Capecitabine Following Administration Alone or in Combination
| Pharmacokinetics Parameter | Sunitinib |
SU12662 |
Sunitinib + SU12662 |
Capecitabine |
5′-DFCR |
5′-DFUR |
FU |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | Geometric Mean Ratio (%) (Test/Ref) | 90% CI | |
| Schedule 4/2 (n = 24) | ||||||||||||||
| Cmax, ng/mL | 112.99 | 94.0 to 135.8 | 108.09 | 90.5 to 129.2 | 111.69 | 94.7 to 131.8 | 101.19 | 65.7 to 155.9 | 79.89 | 56.2 to 113.5 | 94.34 | 64.0 to 139.1 | 144.64 | 88.55 to 236.27 |
| AUC, ng · h/mL | 109.56 | 90.8 to 132.2 | 104.13 | 87.4 to 124.0 | 108.05 | 91.7 to 127.4 | 114.96 | 88.7 to 149.0 | 89.61 | 72.8 to 110.4 | 90.30 | 72.3 to 112.8 | 159.83 | 115.84 to 220.53 |
| Cl/F, L/h | 91.27 | 77.0 to 108.2 | N/A | N/A | N/A | N/A | 83.34 | 63.5 to 109.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| Schedule 2/1 (n = 17) | ||||||||||||||
| Cmax, ng/mL | 93.69 | 74.6 to 117.6 | 76.64 | 58.5 to 100.5 | 89.72 | 72.00 to 111.9 | 86.82 | 52.9 to 142.5 | 74.64 | 44.7 to 124.5 | 97.64 | 67.7 to 140.9 | 161.11 | 100.1 to 259.3 |
| AUC, ng · h/mL | 90.26 | 71.5 to 114.0 | 75.80 | 57.3 to 100.3 | 86.61 | 68.9 to 108.9 | 88.87 | 62.1 to 127.3 | 83.00 | 51.0 to 135.0 | 100.98 | 78.6 to 129.8 | 173.55 | 131.1 to 229.7 |
| Cl/F, L/h | 110.80 | 87.5 to 140.4 | N/A | N/A | N/A | N/A | 116.00 | 72.8 to 184.8 | N/A | N/A | N/A | N/A | N/A | N/A |
NOTE. Test: Parameter value obtained when sunitinib and capecitabine were administered in combination. Ref: Parameter value obtained when either sunitinib or capecitabine were administered alone. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment.
Abbreviations: SU12662, active metabolite of sunitinib; 5′-DFCR, 5′-deoxy-5-fluorocytidine; 5′-DFUR, 5′-deoxy-5-fluorouridine; FU, fluorouracil; Cmax, maximum plasma concentration; AUC, area under the [concentration-time] curve (0 to 24 hours for sunitinib and SU12662, and 0 to 12 hours for capecitabine and its metabolites); Cl/F, apparent oral clearance; N/A, not applicable.
Table A4.
Dose-Corrected Trough Plasma Concentrations for Sunitinib, SU12662, and Sunitinib plus SU12662 at the MTDs
| Schedule/Cycle | No. of Patients | Trough Plasma Concentration (ng/mL) |
|||||
|---|---|---|---|---|---|---|---|
| Sunitinib |
SU12662 |
Sunitinib + SU12662 |
|||||
| Median | Range | Median | Range | Median | Range | ||
| Schedule 4/2 | |||||||
| C1 D29 | 6 | 35.0 | 10.9-63.8 | 11.8 | 10.1-17.4 | 46.5 | 23.0-80.7 |
| C2 D28 | 4 | 40.0 | 0.7-40.5 | 11.3 | 1.0-21.4 | 43.8 | 1.7-58.9 |
| C3 D28 | 1 | 62.9 | 62.9-62.9 | 15.7 | 15.7-15.7 | 78.6 | 78.6-78.6 |
| Schedule 2/1 | |||||||
| C4 D14 | 2 | 31.9 | 3.4-60.4 | 9.1 | 2.6-15.6 | 41.0 | 5.9-76.0 |
| C10 D14 | 1 | 4.1 | 4.1-4.1 | 2.6 | 2.6-2.6 | 6.7 | 6.7-6.7 |
| Continuous daily dosing schedule | |||||||
| C1 D1 | 17 | 0 | 0-5.7 | 0 | 0-2.3 | 0 | 0-7.9 |
| C2 D1 | 9 | 45.3 | 20.0-81.9 | 10.1 | 6.4-45.6 | 54.6 | 27.4-125.4 |
| C3 D1 | 5 | 29.5 | 4.8-52.7 | 10.4 | 7.9-18.6 | 37.9 | 15.2-71.3 |
| C4 D1 | 4 | 27.4 | 1.9-42.7 | 7.8 | 1.9-12.3 | 35.1 | 3.9-55.0 |
| C5 D1 | 3 | 34.1 | 28.4-66.3 | 12.1 | 6.2-20.9 | 40.4 | 40.3-87.2 |
NOTE. Schedule 4/2, 4 weeks on treatment, 2 weeks off treatment; Schedule 2/1, 2 weeks on treatment, 1 week off treatment. Schedule 4/2 maximum-tolerated dose (MTD): sunitinib 37.5 mg + capecitabine 1,000 mg/m2. Schedule 2/1 MTD: sunitinib 50 mg + capecitabine 1,000 mg/m2. Continuous daily dosing schedule MTD: sunitinib 37.5 mg + capecitabine 1,000 mg/m2.
Abbreviations: SU12662, active metabolite of sunitinib; C, cycle; D, day.
Footnotes
Supported by Pfizer, La Jolla, CA.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology (ASCO), June 1-5, 2007, Chicago, IL; Annual European CanCer Organisation, September 23-27, 2007, Barcelona, Spain; 44th Annual Meeting of the ASCO, May 30-June 3, 2008, Chicago, IL; and the ASCO Breast Cancer Symposium, September 5-7, 2008, Washington, DC.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00618124.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Lesley Tye, Pfizer (C); Katherine F. Liau, Pfizer (C); Akintunde Bello, Pfizer (C); Richard Chao, Pfizer (C) Consultant or Advisory Role: None Stock Ownership: Lesley Tye, Pfizer; Katherine F. Liau, Pfizer; Akintunde Bello, Pfizer; Richard Chao, Pfizer Honoraria: None Research Funding: Suzanne Jones, Pfizer; Melanie Royce, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Christopher J. Sweeney, Suzanne Jones, Lesley Tye, Richard Chao, Howard A. Burris
Provision of study materials or patients: Christopher J. Sweeney, E. Gabriela Chiorean, Claire F. Verschraegen, Fa Chyi Lee, Suzanne Jones, Melanie Royce, Howard A. Burris
Collection and assembly of data: Christopher J. Sweeney, Claire F. Verschraegen, Suzanne Jones, Melanie Royce, Lesley Tye, Katherine F. Liau
Data analysis and interpretation: Christopher J. Sweeney, E. Gabriela Chiorean, Claire F. Verschraegen, Suzanne Jones, Lesley Tye, Katherine F. Liau, Akintunde Bello, Richard Chao, Howard A. Burris
Manuscript writing: Christopher J. Sweeney, Claire F. Verschraegen, Katherine F. Liau, Akintunde Bello, Richard Chao
Final approval of manuscript: Christopher J. Sweeney, E. Gabriela Chiorean, Claire F. Verschraegen, Fa Chyi Lee, Suzanne Jones, Melanie Royce, Lesley Tye, Katherine F. Liau, Akintunde Bello, Richard Chao, Howard A. Burris
REFERENCES
- 1.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 3.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 4.Figg WD, Folkman J. New York, NY: Springer; 2008. Angiogenesis: An Integrative Approach From Science to Medicine. [Google Scholar]
- 5.Abrams TJ, Lee LB, Murray LJ, et al. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 6.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 7.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 8.Genentech USA. Xeloda prescribing information. http://www.xeloda.com/pdf/patient-pi.pdf.
- 9.Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 10.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 11.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 13.Venturini M, Paridaens R, Rossner D, et al. An open-label, multicenter study of outpatient capecitabine monotherapy in 631 patients with pretreated advanced breast cancer. Oncology. 2007;72:51–57. doi: 10.1159/000111094. [DOI] [PubMed] [Google Scholar]
- 14.Osako T, Ito Y, Takahashi S, et al. Intermittent capecitabine monotherapy with lower dose intensity in heavily pretreated patients with metastatic breast cancer. Tumori. 2007;93:129–132. doi: 10.1177/030089160709300203. [DOI] [PubMed] [Google Scholar]
- 15.Reichardt P, Von Minckwitz G, Thuss-Patience PC, et al. Multicenter phase II study of oral capecitabine (Xeloda®) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14:1227–1233. doi: 10.1093/annonc/mdg346. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications (ed 2) Philadelphia, PA: Lea and Febiger; 1989. [Google Scholar]
- 18.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 19.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118–2123. doi: 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 20.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy J, Twelves C, Van Cutsem E, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–575. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 24.Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86:1367–1372. doi: 10.1038/sj.bjc.6600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 26.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12:131–141. doi: 10.1177/1078155206069242. [DOI] [PubMed] [Google Scholar]
- 27.Mackean M, Planting A, Twelves C, et al. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998;16:2977–2985. doi: 10.1200/JCO.1998.16.9.2977. [DOI] [PubMed] [Google Scholar]
- 28.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 29.Pfizer. SUTENT (sunitinib) prescribing information, 2009. http://www.pfizer.com/files/products/uspi_sutent.pdf.
- 30.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Peters GJ, van der Wilt CL, van Triest B, et al. Thymidylate synthase and drug resistance. Eur J Cancer. 1995;31A:1299–1305. doi: 10.1016/0959-8049(95)00172-f. [DOI] [PubMed] [Google Scholar]
- 32.Milano G, Etienne MC, Pierrefite V, et al. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br J Cancer. 1999;79:627–630. doi: 10.1038/sj.bjc.6690098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27:4406–4412. doi: 10.1200/JCO.2008.21.7679. [DOI] [PubMed] [Google Scholar]