Abstract
Anoikis is a mode of apoptotic cell death, consequential to insufficient cell-matrix interactions and a critical player in tumor angiogenesis and metastasis. The events involved in tumor cell progression toward metastasis potential are mediated by integrins, which upon engagement with components of the extracellular matrix (ECM), reorganize to form adhesion complexes. Targeting apoptotic players is of immense therapeutic significance since resistance to apoptosis is not only critical in conferring therapeutic failure to standard treatment strategies, but anoikis (apoptosis upon loss of anchorage and detachment from ECM also plays an important role in angiogenesis and metastasis. The ability to survive in the absence of adhesion to the ECM, enables tumor cells to disseminate from the primary tumor site, invade a distant site and establish a metastatic lesion. Tumor cells can escape from detachment-induced apoptosis by controlling anoikis pathways, including the extrinsic death receptor pathway and the ECM-integrin mediated cell survival pathway. Considering the functional promiscuity of individual signaling effectors, it is critical to dissect the molecular networks mechanistically driving tumor cells to evade anoikis and embark on a metastatic spread. Resistance to die via anoikis dictates tumor cell survival and provides a molecular basis for therapeutic targeting of metastatic prostate cancer. Further dissection of critical anoikis signaling events will enable the therapeutic optimization of anoikis targeting to impair prostate cancer metastasis prior to its initiation. This review will discuss the molecular understanding of anoikis regulation in the tumor microenvironment and the in vivo pharmacological implementation of a novel class of antitumor-drugs to optimize apoptotic-based therapeutic targeting, bypassing anoikis-resistance to impair prostate cancer progression to metastasis. Potential combination strategies targeting tumor vascularity (via anoikis) and impairing tumor initiation (via “classic” apoptosis), provide strong therapeutic promise for metastatic prostate cancer by preventing the onset of metastasis.
Keywords: Tumor progression, Invasion, Focal adhesion complex, Integrins, Akt Survival signaling
1. INTRODUCTION
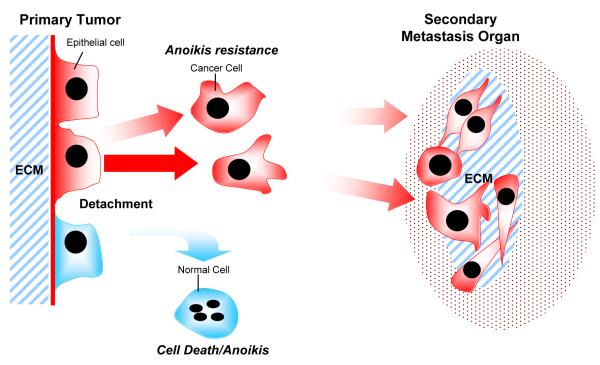
Metastasis is the spread of tumor cells from a primary tumor to a secondary site in the body remains one of the most life-threatening pathological events, responsible for 90% of cancer death in humans (Weigelt et al., 2005; Yilmaz and Christofori, 2009). Cancer metastasis is a multistep and complex process that involves dissociation of the tumor cells from the organ of origin, degradation of the extracellular matrix (ECM), cell migration, anchorage-independent growth, apoptosis evasion, angiogenesis, invasion of surrounding tissues, cell adhesion, movement and colonization to distant sites in the body (Fornaro et al., 2001) (Fig. 1).
Figure 1.
Acquisition of migratory and invasive potential enable cancer cell to detach from the primary site and enter circulatory and lymphatic system. Upon detachment, most of the cells lose the ECM mediated cell survival signals and subsequently lead to anoikis. However, some cancer cells acquire the ability to survive after loss of cell adhesion due to activation of anoikis resistance mechanisms and embark on the metastatic journey. These cancer cells adhere to the distant sites, proliferate and recruit a vascularity network at the secondary organ as distant metastasis tumors.
Prostate cancer is a heterogeneous cancer with a natural history of progression from prostatic intraepithelial neoplasia (PIN) to locally invasive androgen-dependent to androgen-independent metastatic disease which is associated with patient mortality. Androgen-independent prostate cancer cells become resistant due to roadblocks in apoptosis and depending on the interactions with the tumor microenvironment, acquire invasive and metastatic properties (McKenzie and Kyprianou, 2006). Overcoming the androgen-independence of prostate tumors is considered the most critical therapeutic endpoint for improving patient survival (Feldman and Feldman, 2001; Debes and Tindal, 2004). Radical prostatectomy, androgen-ablation monotherapy and radiation therapy are considered curative for localized prostate cancer, but no effective treatment for patients with metastatic disease is currently available. There are two primary contributors towards the emergence of androgen-independent metastatic prostate cancer: activation of survival pathways, including apoptosis suppression and anoikis resistance and increased neovascularization (McKenzie and Kyprianou, 2006; Wang et al., 2007). Both tumor epithelial and endothelial cells require attachment to the ECM for survival and upon loss of adhesion, cells undergo detachment-induced cell death, or anoikis. Molecular targeting of apoptotic signaling pathways has been extensively studied in recent years and directed towards the development of effective therapeutic modalities for treating advanced androgen-independent prostate tumors. The majority of therapeutic agents act through intrinsic mitochondrial, extrinsic death receptor pathways or endoplasmic reticulum (ER) stress pathways to induce apoptosis. Anoikis induction may provide an alternative to treat patients with metastatic disease. Insightful dissection of the signaling pathways associated with the novel compounds may lead to effective exploitation of anoikis for therapeutic targeting of metastastic prostate tumors. Targeting tumor cell metastasis holds considerable therapeutic promise for the benefit of patients with advanced prostate cancer. This review focuses on the therapeutic value of anoikis targeting and the mechanisms conferring anoikis resistance in metastatic prostate tumors.
2. EXPLOITATION OF APOPTOSIS IN CANCER THERAPY
2.1 Anoikis: Cells Gain Freedom and Meet Death
Anoikis is a Greek word meaning “loss of home”, was originally defined by Frisch and Ruoslathi a decade ago as a unique phenomenon reflecting apoptotic cell death upon insufficient cell-matrix interactions (Frisch and Ruoslahti, 1997; Frisch and Screaton, 2001). This unique mode of cell death was later recognized as a significant player in tumor angiogenesis and metastasis (Frisch and Ruoslahti, 1997; Frisch and Screaton, 2001; Rennebeck et al., 2005) Upon detachment, epithelial and endothelial cells normally undergo apoptosis; thus anoikis can suppresses the expansion of oncogenically transformed cells. However acquisition of resistance to this process by the tumor cells, facilitates their ability to migrate to secondary sites and establish metastatic lesions. One can describe that during metastatic progression, cells are in a dynamic state lacking firm attachment to the ECM and susceptible to anoikis (Rennebeck et al., 2005). During the process of anoikis, both death receptor pathways and mitochondrial pathway are activated (Frisch and Ruoslahti, 1997; Frisch and Screaton, 2001). Cell detachment simultaneously triggers down-regulation of Bcl-xL, an anti-apoptotic component of the mitochondrial pathway (Rosen et al., 2000), and up-regulation of Fas ligand (FasL), an activator of the death receptor pathway (Rosen et al., 2002) within a minutes after detachment (Loza-Coll et al., 2005). However, a subpopulation of cancer cells become resistant to detachment induced pro-apoptotic stimuli and after entering the blood stream successfully metastasize to locations distant from the primary site. The contribution of key signaling effectors of the anoikis mode of cell death, to the development of anoikis resistance prostate in tumor epithelial cells, resulting in the metastatic spread has not been explored.
2.2 Mechanistic Insights into Anoikis Signaling
2.2.1 The Death Receptors Lead
The death receptor pathway is the major cell death pathway in responsible for anoikis in non malignant and malignant cells. Death receptor pathway is activated by the binding of the death ligands such as FAS or TRAIL at the extracellular domain of the death receptor causing oligomerization of the receptors. Upon ligand binding, death effector domain (DED) of Fas-associated death domain (FADD) binds with caspase-8 and mediates formation of death inducing signaling complex (DISC). Formation of DISC, further stimulate the caspase-8 dimerization and get cleaved to become active. The active form of caspase-8 is released to the cytoplasm where it activates and cleaves downstream effectors, such as caspase-3 and 7 to induce apoptosis (Simpson et al., 2008).
The primary endogenous inhibitor of the death receptor pathway is FLIP (FLICE-inhibitory protein). This effector consists of two DEDs and a C terminus caspase-like domain where provide the structural similarity to caspase-8. Upon binding, FLIP substitutes active-site cysteine residue to tyrosine, leading to inactivation of caspase-8. Functionally FLIP has higher affinity to the DISC compared to caspase-8, thus blocking caspase-8 recruitments and subsequent activation (Scaffidi et al., 1999). In normal cells, upon loss of cell contact with ECM, Fas ligands and Fas receptor are up-regulated, while FLIP expression is down-regulated. These changes in apoptosis signaling effectors trigger downstream caspase-8 activation in a FADD dependent manner, leading to apoptosis (Aoudjit and Vuori, 2001). In malignant cells, even though cells lost contact with the ECM, the system becomes dysfunctional, as tumor epithelial cells fail to activate the death receptor mediated caspase-8 activation despite increased expression of FAS ligands and FAS receptor, ultimately conferring anoikis resistance. The driving molecular mechanism of this effect can be partially explained by the failure of cancer cells to down-regulate FLIP expression after detachment (Simpson et al., 2008). This provides the first basis for targeting FLIP in anoikis resistant cells towards the formulation of a novel therapeutic approach to treat metastatic prostate cancer. The emerging evidence is indeed very promising: Using small molecules, chemical inhibition of FLIP sensitized cells to apoptosis stimuli and reversed the anoikis resistance in malignant cells, without inducing apoptosis in adherent cells (Mawji et al., 2007a, 2007b).
2.2.2 Integrins: Vital Connections “Unplugged”
Attachment of epithelial and endothelial cells to the ECM is essential for the tight regulation of cell proliferation, cell survival, and migration. The functional contribution of integrins is essential for such processes, as integrins recognize the major adhesive ECM components, fibronectin and laminin, (Giancotti and Ruoslahti, 1999; Goel and Languino, 2004). Integrins are transmembrane proteins that serve a role as primary mediators of cell-ECM interactions that are functionally involved in determining tumor angiogenic response during cancer progression to metastatic disease (Fornaro et al., 2001; Goel and Languino, 2004; Goel et al., 2008). Integrins contribute for signal transduction from the extracellular environment to the intracellular network mediated by integrin-activated signaling molecules, such as focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI 3-kinase), and members of the extracellular signal-regulated kinase 1 and 2/mitogen activated protein (ERK1 and 2/MAP) kinase family to regulate cell proliferation, migration, and apoptosis of tumor and endothelial cells (Fornaro et al., 2001; Nikolopoulos et al., 2004). Loss of integrin mediated epithelial cell-ECM interactions, cause loss of phosphorylation in downstream effectors such as FAK, PI3-K, ERK1 and MAP kinase, which are known to mediate cell susceptibility to anoikis. Among the integrin family, especially, integrins β1, β3 and β6 were found to be up-regulated during prostate cancer progression to metastasis (Goel et al., 2008).
The SRC family of kinases (SFK) is the largest family of nonreceptor protein tyrosine kinases, that consists of nine members including, Blk, Fgr, Fyn, Hck, Lck, Lyn, Src, Yes, and Yrk. SFK are responsible for signal transduction mediated through growth factor receptors, integrin, guanosine phosphate-binding protein-coupled receptors (GPCRs), cytokine receptors, immunological recognition receptors (Fizazi, 2007). Within the SFK family, Src is the most widely studied protein and play significant role during cancer progression (Fizazi, 2007). A highly activated viral version of Src (v-Src) is the first oncogene to be discovered and responsible for the transforming properties of the oncogenic Rous sarcoma virus (Yeatman, 2004). In focal adhesion complex, Src activation is mainly mediated through β1 integrin. Upon cell adhesion, ECM-integrin mediated signals activate Src through FAK and loss of adhesion resulted in inactivation of Src. As Src activation resulted in activation of PI3-K/Akt signals, loss of SRC activation induces anoikis (Giannoni et al., 2008, 2009). Activation of SRC has been linked to anoikis resistance in various cancer including osteosarcoma, pancreatic carcinoma and prostate cancer (Diaz-Montero et al., 2006; Duxbury et al., 2004; Giannoni et al., 2008, 2009). Src is highly expressed in prostate cancer and its targeting has already shown evidence of therapeutic value by significantly impairing prostate cancer metastases to the lymph nodes (Park et al., 2008). Moreover, Src has been shown to be functionally linked to the transition of androgen-independent growth of prostate tumors, while up-regulation of FGR (SFK member) is frequently observed in castration-resistant androgen-independent prostate cancer (Edwards et al., 2003). Since Src is a core player during bone turn over by regulating both osteoclastic and osteoblastic activities, Src plays essential role during bone metastasis (Fizazi, 2007). Indeed inhibition of Src delayed the appearance of prostate tumor bone metastasis via IGF and IGF-1 binding protein mediated signals (Fizazi et al., 2003). The multiplicity of the role of Src during cancer progression, justifies the intense focus on a specific Src inhibitor for the treatment of advanced prostate cancer.
The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a highly conserved tumor suppressor gene that induces cellular apoptosis through its modulation of the P13K/AKT signal transduction pathway. (Hlobilkova et al., 2003). PTEN directly suppress the phosphorylation of AKT, which is essential for its activation and targeting of its many downstream effectors (Wang et al., 2003). Loss of PTEN is commonly observed in treatment resistant and poorly differentiated prostate cancers which mediate constitutive activation of the P13K/AKT pathway and subsequent apoptotic resistance (Davies et al., 1999). Restoration of PTEN activity in PTEN deficient prostate cancer cell lines increases sensitivity to FADD mediated caspase-8 driven apoptosis as well as to facilitate BIDD cleavage allowing for cytochrome c release and subsequent mitochondrial driven apoptosis (Yuan and Whang, 2002).
AKTs are activated by second messengers via phosphatidylinositol 3′-kinases (P13Ks), which phosphorylation is counteracted by the activity of PTEN phosphatases (Stern, 2004). In prostate cancer, AKT phosphorylation occurs constitutively through loss of PTEN activity, or is up-regulated in PTEN positive tumors through autocrine and paracrine cell membrane receptor-ligand interactions (Pfeil et al., 2004). Recent evidence indicates that both PTEN inactivation and AKT phosphorylation are hallmarks of aggressive prostate cancer. Although PTEN inactivation is present in only 10%–15% of primary prostate cancers, PTEN loss is detected in 30%–50% of hormone-refractory tumors, as well as 60% of xenograft models derived from metastatic prostate cancer cells (Wang et al., 2003; Pfeil et al., 2004). Furthermore, loss of PTEN correlates with aggressive local disease (T3b-T4 tumors) and Gleason score >6 (McMenamin et al., 1999). Activation of AKT induces AR (androgen receptor) phosphorylation, as well as AR up-regulation, that can hinder androgen-deprivation-induced apoptosis of prostate cancer cells (Lin et al., 2001; Ghosh et al., 2003).
Integrin-linked kinase (ILK) is a serine/threonine protein kinase, that interacts with cytoplasmic domain of β1-integrin and β3-integrin and has been functionally linked to integrin and Wnt signaling pathways (Hannigan et al., 1996; Li et al., 1997; Wu and Dedhar, 2001). ILK regulates several integrin-mediated cellular processes including cell adhesion, fibronectin-ECM assembly and anchorage-dependent cell growth (Hannigan et al., 1996; Radeva et al., 1997; Cieslik et al., 1998). Upon cell adhesion, ILK is transiently activated and directly phosphorylates AKT Ser473 (Lynch et al., 1999) and glycogen synthase kinase-3 (GSK3) (Cieslik et al., 1998); in contrast, inhibiting ILK in cancer cells inhibits AKT phosphorylation and cell survival. Recent evidences indicated that ILK-1 mediates anoikis resistance by inhibiting apoptosis signals such as caspase-3 and 8 even without activation of integrin/ECM signals, possibly via recruiting through α-parvin-mediated targeting of AKT to lipid rafts (Attwell et al., 2000; Fukuda et al., 2003; Hannigan et al., 2005). In prostate cancer, ILK expression increases significantly with tumor progression and related to the increased proliferative index during progression. ILK expression inversely correlated with 5-year patient survival (Graff et al., 2001).
2.2.3 Epithelial-Mesenchymal-Transition: A State-of-Action Controlling Anoikis?
The functional outcome of epithelial-mesenchymal transition (EMT) in prostate cancer progression to castration-resistant disease is complex, given the uncertainty surrounding the contribution of the androgen axis to prostate cancer metastasis. Indeed the impact of androgen suppression to metastatic dissemination of prostate cancer cells is still a subject of debate, with the notion that androgen deprivation therapy may down-regulate the AR levels in prostate tumors. One could speculate that a threshold low AR level may promote EMT, ultimately facilitating metastatic spread of prostate tumor epithelial cells. The inhibition of EMT response to androgens by AR overexpression, points to: (a) an inverse relationship between AR content and EMT induction and (b) a potential biochemical basis for the metastatic behavior of prostate cancer cells from recurrent castration-resistant tumors. Since long term androgen deprivation may down-regulate AR expression, this threshold of “low” AR status facilitates androgen-induced EMT, thus promoting cancer metastasis. Recently gathered data in our lab demonstrate that AR antagonists reverse the EMT changes triggered by androgens in prostate cancer cells thus supporting the ability of elevated AR to prevent androgen-induced E-cadherin reduction and N-cadherin-induction (Zhu and Kyprianou, 2009). The clinical evidence that intermittent androgen deprivation therapy benefit patients in prostate cancer progression (Boccon-Gibod et al., 2007), certainly provides additional support for this concept. Snail, is a transcriptional repressor of E-cadherin, that plays a critical role in EMT, thus being recruited in the EMT molecular profiling. A direct functional connection to anoikis was recently documented, as Snail can also tightly regulate anoikis by enhancing both cell detachment (from the primary tumor site) and reattachment (to the secondary site), via targeting the basement membrane proteins such as laminin (LN) α3, β3, and γ2 (laminin-5/LN-5) and receptors for LN-5, including integrins α3, α6, or β4 (Haraguchi et al., 2008). Moreover, Snail has also been linked to the development of apoptosis resistance by activating cell survival pathway such as the phosphatidylinositol 3(PI3)/Akt signaling and by inhibiting caspse-3 pathway (Barrallo-Gimeno and Nieto, 2005). One cannot ignore the striking presence of transforming growth factor-β (TGF-β) in the cellular landscape of EMT and apoptosis milieu in prostate epithelial cells (Rennebeck et al, 2005). Considering the autocrine regulation of expression of this multifunctional cytokine as well as the ill-fated alliance between TGF-β signaling and tumor progression, the challenge is to identify modalities that do not interfere with the positive effects of TGF-β as a tumor suppressor (apoptosis induction) at the onset of malignant transformation and tumorigenic growth, while inhibiting the dysfunctional TGF-β in advanced prostate cancer (metastasis promoter).
2.2.4 Intrinsic Apoptosis Signaling
The mitochondrial pathway activates downstream caspases as a result of releasing cytochrome c, Smac/DIABLO and Omi/Htra2 from the mitochondria into the cytoplasm. Released proteins mediated activation of caspase-3, -7 and -9, and induce apoptosis. Cytochrome c mediate formation of apoptosome by recruiting apoptotic protease activating factor (Apaf-1) and pro-caspase-9 where caspase-9 get cleaved and become activated and cleaved. Caspase-9 cleavage mediates the further downstream activation of caspase-3 and -7 (Simpson et al., 2008; Slee et al., 2001).
The mitochondrial apoptotic signaling is regulated through the pro-apoptotic and anti-apoptotic protein in Bcl-2 family (DiPaola et al., 2001). Pro-apoptotic members of the Bcl-2 family such as Bax, Bad, and Bid, allowing for mitochondrial cytochrome c release and caspase cascade activation (DiPaola et al., 2001). Their activities are antagonized by anti-apoptotic members, most notably Bcl-2 and Bcl-xL, inhibiting the release of cytochrome c from the mitochondria, consequently inhibiting mitochondrial-induced apoptosis (DiPaola et al., 2001). Tumor cell survival is dictated through the intrinsic balance between pro-apoptotic to anti-apoptotic family members. Recent evidence implicates the Bcl-2 family of apoptosis signaling effector proteins (intrinsic pathway) play active role in anoikis. For non-transformed cells, detachment from the ECM induces down regulation of Bcl-xL and stimulates release of mitochondrial Omi/HtrA2, thus mediating anoikis. However, in Ras transformed cells, detachment fails to induce Bcl-xL down regulation, but leads to Bak down-regulation, which subsequently blocks the release of Omi/HtrA2 resulting in anoikis resistance (Simpson et al., 2008).
Ample evidence indicates that in poorly differentiated prostate tumors, Bcl-2 and other anti-apoptotic members of its family are significantly up-regulated (Kajiwara et al., 1999; McCarty, 2004). Over-expression of Bcl-2 and Bcl-xL has been related to resistance to both chemotherapy and radiation therapy (McCarty, 2004). Bcl-2 family also plays a critical role in the androgen-signaling axis operating in prostate cancer cells. In androgen-responsive prostate cancer cells, androgens down-regulate expression of pro-apoptotic Bcl-2 family members such as Bax (Coffey et al., 2002). Increased Bcl-2 and Bcl-xL expression in androgen-independent tumors (Furuya et al., 1996) is directly linked to the ability of prostate cancer cells to survive in an androgen-free environment (Kajiwara et al., 1999). Taken together these lines of evidence emphasize the functional significance and predictive value of Bcl-2/Bcl-xL over-expression as potential key regulators of androgen-independent recurrence in prostate tumors after prolonged androgen ablation therapy (McCarty, 2004).
The BH3-only proteins including Bid, Bad, Bim, Bmf and Noxa are essential during mitochondrial-mediated apoptosis (Bouillet and Strasser, 2002). Bim is the most characterized to be the potent pro-apoptotic protein that functions in the intrinsic apoptosis pathway, triggered via withdrawal of growth factor signals (Biswas and Greene, 2002; Gilley et al., 2003; Wang et al., 2004) or exposure to pro-apoptotic triggers (Enders et al., 2003; Sunters et al., 2003; Wang et al., 2004). When epithelial cells detach from the ECM, Bim translocates to the mitochondria, where a vital interaction with Bcl-xL, further blocks its pro-survival function, while promoting Bax activation (Reginato et al., 2003). Upon attachment to the ECM, integrin-mediated signals promote extracellular signal-regulated protein kinases (ERKs) and phosphatidylinositol 3-kinase (PI3K)/Akt-mediated phosphorylation of Bim, mediating its proteosome-dependent degradation. The onset of anoikis is followed by inhibition of the ERK and PI3K/Akt downstream signals and concomitant stimulation of Bim phosphorylation, which prevents Bim from degradation and intracellular accumulation and subsequently promoting anoikis (Giannoni et al., 2008, 2009; Wang et al., 2004).
The family of inhibitors of apoptosis proteins (IAPs) inhibits caspase-3, -7, and -9 activation, resulting in decreased apoptosis (Schimmer, 2004). Thus far, eight human IAPs have been identified including X-linked inhibitor of apoptosis protein (XIAP), inhibitor of apoptosis protein 1 (IAP1), inhibitor of apoptosis protein 2 (IAP2), and survivin (Krajewska et al., 2003). While they all appear capable of inhibiting effector caspases, IAP1 and IAP2 can directly and potently up-regulate NF-κB expression (McEleny et al., 2001). Elevated expression of all four IAPs has been shown in both animal models of prostate cancer and prostate tumors from patients undergoing prostatectomy, and this elevation appears to be present early in prostate cancer development (McEleny et al., 2001). Indeed, recent evidence suggests that XIAP inhibition enhances chemotherapy sensitivity in otherwise resistant prostate cancer cell lines (Amantana et al., 2004). Another small study consisting of 23 patients revealed that expression of both IAP1 and IAP2 was dramatically up-regulated in patients receiving neoadjuvant androgen ablation, implicating IAPs in androgen independence (McEleny et al., 2001).
TrkB is a neurotrophic tyrosine kinase receptor 4, 5, that was identified as a potent and specific suppressor of caspase-associated anoikis through the genome-wide functional screen of anoikis suppression (Douma et al., 2004). TrkB confer anoikis resistance via activating the phosphatidylinositol-3-OH kinase/protein kinase (PI3-kinase) pathway. TrkB induced the formation of large cellular aggregates that survive and proliferate in suspension in vitro and induced rapidly growing tumors that infiltrated lymphatic and blood vessels to colonize distant metastasis in vivo. Overexpression of TrkB has been documented in several tumors including neuroblastoma and pancreatic cancer (Brodeur et al., 1997; Sclabas et al., 2005). In prostate cancer, TrkB overexpression was observed in 22 out of 32 cases of primary tumors and 6 out of 10 bone metastases (Dionne et al., 1998).
3. Therapeutic Value: Overcoming Anoikis Resistance in Metastatic Tumors
3.1 Novel Quinazoline-based Compounds
Experimental and clinical studies documented that the clinically available quinazoline-based α1-adrenoceptor antagonists, doxazosin and terazosin, exert potent anti-tumor growth effects via induction of apoptosis in prostate epithelial, smooth muscle and endothelial cells. Suppression of prostate tumor growth by these drugs proceeds via a α1-adrenoceptor-independent mechanism, mediated by receptor-mediated apoptosis involving death-inducing signaling complex (DISC) formation/caspase-8 activation and inhibition of Akt activation. (Keledjian et al., 2005; Keledjian and Kyprianou, 2003; Rennebeck et al., 2005). Pharmacological exploitation of the existing FDA-approved drugs resulted in the structural optimization of quinazoline’s chemical nucleus and structure-function analysis led to the development of a novel class of apoptosis-inducing and angiogenesis-targeting agents. The two lead compounds, DZ-3 and DZ-50, are shown to be effective at inhibiting epithelial and endothelial cell survival by targeting the Akt survival pathway and preventing angiogenesis. DZ-50 reduces tumor cell adhesion to the ECM by promoting anoikis and inhibits tumor growth and neovascularization “in vivo”. The parent compound, doxazosin induces cell death via the death receptor mediated apoptotic signaling and inhibition of Akt survival signaling (Garrison and Kyprianou, 2006). The novel quinazoline compounds induce anoikis in both prostate tumor epithelial and endothelial cells with higher potency than the parent drug, doxazosin (Garrison et al., 2007). The mechanism driving this effect involves targeting of the VEGF-mediated angiogenic response and integrin mediated focal-adhesion complex. One of the lead drugs of this second generation compounds significantly impaired the metastatic potential in an in vivo prostate metastasis model by specifically targeting tumor vascularity without affecting their proliferative dynamics (Garrison et al., 2007). Moreover, the experimental evidence gathered so far points to a potent primary prevention activity of the lead drugs in impairing the onset of prostate tumor development in a xenograft model. These quinazoline-derived drugs may have therapeutic potential in preventing distant metastasis via inducing anoikis in tumor epithelial as well as endothelial cells (targeting vascularity).
3.2 PPARγ Inhibitor
Peroxisome proliferator-activated receptor gamma (PPAṚ-γ) belongs to the nuclear hormone receptor family; a protein mainly expressed in adipose tissue, plays a central role in adipocyte differentiation and insulin sensitivity (Desvergne and Wahli, 1999); recently PPAṚ-γ is implicated as a putative therapeutic target for cancer in a variety of tumors due to its ability to inhibit tumor cell growth (Martelli et al., 2002; Panigrahy et al., 2003). Inhibition of PPAR-γ̣-induced anoikis in squamous carcinoma and hepatocellular carcinoma (Schaefer et al., 2005) via suppressing integrin α5 expression and blocking FAK signaling.
3.3 TrkB Inhibitor
Tropomyosin-related kinases (Trks) are a family of receptor tyrosine kinases activated by neurotrophins. Trks play important roles in tumor cell growth and survival signaling. Thus, inhibitors of Trk receptor kinases hold considerable therapeutic promise in enabling targeted anti-tumor strategies. The Pan-Trk inhibitors have been used in tumor xenograft and transplantation models and suppressed the xenograft growth in a number of cancer models including neuroblastoma, medulloblastoma, prostatic and pancreatic cancer cell lines. Moreover, Trk inhibitors have already been used in phase I clinical trials (Marshall et al., 2005; Undevia et al., 2004). They appear to be tolerated well, but fail to elicit a tumor response in a limited number of patients suffering from mostly solid tumors. Phase II clinical trials are currently under way, which may bring additional data on the suitability of Trks as anti-cancer therapeutic targets (Desmet and Peeper, 2006).
3.4 SRC Inhibitor
The Src family of kinases is currently being investigated as valuable therapeutic targets for cancer treatment including prostate cancer. In orthotopic nude mouse models, dasatinib treatment effectively inhibit both tumor growth and development of lymph node metastases in both androgen-sensitive and androgen-resistant prostate cancer (Park et al., 2008). Dasatinib suppresses cell adhesion, migration, and invasion of prostate cancer cells by blocking the kinase activities of the SFKs, Lyn, and Src, in human prostate cancer cells at low concentrations. Moreover, focal adhesion kinase and Crk-associated substrate (p130(CAS)) signaling downstream of SFKs are also inhibited (Nam et al., 2005). Several clinical trials with the SRC inhibitor Dastanib, are being developed. Phase II clinical trials are ongoing for the treatment of patients with castration-resistant prostate cancer with Dastanib (alone) (Clincaltrials.gov). Dasatinib in combination with docetaxel, for the treatment of metastatic castration-resistant prostate cancer (CRPC) is currently under a Phase III clinical trial (Clinicaltrial.gov). Neoadjuvant Dasatinib Plus LHRH Analogue Therapy in High-Risk Localized Prostate Cancer is in phase II clinical trial (Clinicaltrials.gov), with anxious anticipation of the final therapeutic impact.
3.5 Talin Resisting Anoikis
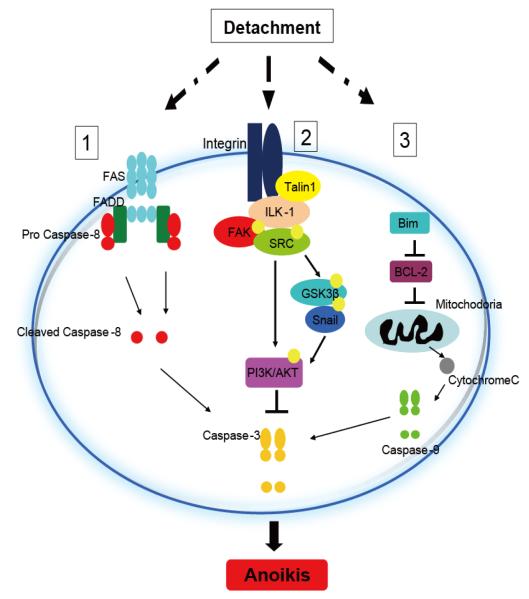
Recent mechanistic findings generated in our laboratory (Sakamoto S, 2009), provide the first evidence that talin1, an integrin-activator might be responsible for the acquisition of the primary tumor cell invasive and metastatic properties leading to prostate cancer metastasis by conferring anoikis resistance (Fig. 2). The significance of anoikis lends tremendous value to the anoikis phenomenon in predicting metastatic potential (by analyzing anoikis signaling effectors such as talin1) in primary prostate tumors/biopsy specimens using microarray analysis or proteomics-based approaches. One could easily consider the possible formulation of a nomogram systematically predicting the metastatic potential of the primary tumor by combining immunohistochemical findings, such as differentiation or Gleason Score plus anoikis related signaling profiling. Indirect as the current evidence might be, the therapeutic promise is appealing: in patients with high risk for metastasis, early targeting with specific inhibitor-based chemotherapy may benefit patient long-term survival by reversing anoikis resistance and ultimately suppressing the tumor metastatic spread, prior to its initiation.
Figure 2.
Programming anoikis induction. Upon cell detachment, anoikis signaling can be activated via three major pathways. (1), death receptor mediated pathway, (2), ECM-integrin cell survival pathway and (3), mitochondrial mediated pathway. (1), Activation of FAS/FADD in death receptor induces cleavage of caspase-8 and subsequently activate downstream caspase-3 to induce apoptosis. (2) ECM-epithelial cell interaction mediates integrin dependent activation of Talin1, FAK/SRC and ILK-1. Activation of ILK-1 mediates phosphorylation of GSK3β and recruitment of Snail. This signaling cascade activates PI3K/AKT cell survival signals. Loss of survival signals upon cell detachment, enhances cell susceptibility to apoptotic stimuli. (3). Cell detachment induces upregulation of pro-apoptotic protein, like Bim and down regulation of anti-apoptotic regulatory proteins such as BCL-2, which activate the cytochrome-C release from the mitochondria and subsequently trigger activation of Caspase-9 and 3 to induce apoptosis.
4. Summary
Prostate cancer progression to advanced metastatic disease is associated with relapse to a castration-resistant state due to impaired apoptotic response to androgen ablation. The biological repertoire of the epithelial and endothelial cells under the control of an array of growth factor signaling mechanisms (such as the TGF-β pathway) is intimately associated with EMT and the anoikis phenomenon towards prostate tumor metastasis. Characterization of the anoikis signaling network operated in response to extrinsic apoptosis triggers in conjunction with intracellular branches of the Akt survival and MAP kinase pathways, has enabled the therapeutic exploitation of this unique mode of cell death during cancer development and progression to metastasis. The current clinical interest surrounding the efficacy of systematically administered short hairpin RNA (shRNA) as an effective therapeutic strategy in human cancers can be applied to anoikis regulators towards impairing metastatic disease. The success of this approach depends on the ability to discern the proteins to be targeted and more importantly, to successfully and selectively deliver the shRNAs to tumor cells. The knowledge gathered so far from molecular dissection of apoptosis signaling would allow for the combination targeting of (classic) apoptosis and anoikis death modes in cell-type dependent content within the microenvironment, towards suppression not only of the primary tumor growth but also of aggressive metastatic spread. The apoptotic action of the novel quinazoline-based compounds via triggering anoikis of prostate cancer cells could be consequential to targeting TGF-β receptors, a mechanism holding major therapeutic promise for treatment of metastasis initiation. Temporal targeting of anoikis signaling pathways should be implemented during the transition from the detachment of transformed malignant cells from the ECM and initiation of the metastatic process. Defining a predictive marker or a molecular signature (in the context of the tumor microenvironment), prior to the onset of metastasis will enable the level of anoikis resistance and the selection of prostate cancer patients likely to exhibit therapeutic benefit in response to anoikis resistance “reversal” agents.
Acknowledgments
This work was supported by an NIH R01 CA107575-06 grant (NK), a Markey Cancer Foundation Grant (NK). Shinichi Sakamoto is an American Urological Association Foundation Research Scholar.
Abbreviations
- ECM
extracellular matrix
- PIN
prostatic intraepithelial neoplasia
- ER
endoplasmic reticulum
- FasL
Fas ligand
- DED
death effector domain
- FADD
Fas-associated death domain
- DISC
death inducing signaling complex
- FLIP
FLICE-inhibitory protein
- SFK
SRC family of kinases
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- ILK
Integrin-linked kinase
- ERKs
signal-regulated protein kinases
- EMT
epithelial-mesenchymal transition
- FAK
focal adhesion kinase
- Trks
Tropomyosin-related kinases
- TGF-β
transforming growth factor-β
- AR
androgen receptor
- IAPs
inhibitors of apoptosis proteins
- PPARγ
Peroxisome proliferator-activated receptor gamma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol. Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–361. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 2002;277:49511–49516. doi: 10.1074/jbc.M208086200. [DOI] [PubMed] [Google Scholar]
- Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U. The role of intermittent androgen deprivation in prostate cancer. BJU Int. 2007;100:738–743. doi: 10.1111/j.1464-410X.2007.07053.x. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu XG, Azar CG, Lee CP, Evans AE. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J. Neurooncol. 1997;31:49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- Cieslik K, Zembowicz A, Wu JL, Tang KK. Transcriptional regulation of endothelial nitric-oxide synthase by lysophosphatidylcholine. J. Biol. Chem. 1998;273:14885–14890. doi: 10.1074/jbc.273.24.14885. [DOI] [PubMed] [Google Scholar]
- Clincaltrials.gov. http://www.clinicaltrials.gov/ct2/show/NCT00570700?term=Dasatinib&rank=27.
- Clinicaltrial.gov. http://www.clinicaltrials.gov/ct2/show/NCT00744497?term=Dasatinib+%2C+prostat e&rank=4.
- Clinicaltrials.gov. http://www.clinicaltrials.gov/ct2/show/NCT00860158?term=Prostate%2C+Dasatinib &rank=5.
- Coffey RN, Watson RW, O’Neill AJ, Mc Eleny K, Fitzpatrick JM. Androgen-mediated resistance to apoptosis. Prostate. 2002;53:300–309. doi: 10.1002/pros.10159. [DOI] [PubMed] [Google Scholar]
- Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl. J. Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell. Mol. Life Sci. 2006;63:755–759. doi: 10.1007/s00018-005-5490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diaz-Montero CM, Wygant JN, McIntyre BW. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur. J. Cancer. 2006;42:1491–1500. doi: 10.1016/j.ejca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Dionne CA, Camoratto AM, Jani JP, Emerson E, Neff N, Vaught JL, Murakata C, Djakiew D, Lamb J, Bova S, George D, Isaacs JT. Cell cycle-independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587) Clin. Cancer Res. 1998;4:1887–1898. [PubMed] [Google Scholar]
- DiPaola RS, Patel J, Rafi MM. Targeting apoptosis in prostate cancer. Hematol. Oncol. Clin. North Am. 2001;15:509–524. doi: 10.1016/s0889-8588(05)70229-x. [DOI] [PubMed] [Google Scholar]
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. A novel role for carcinoembryonic antigen-related cell adhesion molecule 6 as a determinant of gemcitabine chemoresistance in pancreatic adenocarcinoma cells. Cancer Res. 2004;64:3987–3993. doi: 10.1158/0008-5472.CAN-04-0424. [DOI] [PubMed] [Google Scholar]
- Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin. Cancer Res. 2003;9:5271–5281. [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Fizazi K. The role of Src in prostate cancer. Ann. Oncol. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Yang J, Peleg S, Sikes CR, Kreimann EL, Daliani D, Olive M, Raymond KA, Janus TJ, Logothetis CJ, Karsenty G, Navone NM. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin. Cancer Res. 2003;9:2587–2597. [PubMed] [Google Scholar]
- Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J. Biol. Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Krajewski S, Epstein JI, Reed JC, Isaacs JT. Expression of bcl-2 and the progression of human and rodent prostatic cancers. Clin. Cancer Res. 1996;2:389–398. [PubMed] [Google Scholar]
- Garrison JB, Kyprianou N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death receptor-mediated pathway. Cancer Res. 2006;66:464–72. doi: 10.1158/0008-5472.CAN-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JB, Shaw YJ, Chen CS, Kyprianou N. Novel quinazoline-based compounds impair prostate tumorigenesis by targeting tumor vascularity. Cancer Res. 2007;67:11344–11352. doi: 10.1158/0008-5472.CAN-07-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh PM, Malik S, Bedolla R, Kreisberg JI. Akt in prostate cancer: possible role in androgen-independence. Curr. Drug Metab. 2003;4:487–496. doi: 10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Buricchi F, Grimaldi G, Parri M, Cialdai F, Taddei ML, Raugei G, Ramponi G, Chiarugi P. Redox regulation of anoikis: reactive oxygen species as essential mediators of cell survival. Cell Death Differ. 2008;15:867–878. doi: 10.1038/cdd.2008.3. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Fiaschi T, Ramponi G, Chiarugi P. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene. 2009;28:2074–2086. doi: 10.1038/onc.2009.77. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell. Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Languino LR. Integrin signaling in cancer. Cancer Treat. Res. 2004;119:15–31. doi: 10.1007/1-4020-7847-1_2. [DOI] [PubMed] [Google Scholar]
- Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr. Relat. Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, Deddens JA, Konicek BW, Colligan BM, Hurst BM, Carter HW, Carter JH. Integrin-linked kinase expression increases with prostate tumor grade. Clin. Cancer Res. 2001;7:1987–1991. [PubMed] [Google Scholar]
- Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Haraguchi M, Okubo T, Miyashita Y, Miyamoto Y, Hayashi M, Crotti TN, McHugh KP, Ozawa M. Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J. Biol. Chem. 2008;283:23514–23523. doi: 10.1074/jbc.M801125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlobilkova A, Knillova J, Bartek J, Lukas J, Kolar Z. The mechanism of action of the tumor suppressor gene PTEN. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2003;147:19–25. [PubMed] [Google Scholar]
- Kajiwara T, Takeuchi T, Ueki T, Moriyama N, Ueki K, Kakizoe T, Kawabe K. Effect of Bcl-2 overexpression in human prostate cancer cells in vitro and in vivo. Int. J. Urol. 1999;6:520–525. doi: 10.1046/j.1442-2042.1999.00102.x. [DOI] [PubMed] [Google Scholar]
- Keledjian K, Garrison JB, Kyprianou N. Doxazosin inhibits human vascular endothelial cell adhesion, migration, and invasion. J. Cell. Biochem. 2005;94:374–388. doi: 10.1002/jcb.20240. [DOI] [PubMed] [Google Scholar]
- Keledjian K, Kyprianou N. Anoikis induction by quinazoline based alpha 1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. J. Urol. 2003;169:1150–1156. doi: 10.1097/01.ju.0000042453.12079.77. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A, Peehl D, Gao GJ, Reed JC. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin. Cancer Res. 2003;9:4914–4925. [PubMed] [Google Scholar]
- Li F, Liu J, Mayne R, Wu C. Identification and characterization of a mouse protein kinase that is highly homologous to human integrin-linked kinase. Biochim. Biophys. Acta. 1997;1358:215–220. doi: 10.1016/s0167-4889(97)00089-x. [DOI] [PubMed] [Google Scholar]
- Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Coll MA, Perera S, Shi W, Filmus J. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene. 2005;24:1727–1737. doi: 10.1038/sj.onc.1208379. [DOI] [PubMed] [Google Scholar]
- Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18:8024–8032. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- Marshall JL, Kindler H, Deeken J, Bhargava P, Vogelzang NJ, Rizvi N, Luhtala T, Boylan S, Dordal M, Robertson P, Hawkins MJ, Ratain MJ. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest. New Drugs. 2005;23:31–37. doi: 10.1023/B:DRUG.0000047103.64335.b0. [DOI] [PubMed] [Google Scholar]
- Martelli ML, Iuliano R, Le Pera I, Sama I, Monaco C, Cammarota S, Kroll T, Chiariotti L, Santoro M, Fusco A. Inhibitory effects of peroxisome poliferator-activated receptor gamma on thyroid carcinoma cell growth. J. Clin. Endocrinol. Metab. 2002;87:4728–4735. doi: 10.1210/jc.2001-012054. [DOI] [PubMed] [Google Scholar]
- Mawji IA, Simpson CD, Gronda M, Williams MA, Hurren R, Henderson CJ, Datti A, Wrana JL, Schimmer AD. A chemical screen identifies anisomycin as an anoikis sensitizer that functions by decreasing FLIP protein synthesis. Cancer Res. 2007a;67:8307–8315. doi: 10.1158/0008-5472.CAN-07-1687. [DOI] [PubMed] [Google Scholar]
- Mawji IA, Simpson CD, Hurren R, Gronda M, Williams MA, Filmus J, Jonkman J, Da Costa RS, Wilson BC, Thomas MP, Reed JC, Glinsky GV, Schimmer AD. Critical role for Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein in anoikis resistance and distant tumor formation. J. Natl. Cancer Inst. 2007b;99:811–822. doi: 10.1093/jnci/djk182. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr. Cancer Ther. 2004;3:349–380. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- McEleny KR, Watson RW, Fitzpatrick JM. Defining a role for the inhibitors of apoptosis proteins in prostate cancer. Prostate Cancer Prostatic Dis. 2001;4:28–32. doi: 10.1038/sj.pcan.4500502. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J. Cell. Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Panigrahy D, Shen LQ, Kieran MW, Kaipainen A. Therapeutic potential of thiazolidinediones as anticancer agents. Expert Opin. Investig. Drugs. 2003;12:1925–1937. doi: 10.1517/13543784.12.12.1925. [DOI] [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- Pfeil K, Eder IE, Putz T, Ramoner R, Culig Z, Ueberall F, Bartsch G, Klocker H. Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. Prostate. 2004;58:259–268. doi: 10.1002/pros.10332. [DOI] [PubMed] [Google Scholar]
- Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J. Biol. Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;5:11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K, Shi W, Calabretta B, Filmus J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. A novel mechanism of Anoikis of intestinal epithelial cells. J. Biol. Chem. 2002;277:46123–46130. doi: 10.1074/jbc.M207883200. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, McCann RO, Dhir R, Ichikawa T, Kyprianou N. Talin1 is a novel mediator of prostate cancer cell migration, invasion, and metastasis. American Urology Association Annual Meeting 2009; 2009. Abstract 1328. [Google Scholar]
- Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- Schaefer KL, Wada K, Takahashi H, Matsuhashi N, Ohnishi S, Wolfe MM, Turner JR, Nakajima A, Borkan SC, Saubermann LJ. Peroxisome proliferator-activated receptor gamma inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res. 2005;65:2251–2259. doi: 10.1158/0008-5472.CAN-04-3037. [DOI] [PubMed] [Google Scholar]
- Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- Sclabas GM, Fujioka S, Schmidt C, Li Z, Frederick WA, Yang W, Yokoi K, Evans DB, Abbruzzese JL, Hess KR, Zhang W, Fidler IJ, Chiao PJ. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin. Cancer Res. 2005;11:440–449. [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- Stern DF. More than a marker…Phosphorylated Akt in prostate carcinoma. Clin. Cancer Res. 2004;10:6407–6410. doi: 10.1158/1078-0432.CCR-04-1783. [DOI] [PubMed] [Google Scholar]
- Sunters A, de Mattos S. Fernandez, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- Undevia SD, Vogelzang NJ, Mauer AM, Janisch L, Mani S, Ratain MJ. Phase I clinical trial of CEP-2563 dihydrochloride, a receptor tyrosine kinase inhibitor, in patients with refractory solid tumors. Invest. New Drugs. 2004;22:449–458. doi: 10.1023/B:DRUG.0000036687.26604.8c. [DOI] [PubMed] [Google Scholar]
- Zhu M, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of Prostate Cancer Cells. The FASEB J. 2009 doi: 10.1096/fj.09-136994. in press, doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Gilmore AP, Streuli CH. Bim is an apoptosis sensor that responds to loss of survival signals delivered by epidermal growth factor but not those provided by integrins. J. Biol. Chem. 2004;279:41280–41285. doi: 10.1074/jbc.C400248200. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Yin L, Rao P, Stein R, Harsch KM, Lee Z, Heston WD. Targeted treatment of prostate cancer. J. Cell. Biochem. 2007;102:571–579. doi: 10.1002/jcb.21491. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Peterse JL, van ’t Veer LJ. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J. Cell. Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat. Rev. Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Whang YE. PTEN sensitizes prostate cancer cells to death receptor-mediated and drug-induced apoptosis through a FADD-dependent pathway. Oncogene. 2002;21:319–327. doi: 10.1038/sj.onc.1205054. [DOI] [PubMed] [Google Scholar]