Abstract
To date, the coculture of motoneurons (MNs) and skeletal muscle in a defined in vitro system has only been described in one study and that was between rat MNs and rat skeletal muscle. No in vitro studies have demonstrated human MN to rat muscle synapse formation, although numerous studies have attempted to implant human stem cells into rat models to determine if they could be of therapeutic use in disease or spinal injury models, although with little evidence of neuromuscular junction (NMJ) formation. In this report, MNs differentiated from human spinal cord stem cells, together with rat skeletal myotubes, were used to build a coculture system to demonstrate that NMJ formation between human MNs and rat skeletal muscles is possible. The culture was characterized by morphology, immunocytochemistry, and electrophysiology, while NMJ formation was demonstrated by immunocytochemistry and videography. This defined system provides a highly controlled reproducible model for studying the formation, regulation, maintenance, and repair of NMJs. The in vitro coculture system developed here will be an important model system to study NMJ development, the physiological and functional mechanism of synaptic transmission, and NMJ- or synapse-related disorders such as amyotrophic lateral sclerosis, as well as for drug screening and therapy design.
Introduction
An in vitro coculture composed of motoneurons (MNs) and muscle would be of immeasurable value for a plethora of fields, ranging from the understanding of neuromuscular junction (NMJ) development, its structural and functional regulation, various disease investigations, to biorobotics and tissue engineering. It would be especially important to establish a system between MNs derived from human stem cells (hSCs) and rat myotubes as rat is the in vivo model of choice to investigate the therapeutic potential of SCs in disease models such as amyotrophic lateral sclerosis (ALS)1,2 and in spinal cord injury.3,4 Due to its simplicity, its inclusion of hSCs, and its correlation to SC implantation studies, it would also be useful in high-content screening modalities.
To date, multiple MN-muscle cocultures have been described in Xenopus,5,6 chick,7–9 mouse,10,11 and rat tissues,12,13 as well as in cross-species investigations between mouse MN and chick muscle11,14 and utilizing embryonic hSC-derived MNs synapsed to myotubes from the C2C12 cell line.15 However, all of these in vitro MN-muscle coculture systems use a serum-containing medium and a biological substrate.7–9,12,13 The presence of many unknown components in the serum-containing medium and the technical difficulties in creating reproducible biological substrates have led to undesired culture variability, also making it impossible to ascertain the minimum factors required for recreating or maintaining the NMJ in vitro. To eliminate these drawbacks, the development of a defined coculture system consisting of a serum-free medium and a synthetic silane substrate would facilitate the study of all the NMJ-related fields mentioned above, especially studies for hSC therapy.
We have previously built defined serum-free systems for the culture of rat skeletal muscle,16 embryonic and adult rat spinal cord neurons,17,18 and the coculture of rat MNs and rat embryonic skeletal muscle (eSKM).19 However, the necessity to create in vitro systems for the development of therapeutic strategies for diseases drives one to build models that could be used directly for application as a human model system.
This study reports the development of a defined in vitro coculture system consisting of MNs differentiated from human spinal cord SCs20 and rat eSKM.21 This system successfully supported the differentiation of both dissociated skeletal muscle cells (obtained from the hind limb of embryonic rat) and the maturation of human MNs from fetal SCs. The coculture was characterized by morphology, immunocytochemistry, and electrophysiology. Further, NMJ formation was demonstrated by immunocytochemistry and videography.
This model system, by introducing human MNs in a defined system, would be more plausible for use in studying the factors regulating NMJ development and function, especially in the study of human-related MN and/or NMJ-related diseases such as ALS, neuronal-tissue engineering, regenerative medicine, and the development of limb prosthetics. The elimination of serum from the medium makes every component of the culture system well defined and highly reproducible, and moreover makes the system feasible for modification and/or testing for use in high-throughput assays. The utilization of a synthetic, patternable surface enables this coculture applicable for any two-dimensional manipulation that would be important for creating functional in vitro systems or in tissue engineering. Overall, this novel system provides an important tool for the study of human NMJs and related diseases, especially as it relates to in vivo implantation studies of SCs in rat model systems.
Materials and Methods
Trimethoxysilylpropyldiethylenetri-amine surface modification
Glass coverslips (6661F52, 22 × 22 mm No. 1; Thomas Scientific) were cleaned using HCl/methanol (1:1) for at least 2 h, rinsed with water, soaked in concentrated H2SO4 for at least 2 h, and rinsed with water. Coverslips were boiled in nanopure water and then oven dried. The trimethoxysilylpropyldiethylenetri-amine (DETA) (T2910KG; United Chemical Technologies Inc.) film was formed by the reaction of cleaned surfaces with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (T2904; Fisher). The DETA-coated coverslips were heated to ∼80°C, and then cooled to room temperature, rinsed with toluene, reheated to approximately the same temperature, and then cured for at least 2 h at 110°C. Surfaces were characterized by contact angle and X-ray photoelectron spectroscopy to verify monolayer function as described previously.17–19
Coculture of human MNs and rat eSKM
The human spinal cord SC line was isolated and established as described in Refs.2,3,22 MNs were differentiated from this cell line as described in Ref.20 Briefly, 1.2–1.5 × 106 human spinal cord SCs were plated in one 60 mm permanox cell culture dish (Nunc, Cat #174888) and differentiated 4 days in the priming medium followed by 6 days in the differentiation medium, and then added to the muscle culture. The composition of the priming medium and differentiation medium is described in Refs.20
Skeletal muscle was removed from the hind limbs of E18 Sprague–Dawley rat fetuses. Single myocytes were then prepared as described in Ref.16 Afterward, the myocytes were resuspended in the serum-free culture medium (Table 1) and cells were counted using the trypan blue method. Myocytes were then plated on DETA coverslips at a density of 600–700 cells/mm2 in the culture medium as in Table 1, in preparation for plating the hSCs to establish the coculture.
Table 1.
Composition of the Enriched Coculture Medium
| Component | Full name | Concentration | Company | Catalog number |
|---|---|---|---|---|
| Neurobasal/neurobasal A | Invitrogen | 10888/21103 | ||
| B27 (50×) | 1× | Invitrogen | 17504-044 | |
| Glutamax (100×) | 1× | Invitrogen | 35050 | |
| GDNF | Glial-derived neurotrophic factor | 10 ng/mL | Cell Sciences | CRG400B |
| BDNF | Brain-derived neurotrophic factor | 20 ng/mL | Cell Sciences | CRB600B |
| Shh | Sonic hedgehog, N-terminal peptide | 50 ng/mL | R&D | 1845-SH-025 |
| RA | Retinoic acid | 0.1 μM | Sigma | R2625 |
| IGF-1 | Insulin-like growth factor-1 | 10 ng/mL | PeproTech | 100-11 |
| cAMP | Adenosine 3′,5′-cyclic monophosphate | 1 μM | Sigma | A9501 |
| CNTF | Ciliary neurotrophic factor | 5 ng/mL | Cell Sciences | CRC400A |
| NT-3 | Neurotrophin-3 | 20 ng/mL | Cell Sciences | CRN500B |
| NT-4 | Neurotrophin-4 | 20 ng/mL | Cell Sciences | CRN501B |
| Vitronectin | 100 ng/mL | Sigma | V8379 | |
| Laminin | Mouse laminin | 4 μg/mL | Invitrogen | 23017-015 |
| G5 (100×) | 1× | Invitrogen | 17503-012 |
Differentiated hSCs were trypsinized and re-plated on the muscle culture at a density of 200 cells/mm2 on the same day. Cocultures were incubated in the medium as described in Table 1 for 4 days, and then were maintained with NbActiv4 medium (Brainbits) by changing half of the medium every 2 days.
Immunocytochemistry and microscopy
Cells on DETA coverslips were fixed in freshly prepared 4% paraformaldehyde for 15 min. For the costainings with BTX-488, cultures were incubated with α-bungarotoxin, Alexa Fluor® 488 conjugate (BTX)-488 (Invitrogen, Cat# B13422) at 1 × 10−8 M for 1 h in a 37°C incubator before fixation. Fixed cells were then immunostained as described in Ref.20 Primary antibodies used in this study include rabbit-anti-β-III Tubulin (Sigma; 1:1000), mouse-anti-β-III Tubulin (Sigma; 1:400), goat-anti-choline acetyl transferase (ChAT) (Chemicon; 1:100), rabbit-anti-glutamate receptor (GlutR; Chemicon; 1:200), and mouse-anti-synaptophysin (Antibodies Inc.; 1:100). Mouse-anti-embryonic myosin (Hybridoma Bank, F1.652; 1:10) was obtained from the Developmental Studies Hybridoma Bank, which is maintained by the University of Iowa (Department of Biological Sciences 52242). Secondary antibodies include donkey-anti-goat-568 (Invitrogen; 1:250), donkey-anti-mouse-488 (Invitrogen; 1:250), donkey-anti-mouse-697 (Invitrogen; 1:250), donkey-anti-rabbit-594 (Invitrogen; 1:250), and donkey-anti-rabbit-488 (Invitrogen; 1:250). All antibodies were diluted in blocking buffer (5% donkey serum + 0.5% BSA in PBS).
Electrophysiological recordings
Electrophysiological properties of spinal cord SC-derived MNs and rat myotubes were investigated after ∼10 days in the coculture using whole-cell patch-clamp recording techniques.18,20 The recordings were performed in a recording chamber located on the stage of a Zeiss Axioscope 2FS Plus upright microscope.23 MNs were identified visually under an infrared differential interference contrast (DIC)-videomicroscope. The largest multipolar or round cells (15–25 μm diameter) with bright illuminance in the culture were tentatively identified as MNs.20,23,24 Patch pipettes with a resistance of 6–10 MΩ were made from borosilicate glass (BF 150-86-10; Sutter Instrument Company) with a Sutter P97 pipette puller (Sutter Instrument Company). Current-clamp and voltage-clamp recordings were made utilizing a Multiclamp 700A amplifier (Axon Instruments). The pipette (intracellular) solution contained K-gluconate 140 mM, MgCl2 2 mM, Na2ATP 2 mM, Phosphocreatine 5 mM, Phosphocreatine kinase 2.4 mg, and HEPES 10 mM (pH 7.2). The NBActiv4 medium was used as extracellular solution. After the formation of a giga-ohm seal and the membrane puncture, the cell capacitance was compensated. The series resistance was typically <23 MΩ, and it was compensated >60% using the amplifier circuitry. Signals were filtered at 3 kHz and sampled at 20 kHz using a Digidata 1322A interface (Axon Instruments). Data recording and analysis were performed with pClamp8 software (Axon Instruments). Membrane potentials were corrected by subtraction of a 15 mV tip potential, which was calculated using Axon's pClamp8 program. Depolarization-evoked action potentials (AP) were recorded in current-clamp mode. Depolarization-evoked inward and outward currents were examined in voltage-clamp mode. APs were evoked with 1 s depolarizing current injections from a −85 mV holding potential.
Video recordings
Functional NMJ formation was investigated in the coculture system 1–2 weeks after plating utilizing video recordings in a chamber located on the stage of a Zeiss Axioscope 2FS Plus upright microscope in NBActiv medium, which was the same medium system used for the electrophysiology experiments. In each experiment, 30 μL of the Glutamatergic agonist (Glut) (Neurostem, Inc., stock 50 mM, final 0.75 mM) or 100 μL of the Nicotinic cholinergic antagonist, (+)-tubocurarine chloride pentahydrate (also known as curare; cat. no. 93750; Sigma) (stock 250 μM, final 12.5 μM) was applied to the bath solution at the center of the optical viewpoint to activate the GlutR on the MNs and to block the acetylcholine receptors (AchRs) present in the NMJs, respectively. These concentrations were chosen based on previous studies.19,25–27 The videos were recorded by a charge coupled device (CCD) video camera (DAGE Technologies, DC 220) at a frame rate of 30 frames/sec using Pinnacle Technologies Video Studio 9 software and hardware. Muscle contraction frequencies after the application of either Glut or Curare are expressed as mean ± standard deviation.
Results
General analysis of the culture
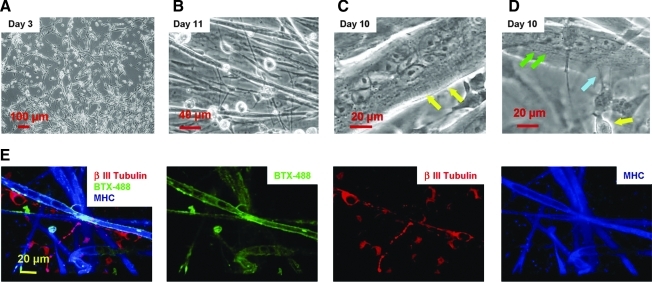
After addition of the differentiated hSCs to the myocyte culture, the SC-eSKM cocultures were maintained for 4 days in the enriched medium (Table 1), in which the spindle-shaped myoblasts proliferated to near-confluence and aligned in preparation for fusion (Fig. 1A). From day 4 onward, the cocultures were fed with Nb4Activ4 medium by changing half of the medium every 2 days, and extensive myotube formation was observed. The SC-derived human MNs matured gradually based on morphological analysis. Until day 10, the neuronal and muscle components in the cultures were distinguished by morphology (Fig. 1B). Large myotubes with striations were frequently observed after day 10 (Fig. 1C). Striations are an indication of the formation of the basic contractile apparatus for skeletal and cardiac muscle. Formation of striations implies that these myofibers are structurally and functionally mature. Colocalization of MNs and myotubes were easily identifiable in the coculture as shown in Figure 1D. Further, processes were observed extending from the MNs to the myotubes.
FIG. 1.
(A–D) Phase pictures of human stem-cell-derived MNs and rat eSKM coculture. (A) Day 3 after plating in the coculture. Myocytes are shown to be proliferating actively at this time period and a number of them have begun to line up to initiate myotube formation. (B) Day 11 after plating. The myotubes and nerve cells are distinguishable easily by morphology. (C) A sample picture for a large myotube with striations (arrows) from day 10 in culture. (D) A micrograph demonstrating coculture of the human stem-cell-derived MNs and rat myotubes from day 10 in culture. The myotube striations are indicated by the green arrows. A nearby MN (indicated by the yellow arrow) sends out processes that approach the myotube (indicated by the blue arrow). (E) Triple staining of the coculture with antibodies against β-III Tubulin, myosin heavy chain, and α-bungarotoxin, Alexa Fluor® 488 conjugate (BTX)-488 to demonstrate the presence of neurons, myotubes, and the distribution of AchRs in a day 9 coculture. MNs, motoneurons; eSKM, embryonic skeletal muscle; AchRs, acetylcholine receptors. Color images available online at www.liebertonline.com/ten.
The identification of the neurons and myotubes in the coculture was demonstrated by immunostaining with β-III Tubulin and embryonic myosin heavy chain, together with staining for AchRs using BTX-488 (Fig. 1E). The immunocytochemical analysis indicated that both the neurons and myotubes expressed the appropriate markers in this coculture system to indicate maturity.
Electrophysiological analysis of the culture system
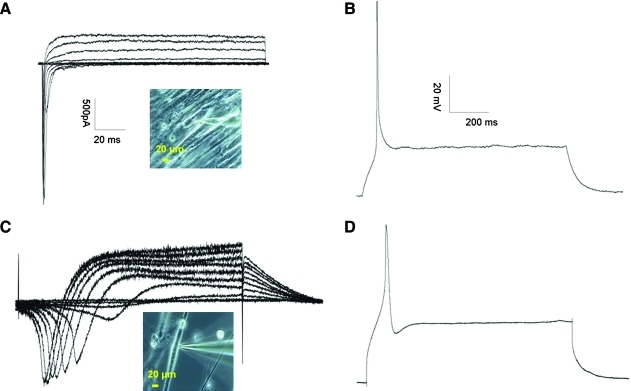
The electrophysiological properties of the MNs and myotubes in the coculture were evaluated using voltage- and current-clamp recordings for each cellular component. Representative voltage- and current-clamp recordings for the MNs and muscle are shown in Figure 2. The electrical properties of the MNs in the coculture system, including cell membrane resistance, resting membrane potential, Na+/K+ current amplitude, the ability to repetitively fire, and the amplitude of the AP, were comparable to results described previously.18,20 The electrical properties for the myotubes were also comparable to previously published results.16
FIG. 2.
Electrophysiological recordings from MNs and eSKM in the coculture. (A, B) Sample traces of a voltage-clamp recording (A) and a current-clamp recording (B) from an MN at day 13 in the coculture. The inset picture indicates the recorded MN. (C, D) Sample traces of a voltage-clamp recording (C) and a current-clamp recording (D) from a myotube at day 26 in the coculture. The inset picture indicates the recorded myotube. The scales are the same in (A) and (B). Color images available online at www.liebertonline.com/ten.
Examination of NMJ formation in the culture by immunocytochemical analysis
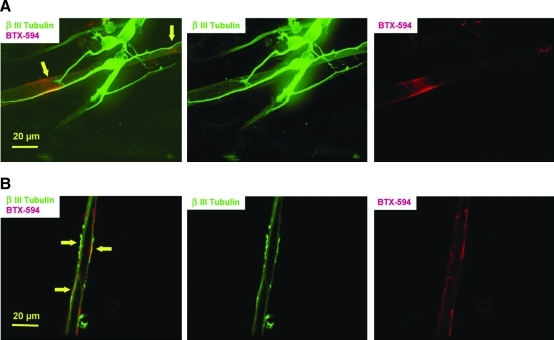
To investigate the formation of NMJs, the cocultures were analyzed utilizing immunocytochemistry and videography. Potential NMJs were first identified by the close appositions of the nerve terminals, demonstrated by β-III Tubulin immunostaining and AchR cluster identification using BTX-488. As shown in Figure 3A, the axonal processes branch at the contact points with the myotube, and the branched terminal is in proximity with the AchR clusters. This image reproduces previous findings during NMJ formation, which indicated that synaptogenesis is a dynamic process directly correlated to the active branching and remodeling of axon terminal arbors.28,29 It has also been shown that the axons of ventral spinal cord neurons can specifically induce AchR aggregation at nerve–muscle contacts.9,12 In Figure 3B, optical sections from confocal images indicated colocalization of β-III Tubulin-staining with AchR clusters marked by BTX-488, strongly suggesting the formation of NMJs in the culture. The β-III Tubulin immunostaining also demonstrated the formation of specialized presynaptic structures resembling varicosities, one of the characteristic structures in differentiated presynaptic terminals.30
FIG. 3.
Coimmunostaining of β-III Tubulin for neurons (green) and BTX-488 for AchRs (red) in a 13-day-old coculture. (A) A sample micrograph demonstrates that a neurite terminal branched upon contact with a myotube (left arrow). Moreover, this contact is surrounded by a cluster of AchRs. Another neurite–myotube contact site (right arrow), indicated by the neurite indentation and slight enlargement, is also in proximity to an AchR cluster. (B) An optical section from a confocal image indicates the close apposition of the neurite terminals and the AchR clusters as illustrated by β-III Tubulin and BTX-488 costaining (indicated by arrows). These nerve terminals presented a varicosity morphology, resembling typical presynaptic terminals. Color images available online at www.liebertonline.com/ten.
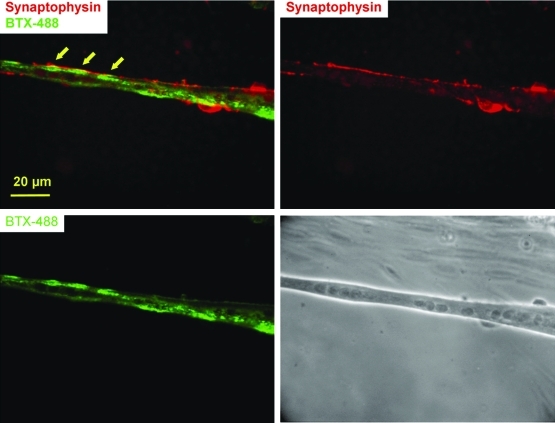
Potential NMJs in the culture were further analyzed by double staining of BTX-488 and synaptophysin, a synaptic vesicle protein. As shown in Figure 4, synaptophysin-positive terminals colocalized with AchR clusters, another strong morphological indication for NMJ formation.
FIG. 4.
Close apposition of a neurite and AchR clusters on the myotube demonstrated by synaptophysin (red) and BTX-488 (green) costaining in a 26-day-old coculture (indicated by arrows). Color images available online at www.liebertonline.com/ten.
Functional investigation of NMJ formation by videography
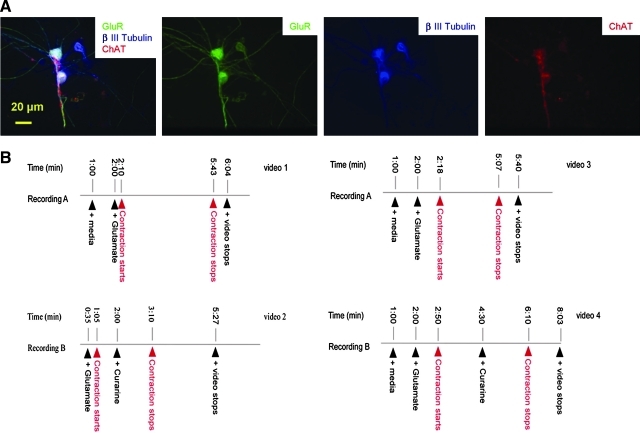
Functional NMJ formation was tested by utilizing the Glut-Curare assay as described below, and the results were recorded by videography. In vivo, MNs receive excitatory input from interneurons or sensory neurons via the neurotransmitter Glut; in vitro, the generation of an inward current, or the depolarization, of MNs by exogenously applied Glut, or its agonists, is a clearly established procedure for MN AP generation.27 Thus, the application of Glut to the electrophysiological recording chamber has been a standard approach utilized to excite spinal MNs.25 Conversely, MNs release the neurotransmitter Ach to induce muscle contraction. If functional NMJ formation has occurred, the addition of Glut to the culture should enable the excitation of the MNs and result in a corresponding myotube contraction. Further, this contraction should be arrested by the application of curare, which specifically blocks AchR. Before the functional assay experiments, the presence of GlutR on the SC-derived MNs was demonstrated by the triple-immunostaining of GlutR, ChAT, and β-III Tubulin to make sure this technique would work with this particular system (Fig. 5A).
FIG. 5.
Glut can specifically induce muscle contractions in the coculture, which can be stopped by curare. (A) Coimmunostaining of β-III Tubulin (blue), choline acetyl transferase (ChAT) (red), and GlutR (green) in a day 31 culture. As indicated, while all the neurons are positive for β-III Tubulin, those that are positive for ChAT also show high level expression of GlutRs. (B) Specific muscle contractions were induced in the MN-eSKM cocultures (Supplemental Videos S1–S4; available online at www.liebertonline.com/ten). GlutRs, glutamate receptors. Color images available online at www.liebertonline.com/ten.
Spontaneous muscle contractions were generally observed in the coculture system at day 10, sometimes as early as day 7. To identify potential NMJ sites, Glut (30 μL 50 mM) was added to the culture and muscle contractions observed at low magnification. The total of 11 tests from 10 coverslips in two different platings indicated that every addition of Glut induced a significantly increased number of contraction loci in the culture. Newly induced contraction sites were then randomly chosen for further analysis. For all the loci that were followed by curare (n = 9), the contractions were stopped by curare application as shown in Supplemental Videos S1–S4 (available online at www.liebertonline.com/ten).
In videograph S1, 30 μL of the medium was first added to the culture at 1:00 min to test whether the addition itself would cause any contractions. One minute after this addition, no contraction was observed. In the next step, 30 μL of Glut was added and the contraction of the central striated myotube started after a 10 s delay, which was followed by the contraction of a number of other myotubes. The contraction of the central striated muscle lasted 3:33 min. After this recording, the culture was washed with the medium and the experiment in videograph S2, focused on the same location, was initiated. The addition of Glut at 0:35 min induced the contraction of the central muscle again but with a relatively longer delay of 30 s. Curare (100 μL, 250 μM) was then added after 55 s of contractions. The addition of curare caused the muscle contraction pattern to be altered immediately. The myotube first contracted very fast and then slowed down and stopped completely after 70 s. Videographs S3 and S4 demonstrate a similar experiment on another coverslip.
On the basis of 11 experiments from individual coverslips, the contraction patterns induced by Glut and Curare were distinctively different. Although Glut was always added to the central view where the target muscle was located, every addition of Glut caused muscle contraction after different time delays (from a few seconds to over a minute), suggesting that they were indirect responses mediated by MNs, with the assumption that the variation in the delay reflected the variation in diffusion and the local concentration of Glut in the medium relative to the position of the innervating MN. Moreover, contractions induced by Glut were generally kept at a stable, moderate frequency (0.9 ± 0.3 Hz, n = 4 coverslips, quantified for the initial 60 s) for longer than 2 min. However, the addition of curare usually caused muscle contraction pattern changes immediately, presumably because it acted on the myotube directly, which was always in the central view of the video and closest to the addition spot. Also, the curare-induced muscle contractions generally started with a spasmodic high frequency (1.9 ± 0.7 Hz, n = 3 coverslips, quantified for the initial 10 s) and quickly slowed and finally stopped completely within 2 min. This temporal pattern is similar to the in vivo toxic response caused by curare.
To confirm that the effect of Glut was mediated by MNs but not by any direct effect on the myotubes, two control experiments were performed. First, not all the myotubes in the MN-eSKM coculture were able to be induced to contract by Glut, presumably because they were not innervated by MNs (Supplemental Video S5, available online at www.liebertonline.com/ten). Second, a culture that contained only eSKMs was tested (Supplemental Videos S6 and S7, available online at www.liebertonline.com/ten). There are a few occasional spontaneous muscle contractions in the eSKM-only culture, and the addition of Glut caused no additional muscle contraction. This result was repeated for three coverslips. Therefore, we believe that these results conclusively indicate that Glut-induced muscle contraction was not initiated by the direct effect of the neurotransmitters on the myotubes, but via the excitation of MNs and the subsequent excitation of the myotubes via the AchRs.
Discussion
This study provides a controlled reproducible system for the investigation of NMJ formation, synaptogenesis, and nerve–muscle interactions. This is the first serum-free model system to recreate mammalian NMJs that contains a human component derived from SCs on synthetic substrates in vitro. The utilization of hSC-derived MNs in coculture with rat myocytes makes this model system more directly applicable for comparison to in vivo implantation studies of SCs in rat, for the investigation of NMJ-related diseases such as ALS, and as model systems for use in high-content drug screening.
The serum-free medium and cell culture scheme was developed on the basis of our previous studies and the literature. The combination of Neurobasal medium, B27, Glutamax, glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), Sonic hedgehog, retinoic acid, insulin-like growth factor-1, adenosine 3′,5′-cyclic monophosphate, ciliary neurotrophic factor, neurotrophin-3 (NT-3), NT-4, Vitronectin, and Laminin has been found to be able to support the growth, differentiation, and long-term survival of MNs derived from hSCs.15,20 Laminins are important components of the extracellular matrix that facilitates synaptogenesis.5 Especially, β2 laminins are concentrated at synaptic sites and are required for their postnatal maturation.31 The addition of the G5 supplement to the coculture medium has been found to significantly enhance myocyte proliferation. However, the continuous presence of these trophic factors, including BDNF, GDNF, NT-3, NT-4, and adenosine 3′,5′-cyclic monophosphate, was found to significantly downregulate agrin deposition along the neurites and at nerve–muscle contacts and thus prevent synaptogenesis.6 So, the trophic factors were gradually withdrawn and the culture was fed using only NbActiv4 medium. The NbActiv4 medium formula was generated by adding three ingredients—cholesterol, estrogen, and creatine—to the medium containing Neurobasal, B27, and Glutamax.32 There is evidence that the addition of these ingredients can significantly promote synaptogenesis.32–35 Therefore, the coculture was first plated in the enriched medium to ensure the survival and growth of MNs and myocytes, followed by the gradual withdrawal of these factors that enabled the reciprocal induction between the MNs and myotubes that naturally occurs in development. The likelihood for NMJ formation to occur under these conditions is strongly supported by current NMJ formation theory. On one hand, muscle cells secret the NTs BDNF, GDNF, and NT-3/4 to support MN survival and attract neurite outgrowth,36–38 as well as provide distinct signals to organize the formation, maturation, and maintenance of motor nerve terminals, which contain laminins, fibroblast growth factors, and collagens.31 On the other hand, motor axons release neuregulin and agrin to increase local AchR synthesis and aggregation, and the neurotransmitter Ach to stabilize and refine the synapses.29,39,40 The Glut experiment in this study, along with the immunocytochemical analysis, indicates that this medium composition and feeding regimen allows for successful trans-species NMJ formation.
Trans-species NMJ formation between human and rat has been reported in one in vivo study in which spinal-transplanted MNs derived from hSCs sent out axons to form synapses with rat muscle.41 The formation of NMJs between different species suggests that the essential components required for NMJ formation are shared in these species. However, there are many mechanisms/components unidentified, which creates a significant obstacle for understanding NMJ-related diseases and designing proper treatments. Our study successfully recreated this process in vitro using a well-defined culture system that delineates the basis for the essential components and provides a starting point for investigating the underlying mechanisms, and later for the development of treatments for diseases affecting the cellular components of the NMJ. This is important because many SC implantation studies targeting spinal cord injuries or diseases are conducted in rats.1–4 Elucidation of essential factors for trans-species NMJ formation utilizing the culture system could greatly facilitate the successful implementation of these studies.
The engineered synthetic substrate DETA used in this study consisted of a glass surface coated with a DETA self-assembled monolayer. This surface has previously been shown to support neuronal, endothelial, and cardiac cell growth,17,18,42–45 and had been used in creating high-resolution in vitro patterned circuits of embryonic hippocampal neurons.46 Moreover, DETA substrates have been shown to promote guided axonal growth and direct axonal and dendritic process extension at the level of a single neuron.47 Therefore, the successful formation of NMJs on this substrate implies that this coculture can be patterned at high resolution to study engineered in vitro NMJs. Especially, this surface modification technique can be used for providing guidance cues for specific NMJ formation.
In summary, this study reports the first defined system that cocultures human MNs with rat eSKMs in a defined system in vitro. The serum-free medium allows controlled system modification that is important for understanding the regulation and process of NMJ formation. The DETA substrate can be easily patterned at a high resolution for dissecting individual NMJs and building in vitro neuron-muscular networks. This system would facilitate not only the studies concerning NMJ development and regulation, both in vitro and in vivo, but also the research fields targeting NMJ-related diseases and treatments.
Supplementary Material
Acknowledgments
This research was funded by National Institutes of Health Grant R01NS050452. We wish to thank NeuralStem for providing the fetal SCs utilized in the project. We also wish to thank Dr. Steven Lambert for his help in this research.
Disclosure Statement
No competing financial interests exist.
References
- 1.Koliatsos V.E. Xu L. Yan J. Human stem cell grafts as therapies for motor neuron disease. Expert Opin Biol Ther. 2008;8:137. doi: 10.1517/14712598.8.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Xu L. Yan J. Chen D. Welsh A.M. Hazel T. Johe K. Hatfield G. Koliatsos V.E. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 3.Cizkova D. Kakinohana O. Kucharova K. Marsala S. Johe K. Hazel T. Hefferan M.P. Marsala M. Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience. 2007;147:546. doi: 10.1016/j.neuroscience.2007.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarasenko Y.I. Gao J. Nie L. Johnson K.M. Grady J.J. Hulsebosch C.E. McAdoo D.J. Wu P. Human fetal neural stem cells grafted into contusion-injured rat spinal cords improve behavior. J Neurosci Res. 2007;85:47. doi: 10.1002/jnr.21098. [DOI] [PubMed] [Google Scholar]
- 5.Lu B. Czernik A.J. Popov S. Wang T. Poo M.M. Greengard P. Expression of synapsin I correlates with maturation of the NMJ synapse. Neuroscience. 1996;74:1087. doi: 10.1016/0306-4522(96)00187-x. [DOI] [PubMed] [Google Scholar]
- 6.Peng H.B. Yang J.F. Dai Z. Lee C.W. Hung H.W. Feng Z.H. Ko C.P. Differential effects of neurotrophins and Schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J Neurosci. 2003;23:5050. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbach G.D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972;28:407. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- 8.Fishbach G.D. Cohen S.A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973;31:147. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- 9.Frank E. Fishbach G.D. Early events in neuromuscular junction formation in vitro. J Cell Biol. 1979;83:143. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper J.M. Krishnan C. Darman J.S. Deshpande D.M. Peck S. Shats I. Backovic S. Rothstein J.D. Kerr D.A. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. PNAS. 2004;101:7123. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miles G.B. Yohn D.C. Wichterle H. Jessell T.M. Rafuse V.F. Brownstone R.M. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels M.P. Lowe B.T. Shah S. Ma J.X. Samuelson S.J. Lugo B. Parakh T. Uhm C.S. Rodent nerve-muscle cell culture system for studies of neuromuscular junction development: refinements and applications. Microsc Res Tech. 2000;49:26. doi: 10.1002/(SICI)1097-0029(20000401)49:1<26::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Dutton E.K. Uhm C.S. Samuelsson S.J. Schaffner A.E. Fitzgerald S.C. Daniels M.P. Acetylcholine receptor aggregation at nerve-muscle contacts in mammalian cultures: induction by ventral spinal cord neurons is specific to axons. J Neurosci. 1995;15:7401. doi: 10.1523/JNEUROSCI.15-11-07401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soundararajan P. Lindsey B.W. Leopold C. Rafuse V.F. Easy and rapid differentiation of embryonic stem cells into functional motoneurons using Sonic hedgehog-producing cells. Stem Cells. 2007;25:1697. doi: 10.1634/stemcells.2006-0654. [DOI] [PubMed] [Google Scholar]
- 15.Li X.J. Du Z.W. Zarnowska E.D. Pankratz M. Hansen L.O. Pearce R.A. Zhang S.C. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 16.Das M. Gregory C.A. Molnar P. Riedel L.M. Hickman J.J. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27:4374. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 17.Das M. Bhargava N. Gregory C. Riedel L. Molnar P. Hickman J.J. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell Dev Biol Anim. 2005;41:343. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 18.Das M. Molnar P. Devaraj H. Poeta M. Hickman J. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol Prog. 2003;19:1756. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 19.Das M. Rumsey J.W. Gregory C.A. Bhargava N. Kang J.F. Molnar P. Riedel L. Hickman J.J. Embryonic motor neuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146:481. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 20.Guo X.F. Johe K. Molnar P. Davis H. Hickman J.J. Characterization of a human fetal spinal cord stem cell line NSI-566RSC and its induction to functional motoneurons. Tissue Eng Regen Med. 2010;4:181. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das M. Rumsey J.W. Bhargava N. Stancescu M. Hickman J.J. Skeletal muscle tissue engineering: an improved model promoting long term survival of myotubes, structural development of E-C coupling apparatus and neonatal myosin heavy chain (MHC) expression. Biomaterials. 2009;30:5392. doi: 10.1016/j.biomaterials.2009.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan J. Xu L. Welsh A.M. Hatfield G. Hazel T. Johe K. Koliatsos V.E. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:318. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B.X. Ziskind-Conhaim L. Development of Glycine- and GABA-gated currents in rat spinal motoneurons. J Neurophysiol. 1995;74:113. doi: 10.1152/jn.1995.74.1.113. [DOI] [PubMed] [Google Scholar]
- 24.Takahasi T. Intracellular recording from visually identified motoneurons in rat spinal cord slices. Proc R Soc Lond B Biol Sci. 1978;202:417. doi: 10.1098/rspb.1978.0076. [DOI] [PubMed] [Google Scholar]
- 25.Burgess C. Lai D. Siegel J. Peever J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep–wake cycle. J Neurosci. 2008;28:4649. doi: 10.1523/JNEUROSCI.0334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clements J.D. Lester R.A. Tong G. Jahr C.E. Westbrook G.L. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 27.Rekling J.C. Funk G.D. Bayliss D.A. Dong X.W. Feldman J.L. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsina B. Vu T. Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 30.Ahmari S.E. Buchanan J. Smith S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 31.Fox M.A. Sanes J.R. Borza D.B. Eswarakuma V.P. Fassler R. Hudson B.G. John S.W.M. Ninomiya Y. Pedchenko V. Pfaff S.L. Rheault M.N. Sado Y. Segal Y. Werle M. Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Brewer G.J. Boehler M.D. Jones T.T. Wheeler B.C. NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J Neurosci Methods. 2008;170:181. doi: 10.1016/j.jneumeth.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goritz C. Mauch D.H. Pfrieger F.W. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Pfrieger F.W. Barres B.A. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 35.Sasahara K. Shikimi H. Haraguchi S. Sakamoto H. Honda S. Harada N. Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funakoshi H. Belluardo N. Arenas E. Yamamoto Y. Casabona A. Persson H. Ibanez C.F. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 37.Henderson C.E. Camu W. Mettling C. Gouin A. Poulsen K. Karihaloo M. Rullamas J. Evans T. McMahon S.B. Armanini M.P. Berkemeier L. Phillips H.S. Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 38.Henderson C.E. Phillips H.S. Pollock R.A. Davies A.M. Lemeulle C. Armanini M. Simpson L.C. Moffet B. Vandlen R.A. Koliatsos V.E. Rosenthal A. Gdnf—a potent survival factor for motoneurons present in peripheral-nerve and muscle. Science. 1994;266:1062. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 39.Sanes J.R. Lichtman J.W. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 40.Sanes J.R. Lichtman J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 41.Gao J. Coggeshall R.E. Tarasenko Y.I. Wu P. Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience. 2005;131:257. doi: 10.1016/j.neuroscience.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 42.Das M. Molnar P. Gregory C. Riedel L. Hickman J.J. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum free medium. Biomaterials. 2004;25:5643. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Kleinfeld D. Kahler K.H. Hockberger P.E. Controlled outgrowth of dissociated neurons on patterned substrates. J Neurosci. 1988;8:4098. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaffner A.E. Barker J.L. Stenger D.A. Hickman J.J. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods. 1995;62:111. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 45.Spargo B.J. Testoff M.A. Nielsen T.B. Stenger D.A. Hickman J.J. Rudolph A.S. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc Natl Acad Sci U S A. 1994;91:11070. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravenscroft M.S. Bateman K.E. Shaffer K.M. Schessler H.M. Jung D.R. Schneider T.W. Montgomery C.B. Custer T.L. Schaffner A.E. Liu Q.Y. Li Y.X. Barker J.L. Hickman J.J. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. J Am Chem Soc. 1998;120:12169. [Google Scholar]
- 47.Stenger D.A. Hickman J.J. Bateman K.E. Ravenscroft M.S. Ma W. Pancrazio J.J. Shaffer K. Schaffner A.E. Cribbs D.H. Cotman C.W. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. J Neurosci Methods. 1998;82:167. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.