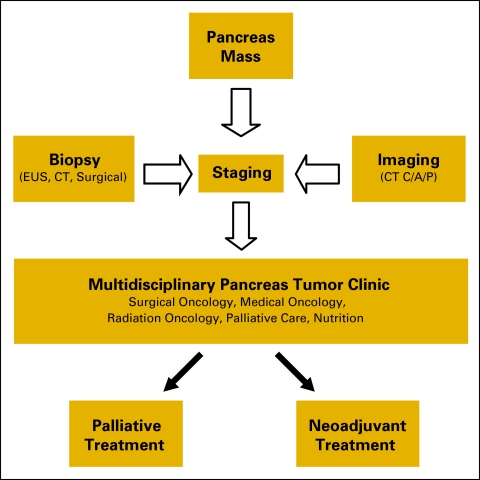
The establishment of a multidisciplinary pancreas tumor clinic led to improved patient access to consultations and shorter time to initial treatment in patients with pancreatic adenocarcinoma undergoing neoadjuvant therapy.
Abstract
Purpose:
Neoadjuvant therapy for pancreatic adenocarcinoma requires referral to multiple specialists before initiating therapy. We evaluated the effect of establishing a multidisciplinary clinic (MDC) for patients with newly diagnosed pancreatic adenocarcinoma on treatment access and time to therapy.
Methods:
Patients with newly diagnosed pancreatic adenocarcinoma diagnosed and treated at our center were included. Two patient groups were defined: preclinic represented those patients diagnosed before 2008 and MDC represented those patients diagnosed since 2009 who were treated in the newly created MDC and were initially candidates for neoadjuvant therapy. The primary outcomes were days from diagnosis to first treatment (initiation of chemotherapy or external beam radiation), days to completion of all required consultations, and number of visits needed before initiation of therapy.
Results:
Ninety-seven patients were diagnosed and treated at our medical center from 2003 to 2008; 22 were treated in 2009 after the implementation of the MDC. Compared with the preclinic group, patients treated in the MDC had shorter times from biopsy to treatment (7.7 days v 29.5 days, P < .001), shorter time to completion of all required pretreatment consultations (7.1 days v 13.9 days, P < .001), and fewer visits to complete all consultations (1.1 v 4.3, P < .001). Thirty-three percent of patients seen in the MDC enrolled onto clinical research trials.
Conclusion:
In patients with pancreatic adenocarcinoma undergoing neoadjuvant therapy, the establishment of a multidisciplinary pancreas tumor clinic led to improved patient access to consultations and shorter time to initial treatment.
Introduction
Pancreatic adenocarcinoma remains a devastating disease, with 5-year mortality rates of approximately 95% and a median time from diagnosis to death of 5 months.1–3 Because mortality rates remain stagnant with the use of up-front operative resection, centers increasingly are treating patients with neoadjuvant chemoradiotherapy before surgery.4–8 Although prospective, randomized comparison with conventional up-front resection is lacking, retrospective studies have demonstrated that neoadjuvant therapy provides better local control of disease and has in specific cases enabled patients with previously unresectable tumors to become candidates for surgery.9
Since 2003, our center has almost exclusively used neoadjuvant chemoradiotherapy in patients with resectable or borderline resectable pancreatic adenocarcinoma. This approach is also used in patients with locally unresectable disease in an attempt to “downstage” for possible operative intervention. Before the initiation of treatment, this therapeutic approach requires endosonographic, pathologic, and radiographic staging, along with medical, surgical, and radiation oncology consultation. In addition, patients are routinely referred for palliative care and nutrition evaluation. Although occasionally these appointments can be scheduled concurrently on the same day, most often patients must present multiple times for consultations before the initiation of treatment. The need to return for multiple visits before initiating therapy, especially in patients who live a long distance from our medical center, can create anxiety, especially if there is a patient perception of treatment delay. Furthermore, scheduling multiple visits can be an inefficient undertaking for administrative staff.
Multidisciplinary care has been increasingly advocated as cancer patients are requiring more complex, multimodality therapy. Multidisciplinary cancer clinics can conceivably facilitate establishment of the correct diagnosis, accurate staging, appropriate therapy, and accrual onto clinical trials.10–12 Although few data exist about the impact of multidisciplinary clinics on important clinical outcomes, one group has reported that after establishing a multidisciplinary pancreatic cancer clinic, 23.6% of their patients had a change in their recommended management and 77.8% of patients enrolled in the National Familial Pancreas Tumor Registry.13
In an effort to streamline time to treatment and improve patient access, our center developed a multidisciplinary pancreas tumor clinic (MDC) in January 2009. The clinic meets weekly and is exclusively for patients with newly diagnosed pancreatic adenocarcinoma. It is coordinated by the Pancreas Disorders Center in the section of Gastroenterology and Hepatology and is staffed by medical, surgical, and radiation oncologists; palliative care specialists; clinical nutritionists; and research coordinators.
Our purpose in performing this study was to evaluate the effect of MDC implementation on important process outcomes including days from diagnosis to treatment, days to completion of all required consultations, and number of visits needed before initiation of therapy in patients before and after establishment of the MDC. We hypothesized that patient access and time to treatment would improve after this intervention.
Methods
Patients were retrospectively identified using records from the Dartmouth-Hitchcock tumor board registry, and this study was approved by the Dartmouth Committee for the Protection of Human Subjects (No. 22,134). Patients were categorized into two groups. The preclinic group represents those patients treated at our center for pancreatic adenocarcinoma between 2003 and mid-2008. We chose 2003 as the initial time of enrollment because at that time our center reached a consensus, on the basis of our own studies and those of others, that neoadjuvant treatment was preferred, even for patients with resectable tumors.2,4–6 All of these patients were treated exclusively at our center (radiation, chemotherapy, operative intervention, and so on). The MDC group represents those patients who were seen in the MDC from the time of its established in January 2009 to January 2010. All included patients were considered candidates for neoadjuvant treatment; however, not all of the patients underwent this therapy after being seen in the MDC. Patients were excluded from the study if they had been diagnosed at another center; sought another opinion before initiation of treatment at our center; or once diagnosed at our center, sought treatment elsewhere.
Patients referred to the MDC were only those with newly diagnosed pancreatic adenocarcinoma. These patients were referred after endoscopic ultrasound fine needle aspiration of a pancreatic mass. The MDC was coordinated via the Section of Gastroenterology and Hepatology, where most referrals originate, as a result of the preference for endoscopic ultrasound-guided fine needle aspiration for diagnosis.14 In the morning before the MDC, all patients' cases were presented in the multidisciplinary gastrointestinal tumor board, where their disease was categorized as resectable, borderline resectable, locally unresectable, or metastatic.
The MDC is held every week and is staffed by physicians from medical oncology, radiation oncology, and surgical oncology, as well as palliative care providers and clinical nutritionists. All patients referred to our center for evaluation of newly diagnosed pancreatic adenocarcinoma were seen in the clinic after its implementation. On the basis of recommendations from the MDC, patients were started on either neoadjuvant treatment or best supportive care palliative chemotherapy.
For the purposes of the study, treatment initiation was defined as the first day of chemotherapy or the day of external-beam radiation simulation. Patients underwent neoadjuvant chemoradiotherapy using one of three regimens: (1) cetuximab 400 mg/m2 intravenous (IV) once followed by 250 mg/m2 IV weekly and gemcitabine biweekly 50 mg/m2 IV over 6 weeks with concurrent radiotherapy 54 Gy over 28 fractions; (2) docetaxel 65 mg/m2 IV and gemcitabine 4,000 mg/m2 IV given on days 1, 15, and 29, followed on day 43 by radiotherapy at 50.4 Gy, with gemcitabine 50 mg/m2 IV twice weekly for 12 doses; (3) radiotherapy at 50.4 Gy in 28 fractions, concurrent with gemcitabine 50 mg/m2 biweekly for 6 weeks. At the completion of neoadjuvant chemoradiotherapy, most patients underwent pancreaticoduodenectomy. Patients with unresectable or metastatic tumors were offered palliative chemotherapy (Figure 1).
Figure 1.
Multidisciplinary pancreas tumor clinic care algorithm.
The primary outcomes were the days from diagnosis to therapy, days to completion of all required pretreatment consultations, and number of visits needed before treatment initiation in patients before and after establishment of the MDC. Patient were excluded from analysis if their therapy was delayed because of laboratory abnormalities (ie, elevated liver tests) or delays in scheduling because of patient request. The secondary outcome was the number of patients seen in the MDC who enrolled subsequently in clinical trials.
Descriptive statistics were used to characterize our population and are reported as means, standard deviations, and 95% confidence intervals. The two-tailed Fisher's exact test was used to compare categorical variables and the two-tailed Student's t test for continuous variables. An alpha level of 0.05% was set for significance. Analysis was performed using Microsoft EXCEL (Microsoft Corporation, Redmond, WA) and Graphpad (Graphpad Software, Inc., San Diego, CA).
Results
From 2003 to mid-2008, we identified 97 patients treated at our center with the intent of pursuing neoadjuvant chemoradiotherapy after the diagnosis of pancreatic adenocarcinoma. Each patient was diagnosed and initiated treatment at our center. For the purposes of the primary outcomes, 54 patients were excluded because they had treatment initiation delayed as a result, for example, of elevated liver tests or patient preference (52) or a desire to seek further consultation elsewhere (two).
From January 2009 to January 2010, 54 patients were seen in the MDC. Of those patients, 27 were either diagnosed at another institution (11), received their initial treatment elsewhere (nine) or did not receive any subsequent therapy after being initially evaluated (seven). Of the 27 remaining patients, five had treatment initiation delayed as a result of either elevated liver tests that precluded therapy or patient request. This left 22 patients for evaluation in the MDC group.
At each visit to the MDC, patients were seen by medical, radiation, and surgical oncology physicians, along with palliative care and nutrition providers if they were deemed as possible candidates for neoadjuvant treatment. Patients offered palliative chemotherapy were seen by medical oncology, palliative care, nutrition, and occasionally surgical oncology.
Table 1 shows the baseline patient characteristics of the two groups. Patients seen in the MDC were older and less likely to undergo neoadjuvant therapy. As of the writing of the manuscript, six patients had undergone pancreaticoduodenectomy, 10 were undergoing neoadjuvant multimodality therapy or palliative chemotherapy, and six had died (median survival after diagnosis, 6.1 month) in the MDC group. The median survival in the preclinic group was 13.2 months.
Table 1.
Baseline Patient Characteristics
| Characteristic | Preclinic | Multidisciplinary Clinic | P |
|---|---|---|---|
| No. of patients | 43 | 22 | |
| Age, years | 63 | 70 | .043 |
| Gender | .072 | ||
| Male | 22 | 8 | |
| Femalel | 21 | 14 | |
| Treatment regimen, %* | |||
| Neoadjuvant therapy | 91 | 64 | < .001 |
| Palliative therapy | 9 | 36 |
Neoadjuvant therapy was used in patients with resectable, borderline resectable, or locally unresectable (without metastases) disease for downstaging before planned pancreaticoduodenctomy. Regimens included single-agent gemcitabine, gemcitabine and docetaxel, and gemcitabine plus cetuximab. Radiation therapy was used in patients considered appropriate for planned pancreaticoduodenectomy. Palliative chemotherapy was used in patients with metastatic or locally unresectable disease who did not wish to pursue a neoadjuvant approach.
The primary clinical outcomes are shown in Table 2. The mean time from biopsy to initiation of treatment in the MDC group was 22 days shorter than in the preclinic group after implementation of the MDC. Consultations were completed in a more timely fashion in the MDC group, and the number of visits to our center before the initiation of treatment was 300% lower in the MDC group.
Table 2.
Primary Clinical Outcomes
| Outcome | Preclinic (n = 43) |
Multidisciplinary Clinic (n = 22) |
P | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Time to first treatment from time of biopsy, days | 29.5 | 27.3 to 31.7 | 7.7 | 2.1 to 13.5 | < .0001 |
| Completion of all required consultations, days | 13.9 | 12.5 to 15.4 | 7.1 | 4.6 to 10.8 | < .001 |
| General surgery | 7.6 | ||||
| Radiation oncology | 9.2 | ||||
| Medical oncology | 10.9 | ||||
| Number of visits to complete all consultations | 4.3 | 1.1 | < .0001 | ||
Thirty-three percent of those patients seen in the MDC enrolled onto a clinical trial at the time of their initial consultations. Accurate data were not available to determine the rate of clinical trial enrollment in patients in the preclinic group.
Discussion
This analysis demonstrates that initiating a multidisciplinary pancreas tumor clinic for patients believed to be candidates for neoadjuvant therapy secondary to pancreatic adenocarcinoma shortens time to treatment, improves wait times for completion of all required consultations, and limits the number of clinic-based visits before therapeutic intervention. The clinic also provides an opportunity for all patients to be seen by palliative care and nutrition services in a coordinated setting. These processes of care improvement are important in patients with such a limited life expectancy. In addition, one third of patients seen in the clinic enrolled onto a research trial.
The multidisciplinary model represents one component of integrated care, which also includes patient centeredness (that patients are actively involved in decisions about their own care) and organization of care (seamless and continuous care is given with optimal coordination and organization of the total care process).15 Multidisciplinary clinics have been used effectively in several diseases, such as diabetes and heart failure, but limited data exist that specifically focus on malignancy.11,15–18 The only report specifically addressing the effect of a multidisciplinary pancreatic cancer clinic on patient care demonstrated that the coordinated effort in the clinic led to a change in management in one quarter of patients.13 However, the article did not highlight any change in important patient process outcomes.
Initiating a neoadjuvant approach in pancreatic adenocarcinoma requires extensive resource allocation and planning. At minimum, patients must receive a tissue diagnosis; appropriate staging, usually with computed tomography and sometimes diagnostic laparoscopy; and serologic testing. Medical oncology, radiation oncology, and surgical oncology consultations need to be completed in the context of patient and familial anxiety about delayed treatment. Palliative care consultation is usually appropriate, as is nutritional evaluation.19 Furthermore, enrollment in clinical trials can potentially be expedited by having a research coordinator present in the clinic.20
We have discovered advantages to using the MDC model for neoadjuvant disease in several key areas that have the immediate clinical impact of improving the quality of care. The MDC allows efficient scheduling at the time of diagnosis, thus helping to allay patient anxieties with regard to delay of care. One visit spread over several hours, as opposed to multiple short appointments over several days, makes it easier for family members to attend all necessary consultations. Our experience is that patients generally do not feel overwhelmed by seeing multiple providers on the same day. Many of our patients travel several hours to visit the clinic, so having to return for multiple visits can be especially burdensome.
Having a weekly clinic also provides the initial diagnostic clinician with a centralized, standardized referral setting. Treating clinicians have the opportunity to collaborate in the clinic between appointments, thereby improving efficiency and potentially altering treatment decisions through shared discussion. Universally, treating provider satisfaction has improved with the initiation of the MDC. Higher patient volumes also likely contribute to better surgical outcomes, especially given the skills required to maintain proficiency with Whipple resection.21 We also have not noted any impediemts to multiple providers billing under the same diagnosis on the same day. Finally, the MDC serves as a focal point for research study enrollment, with one third of our patients enrolling onto clinical trials.
This study has potential weaknesses that may limit the clinical application of its results. Because of the aggressive nature of pancreatic adenocarcinoma, we believe improved time to treatment and patient access improves patient satisfaction, but we did not specifically evaluate this outcome. We also did not evaluate whether the MDC resulted in a change in the number of patients referred to our center. Finally, more patients in the preclinic group underwent neoadjuvant chemotherapy. The reason for this finding is likely the improved quality of care offered by the MDC, as only patients who received all of their care at our center (and therefore underwent neoadjuvant therapy) were included in the preclinic group. Patients in the MDC group were more likely to have palliative therapy administered at our center, probably because of the efficiency of their care.
In conclusion, this study demonstrates that implementing a multidisciplinary pancreas tumor clinic shortened our patient's time to therapy, shortened the time to complete all required consultations, and resulted in a drastic reduction in the number of pretreatment visits. This focused intervention dramatically improved our ability to efficiently evaluate and treat patients with newly diagnosed pancreatic adenocarcinoma. Although implementation of this clinic alone is not likely to result in improved patient mortality, we believe it has improved the quality of care we provide. As neoadjuvant treatment for pancreatic adenocarcinoma is becoming a more popular therapeutic modality, the multidisciplinary clinic model should be considered by high-volume centers.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Timothy B. Gardner, Brian R. Boulay, Gregory H. Ripple, Thomas A. Colacchio, Ira R. Byock, Stuart R. Gordon
Administrative support: Timothy B. Gardner, Thomas A. Colacchio
Provision of study materials or patients: Timothy B. Gardner, Richard J. Barth Jr, Bassem I. Zaki, Margit M. McGowan, John E. Sutton, Gregory H. Ripple, Thomas A. Colacchio, Kerrington D. Smith, Ira R. Byock, Marsha Call, Michael J. Tsapakos, Jeannine B. Mills, Amitabh Srivastava, Mikhail Lisovsky, Stuart R. Gordon, J. Marc Pipas
Collection and assembly of data: Timothy B. Gardner, Brian R. Boulay, Margit M. McGowan, Marsha Call, Arief A. Suriawinata, Amitabh Srivastava, Maureen Stannard, Mikhail Lisovsky, Stuart R. Gordon, J. Marc Pipas
Data analysis and interpretation: Timothy B. Gardner, Richard J. Barth Jr, Brian R. Boulay, Marsha Call, Arief A. Suriawinata, Mikhail Lisovsky, Stuart R. Gordon, J. Marc Pipas
Manuscript writing: Timothy B. Gardner, Richard J. Barth Jr, Margit M. McGowan, J. Marc Pipas
Final approval of manuscript: Timothy B. Gardner, Richard J. Barth Jr, Bassem I. Zaki, Brian R. Boulay, Margit M. McGowan, John E. Sutton, Gregory H. Ripple, Thomas A. Colacchio, Kerrington D. Smith, Ira R. Byock, Marsha Call, Arief A. Suriawinata, Michael J. Tsapakos, Jeannine B. Mills, Amitabh Srivastava, Maureen Stannard, Mikhail Lisovsky, Stuart R. Gordon, J. Marc Pipas
References
- 1.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 2.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 3.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial or preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: An Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 5.Pipas JM, Mitchell SE, Barth RJ, Jr, et al. Phase I study of twice-weekly gemcitabine and concomitant external-beam radiotherapy in patients with adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2001;50:1317–122. doi: 10.1016/s0360-3016(01)01580-2. [DOI] [PubMed] [Google Scholar]
- 6.Pipas JM, Barth RJ, Jr, Zaki B, et al. Doxetaxel/gemcitabine followed by gemcitabine and external beam radiotherapy in patients with pancreatic carcinoma. Ann Surg Oncol. 2005;12:995–1004. doi: 10.1245/ASO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 7.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 8.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 9.Greer SE, Pipas JM, Sutton JE, et al. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. J Am Coll Surg. 2008;206:451–457. doi: 10.1016/j.jamcollsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Chang JH, Vines E, Bertsch H, et al. The impact of a multi-displinary breast cancer center on recommendations for patient management: The Univeristy of Penssylvania experience. Cancer. 2001;91:1231–127. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107:2346–2351. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 12.Gutmann EJ. Pathologists and patients: Can we talk? Mod Pathol. 2003;16:515–518. doi: 10.1097/01.MP.0000068260.01286.AC. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–208. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner TB, Chari ST. Endoscopic ultrasonography and pancreatic cancer. Minerva Gastroenterol Dietol. 2008;54:161–176. [PubMed] [Google Scholar]
- 15.Ouwens M, Hulscher M, Hermens R, et al. Implementation of integrated care for patients with cancer: A systematic review of interventions and effects. Int J Qual Health Care. 2009;21:137–144. doi: 10.1093/intqhc/mzn061. [DOI] [PubMed] [Google Scholar]
- 16.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: The chronic care model, Part 2. JAMA. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 17.Mur-Veeman I, Hardy B, Steenbergen M, et al. Development of integrated care in England and the Netherlands: Managing across public–private boundaries. Health Policy. 2003;65:227–241. doi: 10.1016/s0168-8510(02)00215-4. [DOI] [PubMed] [Google Scholar]
- 18.Ouwens M, Wollersheim H, Hermens R, et al. Integrated care programmes for chronically ill patients: A review of systematic reviews. Int J Qual Health Care. 2005;17:141–146. doi: 10.1093/intqhc/mzi016. [DOI] [PubMed] [Google Scholar]
- 19.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–227. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]