The optimal combination and sequencing of new chemotherapies and biologic agents in the treatment of metastatic colorectal cancer are yet to be determined. This study examined the extent and pattern of use by line of treatment.
Abstract
Purpose:
With the emergence of new chemotherapies and biologic agents in the treatment of metastatic colorectal cancer (mCRC), the optimal combination and sequencing of these therapies are yet to be determined. This study examined the extent and pattern of chemotherapy and biologic therapy use by line of treatment. Biologic continuation and dose escalation were also examined.
Methods:
This study used an integrated electronic medical record database of 91 US oncology practices. Records were analyzed for 1,655 adult patients with mCRC who were treated from January 1, 2004 to January 31, 2008 with systemic therapy and could be observed for ≥ 3 months beyond their diagnosis of metastatic disease. Combination and sequence of individual drugs and regimens were examined.
Results:
For first-line therapy, the most common chemotherapy backbone was infused fluorouracil, leucovorin, and oxaliplatin (FOLFOX; 40.5% of patients), and the most common treatment regimen was FOLFOX plus bevacizumab (26.2%). For second-line therapy, fluorouracil, leucovorin, and irinotecan (FOLFIRI) was the most common chemotherapy backbone (25.7%), and FOLFIRI plus bevacizumab was the most common treatment regimen (18.3%). Across the study period, 68.6%, 22%, and 7% of patients received bevacizumab, cetuximab, and panitumumab, respectively. Among 412 patients receiving bevacizumab-containing regimens as first-line therapy who then received second-line therapy, 58% continued receiving bevacizumab, with dose escalation observed in 44%.
Conclusion:
The most commonly used chemotherapy backbones for mCRC treatment were first-line FOLFOX and second-line FOLFIRI. Bevacizumab was the most frequently administered biologic therapy. Continuation and dose escalation with bevacizumab were frequently observed across lines of therapy.
Introduction
Over the last decade, the median overall survival for patients diagnosed with metastatic colorectal cancer (mCRC) has doubled because of advancements in diagnostic and surgical techniques coupled with the development of new chemotherapies and targeted biologic therapies.1–3 The treatment of mCRC has evolved significantly, with five classes of drug therapies currently available. Three chemotherapeutic classes, including fluoropyrimidine, irinotecan, and oxaliplatin, are commonly used to treat mCRC. Bevacizumab, a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), combined with fluoropyrimidine-based chemotherapy is approved for the treatment of mCRC. Cetuximab, a chimeric monoclonal antibody targeting the epidermal growth factor receptor (EGFR), is approved as monotherapy in patients with disease progression after treatment with irinotecan or oxaliplatin and in patients intolerant to irinotecan. Cetuximab is also approved in combination with irinotecan in irinotecan-resistant patients. Panitumumab, a fully human monoclonal antibody against EGFR, is approved as monotherapy for the treatment of mCRC with disease progression during or after treatment with fluoropyrimidine, oxaliplatin, and irinotecan-containing chemotherapy regimens.
The benefits of combination chemotherapy over monotherapy for first-line treatment of mCRC have been demonstrated.1 Doublet chemotherapy regimens improve objective response and progression-free survival rates and may improve overall survival compared with fluoropyrimidine monotherapy. Chemotherapy regimens comprising fluorouracil plus leucovorin in combination with oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) have emerged as the chemotherapy standard for the treatment of mCRC. FOLFOX is often chosen over FOLFIRI as first-line therapy on the basis of a direct comparison study demonstrating that more patients underwent curative resection with FOLFOX.4
National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology recommend approved biologics in combination with common chemotherapy backbones as treatment choices for mCRC.5–7 Adding bevacizumab to first- and second-line chemotherapy regimens improves patient prognosis; however, there is a lack of evidence from randomized trials to support the practice of biologic continuation (ie, continuation from first- to second-line therapy) with monoclonal antibodies, including bevacizumab.1,8 Although an observational cohort study suggests that continuing bevacizumab beyond first progression could be associated with a relevant clinical benefit,9 investigator and selection biases may have influenced the study design.10 Likewise, evidence to support dose escalation of monoclonal antibodies within or between lines of therapy for the treatment of mCRC is not available from randomized studies.
Although the benefit of adding biologics to chemotherapy backbones has been demonstrated in clinical trials, the optimal combinations and sequencing of the chemotherapeutic and biologic agents have not been established.6,7,11,12 Furthermore, little is known about the patterns of use of the five classes of treatment in clinical practice. The degree to which clinical use of chemotherapy and biologic therapy is consistent with approved indications or reflects treatment combinations demonstrated to be effective in clinical trials is unknown. This retrospective, observational study examined usage patterns of chemotherapy and biologic therapy by line of treatment in oncology practices in the United States. In addition, the extent of continuation and dose escalation for biologic agents were examined. This study was designed to provide insight into the real-world use of new biologic therapies in the context of standard chemotherapeutic agents in a large sample representative of US practice patterns.
Methods
Data Source
In this retrospective, observational study, data were extracted from an integrated database of electronic medical records (EMRs: Varian Medical Systems13 and IMPAC14) of 304,654 patients with cancer from 91 US oncology practice sites across 19 states to examine the frequency, patterns of use, and dosing of chemotherapy and biologic therapy for mCRC across lines of therapy. The practices were generally community based, staffed by five or more oncologists, and used one of the specified EMR systems. The database includes all treated patients with cancer.
At patient visits to the clinic, staff entered into the database information including patient demographics, insurer, diagnosis, and type of treatment. Demographic information included patient year of birth, gender, and geographic region of the clinic. Diagnosis data included date of initial CRC diagnosis; Tumor, Node, Metastasis (TNM) stage at diagnosis; date of metastases; and tumor characteristics. Treatment data included orders or prescriptions for medications, such as specific oncology drugs provided in the clinic; dosage; route of administration; and drug administration date. Laboratory test results that included test date, name, and results were captured directly from the laboratory system or manually entered into the EMR system. Various treatment guidelines were electronically available in the EMRs with the clinician determining the treatment plan and final orders.
Patient Selection Criteria
Patients were included in the study if they were (1) newly diagnosed as of their first practice visit with mCRC or were diagnosed with metastatic disease between January 1, 2004 and January 31, 2008; (2) ≥ 18 years old at diagnosis; (3) receiving chemotherapy and/or biologic treatment; and (4) could be observed for ≥ 3 months beyond the date of diagnosis of metastatic disease. Patients were excluded if they had diagnoses of multiple primary tumors or were in a clinical trial at any time during the study period.
CRC was identified on the basis of the International Classification of Diseases, ninth revision (ICD-9) diagnosis code of 153 (excluding 153.5, 154, 154.0, 154.1, or 154.8; Appendix Table A1, online only). The metastatic date was the date at which a patient was given an ICD-9 diagnosis code of a secondary neoplasm (Appendix Table A1), if the clinical data field was marked as “stage IV,” or if a distant metastasis (M = 1) was noted according to the classifications established by the American Joint Committee on Cancer.8,15 If a patient met more than one metastatis-identifying criterion, then the earliest recorded date of metastasis was used.
Patterns of Chemotherapy and Biologic Therapy Use
Treatment patterns were analyzed by line of therapy. Within each line of therapy, the frequency and length of therapy for chemotherapy and biologic drug regimens were examined. Second, the sequences of the most commonly used chemotherapy and biologic regimens across lines of therapy were investigated. In addition, the rates of continuation and dose escalation of bevacizumab across lines of therapy were evaluated, as it is the most commonly used monoclonal antibody therapy and is indicated for use in earlier lines.
Rules for Defining Lines of Therapy
To identify regimens by line of therapy, the daily drug use profile for each patient was examined. Each treatment regimen was defined by the chemotherapy and/or biologic agents given to a patient within a 4-day period starting from the date of the first chemotherapy or biologic administration. Lines of therapy were defined by the temporal relationship and sequencing of treatment regimens by using the dates of initiation and discontinuation of chemotherapy and/or biologic therapy. The initial treatment regimen, subsequent treatment regimens, and length of therapy for each regimen were determined.
First-Line Therapy
For each patient, the first-line treatment was defined as all chemotherapy and/or biologic drugs given to a patient during the first 28 days after initiation of treatment. The regimen had to be administered for at least two cycles.
Subsequent Lines of Therapy
After two cycles of treatment with the initial regimen, two consecutive cycles of drug regimens were compared to identify any changes in therapy. The treatment was considered advanced to the next line of therapy when an addition or substitution of chemotherapy or biologic agent was observed and the resulting drug regimen lasted ≥ 28 days and was administered for two or more cycles. Discontinuation of a single drug from a combination regimen was not considered as a change in line of therapy. Finally, if the time window without chemotherapy and/or biologic treatment between two consecutive cycles was > 90 days, a new line of therapy was created.
Biologic Continuation and Dose Escalation
The use of bevacizumab in all lines of therapy was examined. Biologic continuation for bevacizumab was defined as use in two consecutive lines of therapy, and dose escalation was defined as a ≥ 5% increase in average monthly dose (in milligram per kilogram body weight). In additional sensitivity analyses, dose escalation was defined as (1) an increase of ≥ 1 mg and (2) a higher value in comparisons between the average milligram doses within each line.
Statistics
Data were summarized with descriptive statistics. In general, categorical variables were reported as frequency and percentage, and continuous variables were reported by mean, median, and standard deviation (SD).
Results
Patient Characteristics
During the study period from January 2004 to April 2008, a total of 1,655 patients with newly diagnosed mCRC met eligibility criteria and were included in the study. Mean age was 62 years; 52% of patients were male. Mean (SD) weight at the first chemotherapy and/or biologic treatment was 79 (20) kg, with mean (SD) body-surface area of 1.9 (0.3) m2. The sample was well distributed across geographic regions, with 31% of patients residing in western, 29% of patients in southern, 21% of patients in eastern, and 19% of patients in central regions of the United States (Table 1).
Table 1.
Demographics and Clinical Characteristics of Patients with mCRC Treated with Chemotherapy and/or Biologics (N = 1655)
| Variable | No. | % | Mean | SD | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 858 | 51.8 | |||
| Female | 795 | 48.0 | |||
| Unknown | 2 | 0.1 | |||
| Age, years | 61.9 | 11.6 | |||
| Weight, kg | 78.5 | 19.8 | |||
| Height, m | 1.68 | 0.11 | |||
| Geographic region | |||||
| Central | 307 | 18.5 | |||
| East | 346 | 20.9 | |||
| South | 482 | 29.1 | |||
| West | 520 | 31.4 | |||
| CRC stage at diagnosis | 821 | 49.6 | |||
| 0, 1, 2 (localized) | 54 | 6.6 | |||
| 3 (regional spread) | 164 | 20.0 | |||
| 4 (distant) | 603 | 73.4 | |||
| Metastatic status | |||||
| Presented as metastatic | 1,064 | 64.3 | |||
| Nonmetastatic to metastatic | 591 | 35.7 | |||
| Days between CRC diagnosis and development of metastasis | 660.7 | 709.9 | |||
| Median | 469.5 | ||||
| Year of metastatic CRC diagnosis | |||||
| Before 2004 | 116 | 7.1 | |||
| 2004 | 252 | 15.2 | |||
| 2005 | 360 | 21.8 | |||
| 2006 | 432 | 26.1 | |||
| 2007 | 448 | 27.1 | |||
| 2008 | 46 | 2.8 | |||
| Site of metastasis | |||||
| Unknown | 325 | 19.6 | |||
| Known | 1,330 | 80.4 | |||
| Liver | 850 | 63.9 | |||
| Lung | 415 | 31.2 | |||
| Bone | 127 | 9.5 | |||
| Ovarian | 38 | 2.9 | |||
| Brain | 31 | 2.3 | |||
| Skin | 8 | 0.6 | |||
| Bladder | 6 | 0.5 | |||
| Breast | 5 | 0.4 | |||
| Kidney/renal cell | 2 | 0.2 | |||
| Other | 564 | 42.4 | |||
| Only “other” | 231 | 41.0 | |||
| Metastasis listed above plus “other” | 333 | 59.0 |
Abbreviations: CRC, colorectal cancer; SD, standard deviation.
The primary tumor site was colon in 73.1% of patients, rectum in 23.0% of patients, and both colon and rectum in 3.9%. The most common metastatic sites were liver (64%), lung (31%), and bone (10%).
Patterns of Chemotherapy and Biologic Therapy Use
Across the study period, 11% of patients received all five therapeutic drug classes (fluoropyrimidine, irinotecan, oxaliplatin, EGFR monoclonal antibody, VEGF monoclonal antibody) and 19% received three or more classes. Among the chemotherapy regimens, FOLFOX and FOLFIRI were most frequently used. The percentages of patients exposed in any line to FOLFOX and FOLFIRI were 49% and 28%, respectively. Among the three biologic therapies, bevacizumab was administered most frequently. The percentages of patients exposed at any time during the study period to bevacizumab, cetuximab, and panitumumab were 69%, 22%, and 7%, respectively.
Treatment Patterns by Lines of Therapy
Table 2 shows treatment regimens and durations by line of therapy. The initial and subsequent regimens patients received across the study period are summarized in Appendix Table A2 (online only). In first-line therapy, FOLFOX, received by 40.5% of patients, was the most common chemotherapy backbone. Among these patients, 14.3% received FOLFOX alone and 26.2% received FOLFOX plus bevacizumab. Mean (SD) length of treatment with FOLFOX in combination with bevacizumab was 4.84 (3.76) months.
Table 2.
Treatment Regimens and Durations by Line of Therapy
| Line of Therapy | No. of Patients | % | No. of Days |
||
|---|---|---|---|---|---|
| Mean | SD | Median | |||
| First | |||||
| FOLFOX + bevacizumab | 434 | 26.2 | 147.2 | 114.4 | 127 |
| FOLFOX | 236 | 14.3 | 92.6 | 68.3 | 72 |
| FOLFIRI + bevacizumab | 153 | 9.2 | 146.2 | 116.5 | 113 |
| Capecitabine | 148 | 8.9 | 59.3 | 54.8 | 37 |
| Bevacizumab + FU/LV | 71 | 4.3 | 152.5 | 108.4 | 134 |
| FU | 56 | 3.4 | 47.8 | 49.1 | 30 |
| FU/LV | 53 | 3.2 | 104.4 | 110.0 | 76 |
| Cetuximab, irinotecan | 52 | 3.1 | 102.9 | 88.0 | 74 |
| FOLFIRI | 47 | 2.8 | 77.1 | 60.5 | 56 |
| Bevacizumab | 43 | 2.6 | 109.9 | 101.3 | 71 |
| Others | 362 | 21.9 | 114.3 | 105.1 | 85 |
| Second | 742 | 44.8 | |||
| FOLFIRI + bevacizumab | 136 | 18.3 | 140.2 | 129.0 | 100 |
| FOLFOX + bevacizumab | 126 | 17.0 | 130.3 | 116.1 | 99 |
| Cetuximab, irinotecan | 63 | 8.5 | 97.3 | 86.3 | 71 |
| Capecitabine | 36 | 4.9 | 64.4 | 84.2 | 41 |
| FOLFOX | 33 | 4.4 | 91.8 | 70.3 | 73 |
| FOLFIRI | 31 | 4.2 | 98.6 | 65.7 | 85 |
| Bevacizumab, capecitabine | 30 | 4.0 | 128.1 | 117.3 | 82 |
| Bevacizumab + FU/LV | 30 | 4.0 | 148.6 | 96.8 | 125 |
| FOLFIRI + cetuximab | 24 | 3.2 | 101.5 | 83.5 | 78 |
| Bevacizumab | 17 | 2.3 | 135.1 | 124.9 | 118 |
| Irinotecan | 17 | 2.3 | 81.9 | 70.8 | 64 |
| Others | 199 | 26.8 | 99.8 | 96.0 | 71 |
| Third | 317 | 19.2 | |||
| Cetuximab, irinotecan | 53 | 16.7 | 84.4 | 77.2 | 57 |
| FOLFIRI + bevacizumab | 29 | 9.1 | 146.8 | 123.5 | 99 |
| FOLFOX + bevacizumab | 23 | 7.3 | 142.8 | 100.5 | 116 |
| Capecitabine | 22 | 6.9 | 67.4 | 54.0 | 54 |
| FOLFIRI | 21 | 6.5 | 77.4 | 68.5 | 57 |
| Panitumumab | 16 | 5.0 | 79.9 | 75.9 | 59 |
| Bevacizumab, irinotecan | 13 | 4.1 | 119.8 | 84.9 | 106 |
| FOLFIRI + cetuximab | 12 | 3.8 | 139.7 | 203.7 | 65 |
| FOLFOX | 12 | 3.8 | 65.3 | 59.9 | 39 |
| Bevacizumab, capecitabine | 11 | 3.5 | 103.9 | 68.6 | 100 |
| Cetuximab | 11 | 3.5 | 60.6 | 32.3 | 59 |
| Others | 94 | 29.7 | 91.8 | 87.4 | 68 |
Abbreviations: FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFIRI, fluorouracil, leucovorin, and irinotecan; SD, standard deviation; FU, fluorouracil; LV, leucovorin.
Of the 1,655 patients who received first-line therapy, 44.8% received second-line therapy and 19.2% received third-line therapy. In second-line therapy, FOLFIRI, received by 25.7% of patients, was the most common chemotherapy backbone. Among these patients, 4.2% received FOLFIRI alone and 21.5% received FOLFIRI in combination with biologics (18.3% with bevacizumab and 3.2% with cetuximab). FOLFOX plus bevacizumab (17.0%) and cetuximab plus irinotecan (8.5%) were also used in second-line treatment. Mean (SD) treatment durations for bevacizumab plus either FOLFIRI or FOLFOX were similar at 4.61 (4.24) and 4.28 (3.82) months, respectively. Mean (SD) treatment duration of cetuximab plus irinotecan was 3.20 (2.84) months.
The most common third-line treatment regimens were EGFR-containing therapies, with 16.7% of patients receiving cetuximab plus irinotecan, 5.0% panitumumab monotherapy, and 3.5% cetuximab monotherapy. Mean (SD) treatment duration was 2.77 (2.54) months for cetuximab plus irinotecan, 2.63 (2.49) months for panitumumab monotherapy, and 1.99 (1.06) months for cetuximab monotherapy.
Treatment Sequence Across Lines of Therapy
Treatment sequences across different lines of therapy for the two most common regimens, FOLFOX plus bevacizumab and FOLFOX alone, in first-line treatment are summarized in Appendix Table A2 (online only). For patients treated with first-line FOLFOX plus bevacizumab (n = 434), less than half (47.2%, n = 205) received subsequent lines of therapy. FOLFIRI-containing regimens were primarily used in subsequent lines of therapy for those who continued systemic treatment (50.7%, n = 103). Among patients treated with first-line FOLFOX alone (n = 236), 52.1% continued to receive subsequent lines of therapy; FOLFOX plus bevacizumab and FOLFIRI-containing regimens were the most commonly used treatment options (52.0% and 17.1%, respectively).
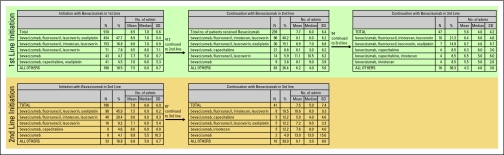
Biologic Continuation and Dose Escalation With Bevacizumab
Figure 1 shows biologic continuation with bevacizumab. Of 910 patients who received bevacizumab-containing regimens in first-line treatment, 412 (45.3%) received second-line treatment, and bevacizumab was used in 58.0% (n = 239). Ninety-four (94) of these 239 patients continued to third-line therapy, and 47 (50.0%) continued to receive bevacizumab-containing regimens. A similar biologic continuation pattern was observed for patients who started bevacizumab-containing regimens beyond the initial line of therapy. Among the 196 patients who started bevacizumab-containing regimens in second-line therapy, 87 continued to receive another line of treatment, with 41 (47%) continuing on bevacizumab in their treatment regimens.
Figure 1.
Biologic continuation with bevacizumab. SD, standard deviation.
Dose increases with bevacizumab were common both within and across lines of therapy, including various sensitivity analyses. On the basis of an increase of ≥ 1 mg, bevacizumab dose increases were recorded in 22% of patients during first-line therapy, 21% during second-line therapy, and 15% during third-line therapy. When based on a comparison between the average millgram dose in each line, among patients who continued bevacizumab from first- to second-line therapy (n = 239), 44% had a dose increase. The average monthly dose increased by 21.2%, from 890 mg to 1,079 mg. Forty-seven (47) patients received bevacizumab across all three lines of therapy; when calculated using a ≥ 5% threshold to define an increase, 76.6% (n = 36) of patients had a bevacizumab dose escalation. The average monthly doses in first-, second-, and third-line therapy were 884, 989, and 1,058 mg, respectively.
Discussion
The treatment of mCRC has become increasingly complex in the past decade because of the rapid proliferation of new chemotherapies and biologics. However, little is known about chemotherapy and biologic treatment patterns for mCRC in real-world clinical practice. This investigation was designed to characterize the treatment patterns of chemotherapy and biologic therapy for mCRC across lines of therapy from 2004 through 2008 in oncology practices in the United States.
Among patients with mCRC treated for their disease, exposure to multiple lines of therapy was common. Of patients who received first-line therapy, approximately half received second-line therapy, and one fifth received third-line therapy. Furthermore, both within and across lines of therapy, patients were typically exposed to multiple classes of therapy. In fact, > 10% of patients received all five therapeutic classes (fluoropyrimidine, irinotecan, oxaliplatin, EGFR monoclonal antibody, VEGF monoclonal antibody). The use of multiple therapeutic classes within and across lines of therapy is consistent with treatment guidelines and recommendations.8,11,12
The data from this study suggest that FOLFOX is currently the standard chemotherapy backbone for first-line therapy, and FOLFIRI is the chemotherapy backbone for second-line therapy in patients with mCRC. Biologic therapy was frequently used in oncology practices in the United States. Most often, biologic therapy was administered in first- and second-line therapy in combination with chemotherapies, after which biologic monotherapy was used.
Bevacizumab, the most commonly administered biologic agent, was used by more than half of the patients with mCRC in first-line therapy in combination with chemotherapies. The frequent use of bevacizumab is likely related to its indication in earlier lines of treatment. During the study period, both cetuximab and panitumumab were approved for use only in later lines of therapy.
Although the pattern of chemotherapy and monoclonal antibody administration in this study was broadly consistent with treatment guidelines,8 the findings show that some aspects of bevacizumab use might not be consistent with treatment guidelines. According to NCCN guidelines, patients who experience progression on first-line bevacizumab-containing regimens should receive an anti-EGFR antibody in subsequent lines of therapy. However, biologic continuation with bevacizumab across lines of therapy was commonly observed in this study population, despite the lack of data from randomized trials to support this practice. A recent observational cohort study suggests that continuing bevacizumab beyond first progression could be associated with a relevant clinical benefit9; however, investigator and selection biases may have influenced the study design.10 Among the 412 patients who initially received a bevacizumab-containing regimen and then received second-line therapy, more than half continued to receive bevacizumab as part of their second-line therapy. Second, trends toward dose-escalation of bevacizumab were observed both within and across lines of therapy. On the basis of an increase of ≥ 1 mg, dose-escalation within a line of therapy was observed in 22% of patients in first-line therapy, 21% in second-line therapy, and 15% in third-line therapy. When calculated as a comparison between the average milligram doses within each line, dose escalations were recorded among 44% of patients who continued bevacizumab from first- to second-line therapy and 39% who continued bevacizumab from second- to third-line therapy.
The results of this study should be interpreted in the context of its strengths and weaknesses. Strengths of the study include the nationally representative sample, the inclusion of recent data, the ability to observe patients longitudinally over time, and the use of EMRs, which include much more detailed information on medication use than the medical claims often used in studies of this type.16 The results provide insight into the real-world use of chemotherapy and biologic therapy for mCRC in a rapidly changing therapeutic environment characterized by the recent introduction of new treatment options and the nearly continuous updating of best practices based on emerging data from clinical trials. In terms of limitations, the line of therapy was defined by the timing and sequence of treatment regimens because of a lack of radiologic information on disease progression in the database. However, not all changes to treatment regimens trigger a change in line of therapy. For example, treatment with only one cycle, cycles lasting fewer than 7 days, or discontinuation of a single drug from a combination regimen would not be considered as an advance to next line of treatment. Second, as potential weight gain among patients was not accounted for during the study period, the dose escalation results may be overstated and should be viewed in this context. For example, Tournigand et al4 reported a weight increase of at least 5% in 35% of patients receiving FOLFIRI and 23% in patients receiving FOLFOX in first-line treatment. In addition, the database-derived study cannot provide information regarding the reasons for change or continuation of therapy components. Potential data entry inaccuracies or omissions (eg, clinical trial participation, or payer/insured) in the EMRs at the practice may have occurred. The effects of data entry inaccuracies were mitigated by plotting frequency distributions and other analytic approaches to identify any potentially inaccurate data. Finally, the use of medical records captured for clinical purposes might be a limitation because the medical records could have lacked precision and consistency compared with records prospectively captured for research purposes. These shortcomings notwithstanding, the results provide important insight into the real-world use of chemotherapy and biologic therapy for mCRC. With the exception of the evidence of bevacizumab biologic continuation and dose escalation, treatment patterns were generally consistent with guidelines and recommendations.
In summary, the current practice for mCRC treatment in this US study appears to be first-line FOLFOX and second-line FOLFIRI as the chemotherapy backbone. Bevacizumab is the most commonly administered biologic therapy, with continuation and dose escalation frequently observed across lines of therapy.
Acknowledgment
This study was funded by Amgen.
Portions of the research described in this article were presented at the Annual Meeting of ASCO, June 29-July 1, 2009, Chicago, IL.
We acknowledge Jane Saiers, PhD, for her medical writing assistance with the manuscript, as well as Susan Dennis and Melissa Pirolli for contributing their expertise and insights in the understanding of oncology electronic medical records. Dr Saiers' work was funded by SDI Health.
Appendix
Table A1.
ICD-9 Codes for Defining CRC and mCRC
| Code | Description |
|---|---|
| Used to define CRC | |
| 153* | Cancer of the colon |
| 154 | Cancer of the rectum |
| 154.0 | Malignant neoplasm rectosigmoid junction |
| 154.1 | Malignant neoplasm rectum |
| 154.8 | Malignant neoplasm rectum, rectosigmoid junction, anus |
| Used to define mCRC in secondary cancer diagnosis | |
| 196 | Secondary and unspecified malignant neoplasm of lymph nodes |
| 196.0 | Lymph nodes of the head, face, and neck |
| 196.1 | Intrathoracic lymph nodes |
| 196.3 | Lymph nodes of the axilla and upper limb |
| 196.5 | Lymph nodes of inguinal region and lower limb |
| 197 | Secondary malignant neoplasm of respiratory and digestive systems |
| 197.1 | Lung |
| 197.2 | Mediastinum |
| 197.3 | Pleura |
| 197.4 | Other respiratory |
| 197.5 | Small intestine, including duodenum |
| 197.6 | Retroperitoneum and peritoneum |
| 197.7 | Liver, specified as secondary |
| 197.8 | Other digestive organs and spleen |
| 198 | Secondary malignant neoplasm of other specified sites |
| 198.0 | Kidney |
| 198.1 | Other urinary |
| 198.2 | Skin |
| 198.3 | Brain |
| 198.4 | Other parts of nervous system |
| 198.5 | Bone and bone marrow |
| 198.6 | Ovary |
| 198.7 | Adrenal gland |
| 198.8 | Other specified sites |
| 198.8 | Breast |
| 198.8 | Genital |
| 198.9 | Other |
| 199 | Malignant neoplasm without specification of site |
| 199.0 | Disseminated |
Abbreviations: ICD-9, International Classification of Diseases, ninth revision; CRC, colorectal cancer; mCRC, metastatic colorectal cancer.
Excluding 153.5 (malignant neoplasm of the appendix).
Table A2.
Treatment Continuum for the Two Most Common First-Line Regimens
| First-Line Therapy | No. | % |
|---|---|---|
| FOLFOX + bevacizumab | 434 | 100 |
| Progressed to subsequent line of therapy | 205 | 47.2 |
| Stopped therapy | 229 | 52.8 |
| Second-line therapy | 205 | 100 |
| FOLFIRI + bevacizumab | 71 | 34.6 |
| Cetuximab + irinotecan | 18 | 8.8 |
| FOLFIRI | 17 | 8.3 |
| Bevacizumab + capecitabine | 12 | 5.9 |
| FOLFOX + bevacizumab | 12 | 5.9 |
| FOLFIRI + cetuximab | 9 | 4.4 |
| Irinotecan | 8 | 3.9 |
| Bevacizumab + FU/LV | 6 | 2.9 |
| FOLFIRI + panitumumab | 6 | 2.9 |
| Bevacizumab | 4 | 2.0 |
| Bevacizumab + capecitabine + irinotecan | 4 | 2.0 |
| Bevacizumab + irinotecan | 4 | 2.0 |
| Capecitabine | 4 | 2.0 |
| Others | 30 | 14.6 |
| First line = FOLFOX + bevacizumab and second line = FOLFIRI + bevacizumab | 71 | 100 |
| Progressed to another line of therapy | 30 | 42.3 |
| Stopped therapy | 41 | 57.7 |
| Third-line therapy | 30 | 100 |
| Cetuximab + irinotecan | 11 | 36.7 |
| Capecitabine | 4 | 13.3 |
| FOLFIRI + bevacizumab | 3 | 10.0 |
| Cetuximab | 3 | 10.0 |
| FOLFIRI + cetuximab | 2 | 6.7 |
| Others | 7 | 23.3 |
| FOLFOX | 236 | 100 |
| Progressed to subsequent line of therapy | 123 | 52.1 |
| Stopped therapy | 113 | 47.9 |
| Second-line therapy | 123 | 100 |
| FOLFOX + bevacizumab | 64 | 52.0 |
| FOLFIRI + bevacizumab | 11 | 8.9 |
| FOLFIRI | 7 | 5.7 |
| FOLFOX | 7 | 5.7 |
| Bevacizumab + 5FU/LV | 6 | 4.9 |
| Capecitabine + oxaliplatin | 4 | 3.3 |
| FOLFOX + cetuximab | 4 | 3.3 |
| Bevacizumab | 3 | 2.4 |
| FOLFIRI + cetuximab | 3 | 2.4 |
| Cetuximab + irinotecan | 3 | 2.4 |
| Others | 11 | 8.9 |
| First line = FOLFOX and second line = FOLFOX + bevacizumab | 64 | 100 |
| Progressed to another line of therapy | 34 | 53.1 |
| Stopped therapy | 30 | 46.9 |
| Third-line therapy | 34 | 100 |
| FOLFIRI + bevacizumab | 6 | 17.6 |
| FOLFIRI | 6 | 17.6 |
| FOLFIRI + cetuximab | 4 | 11.8 |
| Bevacizumab + irinotecan | 3 | 8.8 |
| Capecitabine | 3 | 8.8 |
| Bevacizumab + capecitabine | 2 | 5.9 |
| Others | 10 | 29.4 |
Abbreviations: FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFIRI, fluorouracil, leucovorin, and irinotecan; FU, fluorouracil; LV, leucovorin.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Peter Feng Wang, Amgen (C); Beth Barber, Amgen (C); Zhongyun Zhao, Amgen (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Gregory P. Hess, Peter Feng Wang, David Quach, Beth Barber, Zhongyun Zhao
Financial support: Peter Feng Wang, Zhongyun Zhao
Administrative support: Gregory P. Hess, Peter Feng Wang, Zhongyun Zhao
Provision of study materials or patients: Beth Barber
Collection and assembly of data: Gregory P. Hess, David Quach
Data analysis and interpretation: Gregory P. Hess, Peter Feng Wang, David Quach, Beth Barber, Zhongyun Zhao
Manuscript writing: Gregory P. Hess, Peter Feng Wang, David Quach, Beth Barber, Zhongyun Zhao
Final approval of manuscript: Gregory P. Hess, Peter Feng Wang, David Quach, Beth Barber, Zhongyun Zhao
References
- 1.Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: A paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12:38–50. doi: 10.1634/theoncologist.12-1-38. [DOI] [PubMed] [Google Scholar]
- 2.Gruenberger T, Schuell B, Puhalla H, et al. Changes in liver surgery for colorectal cancer liver metastases under neoadjuvant treatment strategies. Eur J Surg. 2004;36:317–321. [Google Scholar]
- 3.Falcone A, Fornaro L, Loupakis F, et al. Optimal approach to potentially resectable liver metastases from colorectal cancer. Expert Rev Anticancer Ther. 2008;8:1533–1539. doi: 10.1586/14737140.8.10.1533. [DOI] [PubMed] [Google Scholar]
- 4.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 5.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: Current options, current evidence. J Clin Oncol. 2005;23:4553–4560. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 7.Board RE, Valle JW. Metastatic colorectal cancer: Current systemic treatment options. Drugs. 2007;67:1851–1867. doi: 10.2165/00003495-200767130-00004. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Colon Cancer version 4.2008. [Accessed December 13, 2008]. http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf.
- 9.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 10.Kopetz S, Abbruzzese JL. Hidden biases in an observational study of bevacizumab beyond progression. J Clin Oncol. 2009;27:1732. doi: 10.1200/JCO.2009.21.2084. [DOI] [PubMed] [Google Scholar]
- 11.Clark JW, Grothey A. Systemic chemotherapy for nonoperable metastatic colorectal cancer: Treatment recommendations. [Accessed December 15, 2008]. http://www.uptodate.com/patients/content/topic.do?topicKey=∼Y.HUfE6yXpcXADM.
- 12.Goldberg RM. Therapy for metastatic colorectal cancer. Oncologist. 2006;11:981–987. doi: 10.1634/theoncologist.11-9-981. [DOI] [PubMed] [Google Scholar]
- 13.VARIAN Medical Systems. ARIA Oncology Information System. [Accessed December 12, 2008]. www.varian.com/us/oncology/radiation_oncology/aria/
- 14.IMPAC Software. The Elektra Group Medical Oncology EMR. [Accessed December 12, 2008]. www.impac.com/productsNEW/medical-oncology/index.html.
- 15.American Joint Committee on Cancer. Colon and rectum. In: Greene FL, editor. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. pp. 113–124. [Google Scholar]
- 16.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors: A population-based analysis. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]