Multidisciplinary meeting recommendations are largely concordant with guidelines in the treatment of lung cancer.
Abstract
Purpose:
Multidisciplinary meetings (MDMs) are increasingly being mandated as essential to oncology practice. However, there is a paucity of data on their effectiveness. The aim of this study was to assess whether MDM recommendations were concordant with guidelines in the treatment of lung cancer.
Patients and Methods:
The Lung Cancer Multidisciplinary Meeting in South West Sydney, Australia, prospectively collects data on all patients whose cases have been presented. New patients with lung cancer who presented between December 1, 2005, and December 31, 2007, were reviewed. Patients were assigned to treatment on the basis of evidence-based guidelines according to pathology, stage, and Eastern Cooperative Oncology Group (ECOG) performance status. MDM recommendations were compared with guideline treatment, and reasons for discrepancy were noted.
Results:
There were 335 patients with a median age of 69 years. Of these, 82% had non–small-cell lung cancer (NSCLC), 14% had small-cell lung cancer, and 4% had no pathologic diagnosis. Eighty-four percent had locally advanced or metastatic disease. Concordance of MDM recommendations with guideline treatment existed in 29 (58%) of 50 cases for surgery, 201 (88%) of 228 cases for radiotherapy, and 200 (77%) of 260 cases for chemotherapy. Overall concordance with guideline treatment was 71% (239 of 335 cases). On multivariate analysis, age greater than 70 years, ECOG performance status of 2 or higher, and stage III NSCLC were associated with the MDM not recommending guideline treatment. The primary reasons for this were physician decision (39%), comorbidity (25%), and technical factors (22%).
Conclusion:
MDM recommendations were largely concordant with guidelines. Physician discretion in not recommending guideline treatment was most often exercised in older patients and those with borderline performance status. Individual factors that may preclude guideline treatment cannot be accounted for by guidelines.
Introduction
Multidisciplinary meetings (MDMs) are commonplace in oncology. They represent a gathering of medical professionals and/or patients to form management plans for patients with a particular type of cancer. Potentially, these MDMs can help streamline care, improve efficiency of health service delivery, and increase adherence to guideline-based treatment. A systematic review of MDMs found that the benefits ranged from increased treatment utilization to reduced time from diagnosis to treatment to improved survival.1 There was also greater enrollment onto clinical trials.2,3
Some lung cancer guidelines state that all patients with lung cancer should be referred to an MDM.4–6 However, there is a paucity of data to support these recommendations. Conron et al7 found a high rate of concordance with guidelines but only three of these specifically related to treatment. Leo et al8 found a low rate of discordance between MDM recommendations and actual treatments received. None of these studies compared whether MDM recommendations followed evidence-based treatment guidelines according to stage of lung cancer.
There are numerous clinical practice guidelines for the management of lung cancer.4–6,9–11 These guidelines have been used to create decision trees for evidence-based use of chemotherapy and radiotherapy.12,13 The aim of this study was to assess concordance of MDM recommendations with evidence-based guidelines that use these decision trees.
Patients and Methods
A weekly MDM on lung cancer is held at the Liverpool and Macarthur Cancer Therapy Centres (South West Sydney, Australia). The meeting takes place during 50 weeks each year and is attended by respiratory physicians, a cardiothoracic surgeon, medical and radiation oncologists, palliative care physicians, a positron emission tomography physician, a radiologist, and lung cancer nurse. This composition has remained largely unchanged since early 2000. The Australian lung cancer guidelines,9 published in 2004, were available to clinicians to guide management decisions but were not actively referenced during the meeting.
Prospective electronic data collection has been in place since December 1, 2005, and includes patient demographics, specific comorbidities, smoking history, ECOG performance status, histology, diagnostic investigations, stage, and MDM consensus. For patients for whom the recommendation was best supportive care alone, the best reason for this was recorded (eg, comorbidity, ECOG performance status, patient decision, clinician decision).
There are models for evidence-based use of radiotherapy12 and chemotherapy13 in lung cancer. These decision trees use guideline recommendations to assign the use of radiotherapy or chemotherapy on the basis of pathology, stage, and performance status (Appendix Table A1, online only). There are no models for the use of surgery in lung cancer; however, this is included in the radiotherapy decision tree.12 Data concerning patients discussed at the MDM were entered into the model to assess guideline-based treatment for each patient. More than one option could be defined as guideline-based treatment if alternative treatments existed.
We performed a retrospective review of MDM decisions to assess the effect of this forum in recommending treatment according to guidelines that existed at the time. Data was retrieved on all patients with newly diagnosed lung cancer whose cases were presented at the MDM between December 1, 2005 and December 31, 2007. MDM recommendations were compared with guideline treatment to assess concordance. For patients for whom the guidelines recommended a combination of therapies, all therapies had to be recommended by the MDM for concordance to exist.
If there was a discordance, one investigator reviewed the electronic medical records to identify the best reason for the difference. The reasons included those already in the database for best supportive care as well as “technical factors,” “alternate treatment modality,” and “physician decision.” Technical factors considered for radiotherapy included large tumor volume, previous chest radiotherapy, proximity of tumor to spinal cord; those factors consider for surgery included proximity of tumor to neurovascular structures; and factors considered for chemotherapy included impaired renal or hematologic function. If no specific reason could be identified for discordance, the reason was recorded as a physician decision.
Non–small-cell lung cancer (NSCLC) was staged according to TNM (version 6) and small-cell lung cancer (SCLC) according to Veterans Administration staging.14,15
Analyses were performed using Statistical Package for the Social Sciences (SPSS) for Windows version 17.0 (SPSS, Chicago, IL). Univariate analyses were performed using χ2 tests, and multivariate analyses used logistic regression.
Results
Patient and Tumor Characteristics
Between December 1, 2005 and December 31, 2007, 335 patients with newly diagnosed lung cancer were presented. This represented 70% of all new patients with lung cancer seen at the cancer therapy centers and 59% of the patient population with lung cancer in South West Sydney.16 Patient and tumor characteristics are listed in Table 1. The median age of patients was 69 years; 64% of patients had good performance status (ECOG 0-1), and 66% had a comorbidity. Of all 335 patients, 82% had NSCLC, 14% had SCLC, and 4% had only been given clinical diagnoses. Most patients had stage III (36%) or IV (34%) NSCLC.
Table 1.
Patient and Tumor Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Male | 218 | 65 |
| Female | 117 | 35 |
| Country of birth | ||
| Australia | 163 | 49 |
| Other | 165 | 49 |
| Unknown | 7 | 2 |
| Language | ||
| English | 275 | 82 |
| Other | 59 | 18 |
| Unknown | 1 | 0 |
| Age, years | ||
| < 50 | 22 | 7 |
| 50-59 | 47 | 14 |
| 60-69 | 110 | 33 |
| 70-79 | 116 | 35 |
| > 80 | 40 | 12 |
| ECOG PS | ||
| 0-1 | 213 | 64 |
| 2 | 76 | 23 |
| 3-4 | 45 | 13 |
| Unknown | 1 | 0 |
| Comorbidity | ||
| Absent | 112 | 34 |
| Present | 221 | 66 |
| Pathology | ||
| Squamous cell | 60 | 18 |
| Adenocarcinoma | 90 | 27 |
| Large cell carcinoma | 109 | 32 |
| NSCLC NOS | 15 | 5 |
| SCLC | 46 | 14 |
| No pathology | 15 | 4 |
| Stage* | ||
| NSCLC | ||
| I | 29 | 9 |
| II | 25 | 7 |
| III | 122 | 36 |
| IV | 113 | 34 |
| SCLC | ||
| Limited stage | 19 | 6 |
| Extensive stage | 27 | 8 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non–small-cell lung cancer; NOS, not otherwise specified; SCLC, small-cell lung cancer.
Patients without pathologic diagnosis were staged with the NSCLC group.
Radiotherapy
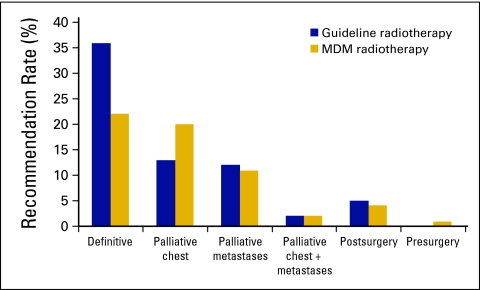
Guideline-based use of radiotherapy in this cohort was 68% (n = 228). The MDM recommended radiotherapy in only 60% (n = 201). There were 28 patients for whom radiotherapy was not recommended. The main reasons were technical factors that precluded safe delivery of radiotherapy and physician decision (Table 2). There was one patient for whom the MDM recommended radiotherapy when such was not the guideline-based treatment. In terms of radiotherapy intent, the main difference between guideline and MDM recommendations was in definitive radiotherapy (36% v 22%, respectively; Appendix Fig 1, online only). Conversely, the MDM recommended palliative chest radiotherapy more often (20% v 13%).
Table 2.
Reasons for the MDM Not Recommending Guideline Treatment, by Treatment Modality and Overall
| Reason | Radiotherapy (n = 28) |
Chemotherapy (n = 64) |
Surgery (n = 21) |
Overall* |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Physician decision | 8 | 29 | 34 | 53 | 4 | 19 | 37 | 39 |
| Technical factors | 8 | 29 | 5 | 7 | 3 | 14 | 21 | 22 |
| Patient decision | 2 | 7 | 1 | 2 | 3 | 14 | 3 | 3 |
| Change in ECOG PS/progressive disease | 1 | 3 | 7 | 11 | 0 | 0 | 9 | 9 |
| Lung comorbidity | 4 | 14 | 7 | 11 | 7 | 34 | 13 | 14 |
| Other comorbidity | 3 | 11 | 9 | 14 | 4 | 19 | 11 | 11 |
| Alternate modality | 2 | 7 | 1 | 2 | 0 | 0 | 2 | 2 |
Abbreviations: MDM, multidisciplinary meeting; ECOG PS, Eastern Cooperative Oncology Group performance status.
The overall patient numbers do not represent the sum of patient numbers for each treatment, as an individual patient could appear in more than one treatment column if multimodality treatment was the optimal treatment.
Chemotherapy
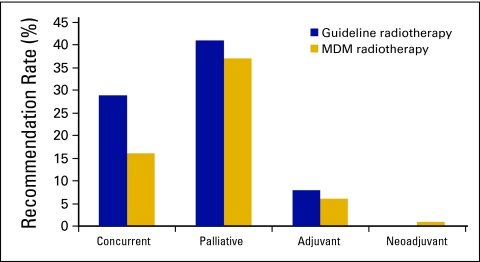
Guideline-based chemotherapy use in this cohort was 78% (n = 260). The MDM recommended chemotherapy in only 60% (n = 200). There were 64 patients for whom chemotherapy was not recommended by the MDM, largely as a result of physician decision or comorbidity (Table 2). The MDM recommended chemotherapy for 4 patients for whom that was not deemed to be guideline treatment by this study. Concurrent chemotherapy with radiotherapy was guideline treatment in 29% of patient cases but was only recommended in 16% (Appendix Fig A2, online only). Although 41% of patients had indications for palliative chemotherapy on the basis of guidelines, palliative treatment was recommended by the MDM for only 37%.
Surgery
The use of surgery as guideline treatment has been limited to patients with stage I and II NSCLC. Although there is a role for surgery in stage IIIA disease, the definition of operability differs from surgeon to surgeon. On the basis of evidence, surgery should have been assigned for 15% of patients (n = 50). The MDM recommended surgery for only 9% (n = 29), primarily because of comorbidities (Table 2). There were an additional 15 patients—all with stage III NSCLC—who were recommended for surgery by the MDM.
Overall Concordance
Overall, the MDM recommended guideline treatment for 71% of patients (239 of 335). The primary reasons for not recommending guideline treatment were physician decision and comorbidity. In those patients for whom the reason was physician decision, median age was 79 years, 56% (n = 20) were ECOG performance status 2, 47% (n = 17) had stage III NSCLC, and 33% (n = 12) had stage IV NSCLC. Respiratory comorbidity was the primary reason for nonguideline recommendations in stage I and II NSCLC (Appendix Table A2, online only), physician decision in stage III and IV NSCLC, and other comorbidity in extensive-stage SCLC. Technical factors were a prominent reason for lack of guideline recommendation in limited-stage SCLC and stage III NSCLC and generally related to large tumor volume precluding safe delivery of radical radiotherapy.
In univariate analysis, recommendation of guideline treatment by the MDM was significantly associated with age, ECOG performance status, disease stage, weight loss, comorbidity, and prior cancer. The median age of those for whom the MDM recommended guideline treatment was 67 years compared with 74 years for those for whom guideline treatment was not recommended. In multivariate analysis, only age, ECOG performance status, and disease stage remained independent predictors of whether the MDM recommended guideline treatment (Table 3). Patients age 70 years and older, those who were ECOG performance status 2, and those with stage III NSCLC were all significantly less likely to have guideline treatment recommended. Conversely, patients who were ECOG performance status 4 or had stage IV NSCLC or extensive-stage SCLC were more likely to have guideline treatment recommended for them, because for many of these patients with advanced disease, the guideline treatment may have been palliative care alone.
Table 3.
Concordance of MDM Recommendations With Guideline-Based Treatment According to Patient Characteristics
| Characteristic | Concordance (%) | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
| Age, years | .002 | |||
| < 50 | 82 | 1 | ||
| 50-59 | 81 | 1.1 | 0.3 to 4.3 | |
| 60-69 | 80 | 1.3 | 0.4 to 4.9 | |
| 70-79 | 63 | 0.5 | 0.1 to 1.8 | |
| > 80 | 55 | 0.4 | 0.1 to 1.6 | |
| ECOG PS | < .0001 | |||
| 0-1 | 78 | 1 | ||
| 2 | 43 | 0.2 | 0.1 to 0.4 | |
| 3-4 | 84 | 2.3 | 0.8 to 6.4 | |
| Stage | .005 | |||
| NSCLC | ||||
| I and II | 72 | 1 | ||
| III | 60 | 0.5 | 0.2 to 1 | |
| IV | 81 | 1.4 | 0.6 to 3.4 | |
| SCLC | ||||
| Limited stage | 68 | 1 | 0.3 to 3.9 | |
| Extensive stage | 82 | 2.3 | 0.6 to 9.1 |
Abbreviations: MDM, multidisciplinary meeting; ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Discussion
It is difficult to measure the effectiveness of MDMs and whether such meetings have an impact on patient outcomes. There have been three retrospective studies that have compared patient outcomes of patients discussed at MDMs with those of patients not discussed at MDMs.3,17,18 Riedel et al3 compared the timeliness of care of 244 patients discussed at an MDM with 101 patients who were not. They found no significant difference between time from presentation to diagnosis and time from diagnosis to treatment. Survival was also similar.
The other two studies have been limited to inoperable stage III and IV NSCLC.17,18 Bydder et al17 compared 81 patients discussed at MDMs with 17 patients not discussed at an MDM. They found increased use of treatment in the MDM cohort and significantly improved 1-year survival (18% v 33%). The authors did acknowledge that biases in selecting patients for MDM discussion could have impacted their findings. Forrest et al18 compared outcomes of patients who were diagnosed four years apart—before (n = 117) and after (n = 126) the introduction of an MDM. Chemotherapy use and median survival improved in the MDM cohort (3.2 months v 6.6 months). However, these findings could also be related to improved staging19 and more evidence supporting the use of chemotherapy over time.
A surrogate measure of MDM effect is the concordance of its recommendations with evidence-based guidelines. Population studies from throughout the world have shown that treatment use varies enormously, from 50% in New Zealand and Ireland to 81% in the United States.20–25 There have been two large population-based studies assessing the use of guideline treatment for patients with NSCLC.26,27
Potosky et al26 evaluated 898 patients with NSCLC from the Surveillance, Epidemiology, and End Results (SEER) database comparing actual management with guideline treatment. Overall, 52% of patients were treated according to guidelines, although concordance ranged from 69% of patients with stage I and II NSCLC to 48% of patients with stage III NSCLC and 41% of patients stage IV NSCLC. Similar to our findings, the use of guideline treatment decreased with age, from 75% for patients age 50 years and younger to 34% for those age 80 years and older.
De Rijke et al27 identified patients with lung cancer from two Dutch cancer registries (n = 803) and evaluated whether they had been treated according to local guidelines. The authors found that 82% of patients with stage I and II NSCLC, 48% of patients with stage IIIA NSCLC, and 54% of patients with stage IIIB NSCLC received guideline treatment. Of the patients with stage IV NSCLC, 64% received any treatment. The presence of comorbidity and age older than 75 years predicted for lack of guideline treatment. ECOG performance status was not a predictor of guideline treatment, although only 4% of the patient population was ECOG performance status 3 or 4.
The patients in the current study included patients with both NSCLC and SCLC. We found that 72% of patients with stage I and II NSCLC, 60% of patients with stage III NSCLC, 81% of patients with stage IV NSCLC, 68% of patients with limited-stage SCLC, and 82% of patients with extensive-stage SCLC received an MDM recommendation for treatment that was concordant with the guidelines. For patients with stage III and IV NSCLC, the concordance is higher than the use of guideline treatment documented in the US and Dutch populations.
All three studies show increasing age to be a negative predictor for guideline treatment, independent of comorbidity or ECOG performance status. This is probably because of a paucity of randomized data on treatment options for the elderly. Patients for whom guideline treatment was not recommended had an older median age of 79 years. Lung cancer guidelines do not have an age cutoff for treatment recommendations.4,6,9–11 Although age is associated with increased incidence of organ dysfunction, guideline treatment for patients with minimal comorbidities and good performance status can result in outcomes equivalent to those experienced by younger patients.28–32
Patients with ECOG performance status 2 were the least likely to receive guideline treatment. The uncertainty of outcome for these patients again reflects the selection criteria of many trials. Specific trials targeting this population do show a clinical benefit in patients with ECOG performance status 2, although survival remains poorer than for those with ECOG performance status 0-1.33,34 Newer biologic agents have an emerging role in this group of patients.35
Concordance of MDM recommendation to guideline treatment was lowest in stage III NSCLC, which represents a heterogeneous population. The optimal management is multimodality treatment. Physician decision was the primary reason for lack of a guideline treatment recommendation in this group, suggesting that doubt still exists about optimal treatment for these patients, especially patients in the older age group. Patient comorbidity and technical factors also meant that patients were unsuitable for guideline treatment.
Concordance between MDM recommendations and guideline treatment varied by treatment modality, with 58% (29 of 50) for surgery, 77% (200 of 260) for chemotherapy, and 88% (201 of 228) for radiotherapy. For surgery and radiotherapy, there were legitimate reasons for not recommending these treatments on the basis of comorbidity or technical factors, such as large tumor volume. Physician decision was the predominant reason for not recommending chemotherapy. It is important to note that this was the collective decision of the MDM, which took individual patient factors into account. The most marked difference was in the recommendation for definitive radiotherapy and concurrent chemotherapy. Although numerous trials have proven the benefit of this treatment, it may not be applicable to many in the general population with lung cancer, because of comorbidity, technical factors, or uncertain applicability in older patients and patients with borderline performance status.
This study simply assessed the management recommendations after discussion of a patient at a single MDM at which there was breadth of specialty attendance, written management protocols, and formalized data collection. The results may not be able to be generalized to other settings, particularly if the composition or process of the MDM differs substantially. The next step is to evaluate whether these patients actually received the treatment recommended by the MDM. Ideally, we would like to compare patterns of care and outcomes of this MDM cohort with the resident population diagnosed with lung cancer during the same time period. Both of these areas are currently the subjects of ongoing research.
In conclusion, MDM discussion resulted in recommendation of guideline treatment in a high proportion of patients. Physician discretion in not recommending guideline treatment was more often exercised for older patients and those with borderline performance status. These patients have been largely excluded from clinical trials, and additional data is necessary to guide treatment decisions. Given that guidelines cannot account for patient or tumor-specific factors that may preclude specific treatment in an individual patient, MDMs are the preferred forum to formulate management plans for patients with lung cancer.
Acknowledgment
We thank Gabriel Gabriel, FAFPHM, for his help with the statistical analysis. Presented in part at the Wold Conference on Lung Cancer, San Fransisco, CA, July-August 2009.
Appendix
Table A1.
Guideline-Based Treatment on the Basis of Pathology, Stage, and Performance Status
| Basis | Guideline Treatment |
|---|---|
| NSCLC | |
| I | |
| ECOG PS 0-2 | Surgery or curative radiotherapy |
| ECOG PS 3-4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| II | |
| ECOG PS 0-2 | Surgery and adjuvant chemotherapy or curative radiotherapy with or without chemotherapy |
| ECOG PS 3-4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| IIIA | |
| ECOG PS 0-2 | Surgery and chemotherapy (adjuvant or neoadjuvant) with or without radiotherapy or curative radiotherapy and chemotherapy |
| ECOG PS 3-4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| IIIB | |
| ECOG PS 0-2 | Curative radiotherapy and chemotherapy or palliative radiotherapy or palliative chemotherapy |
| ECOG PS 3-4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| IV | |
| ECOG PS 0-2 | Palliative chemotherapy with or without palliative radiotherapy to symptomatic sites |
| ECOG PS 3-4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| SCLC | |
| Limited | |
| ECOG PS 0-3 | Curative chemotherapy and radiotherapy |
| ECOG PS 4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
| Extensive | |
| ECOG PS 0-3 | Palliative chemotherapy with or without palliative radiotherapy to symptomatic sites |
| ECOG PS 4 | Best supportive care with or without palliative radiotherapy to symptomatic sites |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Data adapted.4–6,9–11
Table A2.
Reasons for the MDM Not Recommending Guideline Treatment According to Stage of Lung Cancer
| Reason | NSCLC |
SCLC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage I and II (n = 15) |
Stage III (n = 49) |
Stage IV (n = 21) |
Limited Stage (n = 6) |
Extensive Stage (n = 5) |
||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Physician decision | 3 | 20 | 18 | 37 | 12 | 57 | 2 | 33 | 2 | 40 |
| Technical factors | 3 | 20 | 12 | 25 | 2 | 9.5 | 4 | 67 | 0 | 0 |
| Patient decision | 2 | 13 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 |
| Change in ECOG PS/progressive disease | 1 | 7 | 6 | 12 | 2 | 9.5 | 0 | 0 | 0 | 0 |
| Lung comorbidity | 4 | 27 | 9 | 18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other comorbidity | 2 | 13 | 4 | 8 | 2 | 9.5 | 0 | 0 | 3 | 60 |
| Alternate modality | 0 | 0 | 0 | 0 | 2 | 9.5 | 0 | 0 | 0 | 0 |
Abbreviations: MDM, multidisciplinary meeting; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group performance status.
Figure A1.
Indications for radiotherapy: Guideline versus multidisciplinary meeting (MDM) recommendation.
Figure A2.
Indications for chemotherapy: Guideline versus multidisciplinary meeting (MDM) recommendation.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Shalini K. Vinod, Mark A. Sidhom
Administrative support: Mark A. Sidhom
Provision of study materials or patients: Shalini K. Vinod
Collection and assembly of data: Mark A. Sidhom
Data analysis and interpretation: Shalini K. Vinod, Mark A. Sidhom, Geoff P. Delaney
Manuscript writing: Shalini K. Vinod, Mark A. Sidhom, Geoff P. Delaney
Final approval of manuscript: Shalini K. Vinod, Mark A. Sidhom, Geoff P. Delaney
References
- 1.Wright FC, De Vito C, Langer B, et al. Multidisciplinary cancer conferences: A systematic review and development of practice standards. Eur J Cancer. 2007;43:1002–1010. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Bouvier AM, Bauvin E, Danzon A, et al. Place of multidisciplinary consulting meetings and clinical trials in the management of colorectal cancer in France in 2000. Gastroenterol Clin Biol. 2007;31:286–291. doi: 10.1016/s0399-8320(07)89375-4. [DOI] [PubMed] [Google Scholar]
- 3.Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1:692–696. [PubMed] [Google Scholar]
- 4.National Collaborating Centre for Acute Care. The diagnosis and treatment of lung cancer. http://www.rcseng.ac.uk/new_rcseng/content/publications/docs/lung_cancer.html.
- 5.The Royal College of Radiologists Clinical Oncology Information Network. Guidelines on the non-surgical management of lung cancer. Clin Oncol (R Coll Radiol) 1999;11(suppl):S1–S53. [PubMed] [Google Scholar]
- 6.American College of Chest Physicians, Health and Science Policy Committee. Diagnosis and management of lung cancer: ACCP evidence-based guidelines—American College of Chest Physicians. Chest. 2003;123S(suppl):1–337. [PubMed] [Google Scholar]
- 7.Conron M, Phuah S, Steinfort D, et al. Analysis of multidisciplinary lung cancer practice. Int Med J. 2007;37:18–25. doi: 10.1111/j.1445-5994.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 8.Leo F, Venissac N, Poudenx M, et al. Multidisciplinary management of lung cancer: How to test its efficacy? J Thorac Oncol. 2007;2:69–72. doi: 10.1097/JTO.0b013e31802bff56. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Council Australia. Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/cp97.pdf.
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer V2. 2006. http://www.nccn.org.
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Small cell lung cancer—V1.2006. www.nccn.org.
- 12.Delaney G, Barton M, Jacob S, et al. A model for decision making for the use of radiotherapy in lung cancer. Lancet Oncol. 2003;4:120–128. doi: 10.1016/s1470-2045(03)00984-7. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Hovey E, Ng W, et al. Estimation of an optimal chemotherapy utilisation rate for lung cancer: An evidence-based benchmark for Cancer Care. Lung Cancer. 2010;69:307–314. doi: 10.1016/j.lungcan.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Sobin L, Wittekind C, editors. TNM Classification of Malignant Tumours. ed 6. New York, NY: Wiley-Liss; 2002. pp. 99–103. [Google Scholar]
- 15.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—What limits limited disease? Lung Cancer. 2003;37:271–276. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 16.Vinod SK, Sidhom MA, Gabriel GS, et al. Why do some lung cancer patients receive no anti-cancer treatment? J Thor Oncol. 2010;5:1025–1032. doi: 10.1097/JTO.0b013e3181da85e4. [DOI] [PubMed] [Google Scholar]
- 17.Bydder S, Nowak A, Marion K, et al. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39:838–841. doi: 10.1111/j.1445-5994.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 18.Forrest LM, McMillan DC, McArdle CS, et al. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93:977–978. doi: 10.1038/sj.bjc.6602825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mac Manus MP, Wong K, Hicks RJ, et al. Early mortality after radical radiotherapy for non-small-cell lung cancer: Comparison of PET-staged and conventionally staged cohorts treated at a large tertiary referral center. Int J Radiat Oncol Biol Phys. 2002;52:351–361. doi: 10.1016/s0360-3016(01)02673-6. [DOI] [PubMed] [Google Scholar]
- 20.Stevens W, Stevens G, Kolbe J, et al. Lung cancer in New Zealand: Patterns of secondary care and implications for survival. J Thorac Oncol. 2007;2:481–493. doi: 10.1097/JTO.0b013e31805fea3a. [DOI] [PubMed] [Google Scholar]
- 21.Mahmud SM, Reilly M, Comber H. Patterns of initial management of lung cancer in the Republic of Ireland: A population-based observational study. Lung Cancer. 2003;41:57–64. doi: 10.1016/s0169-5002(03)00148-x. [DOI] [PubMed] [Google Scholar]
- 22.Vinod SK, O'Connell D, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol. 2008;3:871–879. doi: 10.1097/JTO.0b013e31818020c3. [DOI] [PubMed] [Google Scholar]
- 23.Richardson GE, Thursfield VJ, Giles GG. Reported management of lung cancer in Victoria in 1993: Comparison with best practice—Anti-Cancer Council of Victoria Lung Cancer Study Group. Med J Aust. 2000;172:321–324. [PubMed] [Google Scholar]
- 24.Erridge SC, Murray B, Price A, et al. Improved treatment and survival for lung cancer patients in South-East Scotland. J Thorac Oncol. 2008;3:491–498. doi: 10.1097/JTO.0b013e31816fca46. [DOI] [PubMed] [Google Scholar]
- 25.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States. Cancer. 1999;86:1867–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 27.de Rijke JM, Schouten LJ, ten Velde GP, et al. Influence of age, comorbidity and performance status on the choice of treatment for patients with non-small cell lung cancer; results of a population-based study. Lung Cancer. 2004;46:233–245. doi: 10.1016/j.lungcan.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Bernet F, Brodbeck R, Guenin MO, et al. Age does not influence early and late tumor-related outcome for bronchogenic carcinoma. Ann Thorac Surg. 2000;69:913–918. doi: 10.1016/s0003-4975(99)01439-3. [DOI] [PubMed] [Google Scholar]
- 29.Gauden SJ, Tripcony L. The curative treatment by radiation therapy alone of Stage I non-small cell lung cancer in a geriatric population. Lung Cancer. 2001;32:71–79. doi: 10.1016/s0169-5002(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 30.Quon H, Shepherd FA, Payne DG, et al. The influence of age on the delivery, tolerance, and efficacy of thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys. 1999;43:39–45. doi: 10.1016/s0360-3016(98)00373-3. [DOI] [PubMed] [Google Scholar]
- 31.Tombolini V, Bonanni A, Donato V, et al. Radiotherapy alone in elderly patients with medically inoperable stage IIIA and IIIB non-small cell lung cancer. Anticancer Res. 2000;20:4829–4833. [PubMed] [Google Scholar]
- 32.Hickish TF, Smith IE, O'Brien ME, et al. Clinical benefit from palliative chemotherapy in non-small-cell lung cancer extends to the elderly and those with poor prognostic factors. Br J Cancer. 1998;78:28–33. doi: 10.1038/bjc.1998.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helbekkmo N, Aasebø U, Sundstrøm SH, et al. Treatment outcome in performance status 2 advanced NSCLC patients administered platinum-based combination chemotherapy. Lung Cancer. 2008;62:253–260. doi: 10.1016/j.lungcan.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Langer C, Li S, Schiller J, Tester W, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol. 2007;25:418–423. doi: 10.1200/JCO.2005.04.9452. [DOI] [PubMed] [Google Scholar]
- 35.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. 2009;27:2253–2260. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]