Summation
The cytocidal potency of a molecule can be augmented by conjugating a radionuclide for molecular targeted radionuclide therapy (MTRT) for cancer. Radioimmunotherapy (RIT) should be incorporated into the management of patients with B-cell non-Hodgkin's lymphoma (NHL) soon after the patients have proven incurable. Better drugs, strategies, and combinations with other drugs seem certain to make RIT integral to the management of patients with NHL and likely to lead to a cure of the currently incurable NHL. These improved drugs, strategies, and combinations thereof also offer opportunities for RIT to become part of the management of solid malignancies, including epithelial cancers. Smaller radionuclide carriers, such as those used for pretargeted strategies, provide dose intensification. The potential of pretargeted RIT to improve patient outcomes is striking.
Key words: radionuclide, radiotherapy, antibody, solid cancers, non-Hodgkin's lymphoma, radioimmunotherapy
Introduction
“Only those who risk going too far will ever know how far they can go.”
—Anonymous
Patients are commonly cured of locoregional malignancies by using surgery, conventional radiotherapy, and chemotherapy but die of disseminated cancer (Fig. 1). Using molecular targeted radionuclide therapy (MTRT), we can cure, the evidence suggests, non-Hodgkin's lymphoma (NHL). It is time to ask the question, “Does MTRT provide a meaningful opportunity in metastatic ‘solid malignancies?’ ” The term “solid malignancies” is often used to refer to malignancies that are not of hematopoietic origin and less radiosensitive. Many are of epithelial origin, such as breast, prostate, colorectal, and lung cancers.
Figure 1.
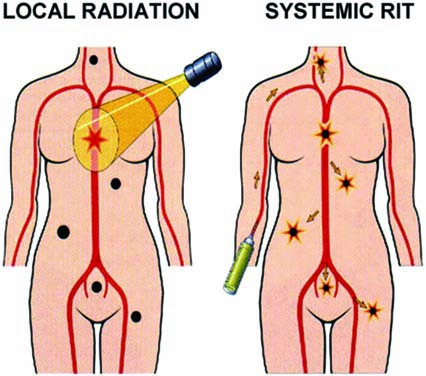
Local external beam radiotherapy versus systemic radioimmunotherapy (RIT) or other forms of molecular targeted radionuclide therapy. External beam radiotherapy is effective for locoregional non-Hodgkin's lymphoma (NHL) (left). NHL commonly presents as a multifocal disease. RIT capitalizes on: (1) specific monoclonal antibody targeting carrying therapeutic radionuclides to all NHL after an infusion (right) and (2) the sensitivity of malignant lymphocytes to radiation (reproduced with permission from DeNardo and O'Donnell (DeNardo GL, O'Donnell RT. Radiolabeled Lym-1 antibody therapy in lymphoma. Biol Ther Lymph 1999;2:8).
RIT has proven highly effective for patients with B-cell NHL, using conventional or direct strategies. Durable response rates of 60%–80% have been achieved in trials using 131I-tositumomab (Bexxar®; Glaxo Smith Kline, Research Triangle Park, NC) or 90Y-ibritumomab tiuxetan (Zevalin®; Biogen Idec, Inc., Cambridge, MA; and Cell Therapeutics, Inc., Seattle, WA). In solid malignancies, response rates to RIT have been about 10%–20% and less durable. The reasons seem mostly related to greater radioresistance of these malignancies; however, solid malignancies are variable in this respect. Some are less radioresistant and, therefore, preferred candidates. Bone marrow toxicity has generally been dose limiting for RIT,1,2 unless stem cell transplantation (SCT) has been used to displace the dose-limiting tissue to one that is more radioresistant. Another strategy for dose intensification separates the delivery of the targeting molecule from that of the radionuclide carrier, using pretargeted strategies. The concept of pretargeted RIT is to deliver a bispecific molecule to the malignancy first, allow for clearance from the circulation, then administer a small radionuclide carrier that readily binds to the targeting molecule in the malignant tissues and clears from normal tissues.
MTRT in NHL
The frequency and incidence of NHL has substantially increased in recent decades. Fortunately, new therapies have become available. In 1997, the United States Food and Drug Administration approved rituximab (Rituxan®; Biogen Idec, Inc.; Cambridge, MA; and Genentech, Inc., South San Francisco, CA) for NHL. Rituximab has been proven useful alone for indolent NHL and, in combination with chemotherapy, for aggressive NHL. The drug is sometimes used as first-line therapy for indolent NHL. Patients refractory or resistant to chemotherapy or rituximab respond and achieve long-term remissions from 131I-tositumomab and 90Y-ibritumomab, monoclonal antibodies (mAbs) conjugated to radionuclides, to deliver radiation to the NHL cells. These mAbs localize to the surface membrane of antigen-expressing NHL cells. Like conventional radiotherapy, NHL almost never comes back at a site treated using these systemic drugs because NHL is highly radiosensitive. Overall response rates and complete remission rates have been substantially higher for RIT than for rituximab alone in both indolent and aggressive NHL; there is evidence for survival advantage. Dose-response relationships have been observed, as expected. In one study, higher concentrations of lymph node radioactivity correlated with better clinical response. 3
Phase III Salvage RIT
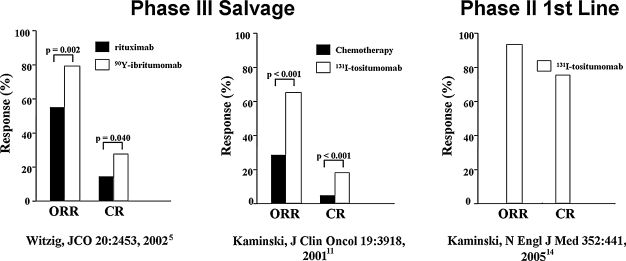
In trials in patients with relapsed or refractory NHL, overall response rates were greater than 60% and complete remission rates ranged from 15% to 50%.4–10 In all instances, the response rates were greater for RIT, using tositumomab or ibritumomab, than they were for rituximab or the most recent regimen of chemotherapy (Fig. 2).5,11 Continuing responses beyond 5 years have been observed in a substantial proportion of the patients that achieved a complete remission, despite a prior failure to respond to other therapy.12,13
Figure 2.
Salvage and first-line radioimmunotherapy (RIT) in non-Hodgkin's lymphoma (NHL).5,11,14 Overall (ORR) and complete (CR) response rates in the pivotal phase III trial of 90Y-ibritumomab versus rituximab in relapsed/refractory low-grade, follicular, or transformed NHL (left). Patients randomized into the 90Y-ibritumomab arm were given a single therapeutic dose of 14.8 MBq/kg (0.4 mCi/kg) of 90Y-ibritumomab on day 7, preceded by 250 mg/m2 of rituximab. Patients randomized into the rituximab arm received rituximab 375 mg/m2 weekly × 4. The efficacy analysis showed an overall response rate (ORR) of 80% for 90Y-ibritumomab versus 56% for rituximab (p = 0.002). The complete remission (CR) was 30% for 90Y-ibritumomab versus 16% for rituximab (p = 0.04). Pivotal phase III trial of iodine-131 (131I)-tositumomab versus chemotherapy in low-grade, or transformed, NHL (middle). Patients who had not responded or had progressed after their most recent chemotherapy were treated with 131I-tositumomab at a dose contributing 75 cGy to the body, preceded by 450 mg of tositumomab. The patients had received a median of four prior chemotherapy regimens. Sixty-five percent (65%) of the patients had a response after 131I-tositumomab, compared with 28% after their last chemotherapy (p < 0.001); 20% of the patients had a complete remission after 131I-tositumomab, compared with 3% after their last chemotherapy (p < 0.001). Phase II first-line trial of 131I-tositumomab in stage III–IV follicular NHL. A single therapy dose of 131I-tositumomab (75 cGy to the body, preceded by 450 mg of tositumomab) led to ORRs and CRs of 95% and 75%, respectively, with very modest toxicity. Median progression-free survival was 6.1 years (graphics generated from data in Witzig et al., 5 Kaminski et al., 11 and Kaminski et al., 14 respectively).
Phase II First Line RIT
Given as a single dose of a single drug to previously untreated patients with advanced follicular NHL, 131I-tositumomab had an overall response rate of 95% and a complete remission rate of 74% (Fig. 2). 14 Toxicity was minimal and the 5-year progression-free survival for these patients was 62%.
Radiation Dose Versus Radiobiologic Response
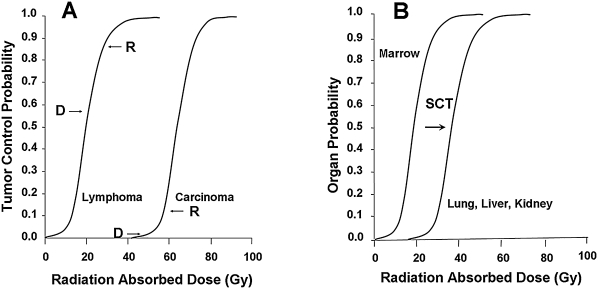
Before addressing dose intensification, several concepts need to be addressed. There is abundant evidence for a radiation dose-tissue response relationship for radiotherapy (Fig. 3), including radionuclide therapy (and for chemotherapy). This relationship can also be described in terms of the radiation-absorbed doses to the malignant and normal tissues. A variety of other surrogates are sometimes used. However, the radiation effect on a tissue reflects both its radiosensitivity and the radiation dose absorbed by the tissue (Fig. 3). As indicated earlier, malignancies of hematopoietic origin are quite radiosensitive. Among normal tissues, one of the most radiosensitive is the bone marrow. Similar to solid malignancies, normal tissues, such as those in the liver, kidney, and lung, are more radioresistant. For radionuclide therapy, in therapeutic indices reflecting the ratio of the radiation doses (or some surrogate thereof) absorbed by the malignant tissue relative to that absorbed by a normal tissue, often the dose-limiting normal tissue (the marrow) can be generated to compare drugs and strategies and to obtain a first approximation of the likely therapeutic advantage, that is, the likelihood of therapeutic effect relative to the likelihood and severity of normal tissue toxicity. These estimates are best performed on the radiation-absorbed doses or surrogates (e.g., the area under the time-activity curves), that is, the activities for the tissues over time in microcurie hours per gram of tissue. Other factors must be considered when judging strategies and drugs. One of these is the absolute activity concentrations in the tissues. Some drugs and strategies have attractive indices, but low absolute activity concentrations, that influence the radiation-absorbed and the activity doses needed for therapy.
Figure 3.
Relationship of radiation dose and tissue tolerance. (A) Tumor control probability versus radiation-absorbed dose for lymphoma and for a solid malignancy. Lymphoma is more radiosensitive than most solid malignancies. Whereas the observed macroscopic radiation doses of ≥2 Gy (D) lead to high response rates (R) in lymphoma, they just approach the steep portion of the dose-response relationship for solid malignancies, as expected. In both cases, observed response rates have exceeded expectations for the estimated radiation doses where response rates are low, probably reflecting differences between macroscopic and cell-level radiation-absorbed doses. Dose intensification for radioimmunotherapy should lead to non-Hodgkin's lymphoma cures and meaningful responses in some of the solid malignancies, because estimated radiation doses are on the steep regions of the curves describing the relationships to tumor control. (B) Probability of a normal tissue effect versus radiation dose for bone marrow versus lung, liver, or kidney. Stem cell transplantation, and analogous methods for dose intensification, displaces the dose-limiting tissue to ones much more radiotolerant (graphics generated from data in multiple sources, including Cronqvist et al., Cronqvist AK, Kallman P, Turesson I, et al. Volume and heterogeneity dependence of the dose-response relationship for head and neck tumours. Acta Oncol 1995;34:851. Fletcher and Shukovsky Fletcher GH, Shukovsky LJ. Interplay of radiocurability and tolerance in the irradiation of human cancers. J Radiol Electrol Med Nucl 1975;56:383; Emami et al. 18 ; and Press et al. 58 )
Solid Malignancies
The use of RIT as an adjuvant to locoregional surgery and radiotherapy to eradicate metastases, whether detectable or undetectable, is sound and was one of the earliest purposes for chemotherapy. RIT has favorably influenced long-term outcomes in solid malignancies, such as colorectal cancer, 15 ovarian, 16 and glioblastoma multiforme, 17 when used as an adjuvant to established therapy. Phase II trials of RIT at fixed doses in these settings have shown benefit but require confirmation in larger, phase III multicenter, randomized trials. Phase I studies in broader settings show evidence that RIT has activity, but the durability and frequency of responses have been low.18–25 Reasons given for the different effectiveness of RIT in solid malignancies versus NHL include different radiation doses, dose rates, uptakes, and activity concentrations in the malignancies; however, these parameters are not substantially different for the solid malignancies and NHL. The most likely explanation is that solid malignancies are less radiosensitive than NHL, just as many normal tissues are less radiosensitive than bone marrow (Fig. 3). Along with dose intensification in larger numbers of patients, less radioresistant solid malignancies should be the focus of future efforts (Table 1). The numbers of patients in trials of SCT and pretargeted RIT for solid malignancies have been insufficient even to reach the maximum tolerated dose in many instances, and more radioresistant solid malignancies have sometimes been selected. Before conclusions can be drawn for the solid malignancies, phase II studies at fixed doses in adequate numbers of patients having less radioresistant types of malignancies, and using current methods for dose intensification, such as pretargeted RIT, are required.
Table 1.
Future Trial Requirements for Solid Malignancies
| • Phase II and III trials |
| • More patients |
| • Better-selected patients |
| • More networking, multiple centers |
Dose Intensification
A method for intensifying the effect of a molecule, even a bioactive one, is to add a radionuclide to serve as a radiation source.5,26–28 Other strategies and drugs have been shown, in preclinical studies, to provide radiation dose intensification (Table 2). Some of these have also been validated in patients but not adequately in patients with solid malignancies. The latter have often involved insufficient numbers of patients studied at a fixed-dose level; better judgments can be made after multicenter, phase II and III trials.
Table 2.
Validated Methods for Intensification of Therapeutic Effects
| • Arm with radionuclide (e.g., 90Y or 131I) |
| • Treat earlier! |
| • Radiation dose intensification: “more and longer” |
| • Larger doses, (e.g., SCT) |
| • More doses (e.g., multiple doses or fractionation) |
| • Better-defined patient population, using imaging |
| • Small molecule radionuclide carriers |
| • Pretargeted RIT |
| • mAb alternatives (e.g., mAb fragments, affibodies, aptamers, SHALs, peptides, etc.) |
SCT, stem cell transplantation; RIT, radioimmunotherapy; mAb, monoclonal antibody; SHALs, selected high-affinity ligands.
Strategies and drugs for dose intensification may reduce the mathematically derived therapeutic index for the malignant and dose-limiting tissues, yet therapeutic advantage may be gained because the dose-limiting tissue is displaced from a radiosensitive one (e.g., the marrow) to a less radiosensitive one (e.g., the liver, kidney, or lungs). For example, SCT and pretargeted strategies displace the dose-limiting tissue from the marrow to the liver, kidney, or lungs, for which the therapeutic index is only about 4-1. Therapeutic advantage occurs because the liver, kidney, or lungs are at least 10 times more radioresistant than the marrow. If multiple doses of the same drug are given using the same strategy, the dose-limiting tissue may be the same, as may be the radiation-dose distribution, for each of the doses. 29 Some studies have actually shown that the radiation dose absorbed by malignant tissue was greater for a subsequently administered dose than for the previous dose. 30
Radiosensitization
The potential to radiosensitize malignant cells (or radioprotect normal cells) has long been appreciated by radiotherapists. The opportunity to disassociate malignant from normal tissue radiation, and to achieve advantage from radiosensitizers, has been described, 31 and was shown to be effective for MTRT, using drugs (taxanes) approved for other purposes.32,33 Other targets that are attractive in this regard include the epidermal growth factor (EGF) family of receptors and related intracellular signaling proteins. 34 Overexpression of EGFR occurs in many malignancies. Drugs that block EGFR expression or inhibit tyrosine kinases have been associated with events favorable for radiotherapy, including cell-cycle arrest.35,36
In addition to its antilymphoma effect in the absence of radiation, rituximab has been shown to radiosensitize lymphoma cells at low levels of radiation exposure.37,38 A purported mechanism of action is the arrest of the cell cycle. 39 Another mAb against solid malignancies has shown remarkable bioactivity and evidence for radiosensitization in patients. 30 Patients given this mAb, radiolabeled, for RIT of advanced breast cancer showed greater therapeutic benefit than have patients with this and other solid malignancies given RIT, using other mAbs. Other protocol factors may dictate that it is preferable to use a different drug for radiosensitization than a bioactive mAb that also serves as the radionuclide carrier.
Hematopoietic SCT
High-dose chemotherapy and SCT are used for chemosensitive, relapsed NHL. This strategy for the dose intensification of chemotherapy is the standard for the salvage of follicular NHL, the most common indolent and second most common type of NHL. An alternative approach is to deliver potentially curative radiation doses, using SCT, to permit the displacement of the dose-limiting tissue from radiosensitive marrow to more resistant normal tissues, such as the lung or liver. Press et al. 40 initiated high-dose RIT for relapsed NHL and achieved remarkable, highly durable response rates. 41 In this salvage setting, overall response rates and complete remission rates have been 95% and 85%, respectively, and remissions have been longer than single-dose, nonmyeloablative RIT.42–44 At 2 years, overall survival was 93% and progression-free survival 62%, both impressive in a salvage setting for NHL. Later follow-up has shown that many of the remissions have lasted more than 5 years. Although the mortality and toxicity for myeloablative RIT was greater than that for nonmyeloablative RIT for NHL, they were substantially less than those for high-dose chemotherapy. 45 Additionally, 5-year overall and progression-free survivals were greater for high-dose RIT than for high-dose chemotherapy with SCT in patients with relapsed follicular NHL; the former also tolerated further therapy better than the latter.
A phase III trial comparing the efficacy of six cycles of chemotherapy followed by 131I-tositumomab versus six cycles of chemotherapy in previously untreated, advanced follicular NHL showed the superiority of the former. Overall and complete response rates were 91% and 69%, respectively, and the progression-free survival was better when 131I-tositumomab was added to chemotherapy. 46
Multiple Doses (Fractionation)
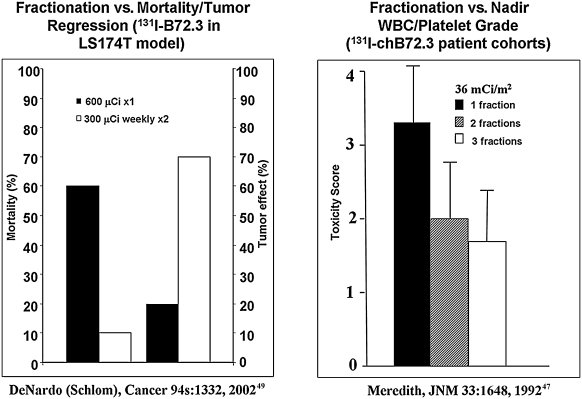
Reflecting the remarkable potency of the drugs, the single therapy dose used in the phase III pivotal trials of 131I-tositumomab and 90 Y-ibritumomab proved effective and led to drug approvals. However, a single therapy dose of a single drug is not consistent with the principles established for chemotherapy and conventional radiotherapy, and likely decreased the opportunities for complete remissions and better long-term outcomes from 131I-tositumomab and 90 Y-ibritumomab. Dose intensification is clearly needed for the solid malignancies. Administration of multiple doses has been shown to be effective and safe (Fig. 4).47–49 A study in mice with human colon cancer xenografts in which 1000 μCi of 131I-labeled mAb fragments was given in a single dose and compared with four daily doses of 250 μCi showed that the “fractionated schedule clearly presented a therapeutic advantage ….” 29 Further, the cumulative radiation dose to the xenografts was 4-fold greater when the activity dose was fractionated. Finally, the multiple-dose strategy can be used with other strategies for dose intensification; it has been used for RIT with SCT,30,32,50 as well as RIT alone.51,52
Figure 4.
Beneficial effects of multiple-dose radioimmunotherapy. Mortality and efficacy after one or two doses of 131I-B72.3 at a weekly interval in mice (left) (graphic generated from data in DeNardo 27 ). Fractionation versus single-dose toxicity in patients (right). Mean toxicity scores (sum of white blood cell count and platelet nadir grade) for groups of patients treated with a total dose of 36 mCi/m2 of 131I-chimeric B72.3 given in one, two, or three weekly fractions (modified from DeNardo et al. 49 and Meredith et al. 47 ).
Pretargeted RIT
To improve the therapeutic ratios of RIT, methods, known as pretargeted RIT and consisting of multiple-step processes that separate the distribution of the targeting molecule from that of the radionuclide delivery system have allowed dose escalation in preclinical and clinical studies (Fig. 5).53–59 In mice and in patients pretargeted RIT has been more effective than direct RIT, even in solid malignancies, because therapeutic indices were higher and larger activity doses of radionuclide could be safely administered (Figure 6).58–62 In a landmark study by Axworthy et al., 63 cures were obtained in mice with lung, colon, or breast cancer xenografts by using this strategy. However, some of the agents used in this strategy are immunogenic. A more general pretargeted RIT strategy, referred to as “dock and lock,” has been described.61,64,65 Preclinical studies have been exciting; this strategy and these agents are ready for application to trials in patients for the dose intensification needed for solid malignancies, as well as that for the cure of NHL. Several exceptional recent reviews are recommended.64–66 Although disadvantages, including the complexity of the strategy, exist, many past disadvantages have been overcome. Ultimately, more direct strategies involving small-molecule radiation-delivery systems are likely to dominate clinical imaging and therapy.
Figure 5.
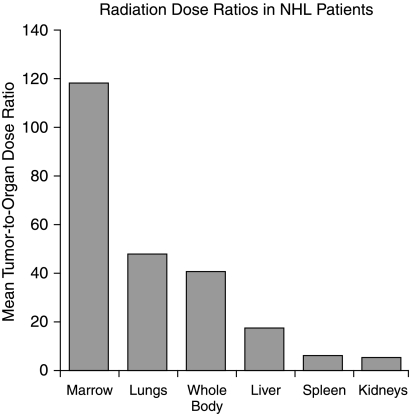
Illustrative therapeutic indices for pretargeted radioimmunotherapy. Tumor-to-tissue radiation-dose ratios for 111In-DOTA-biotin after pretargeted C2B8-SA in non-Hodgkin's lymphoma patients (reproduced with permission from Weiden et al. 59 )
Figure 6.
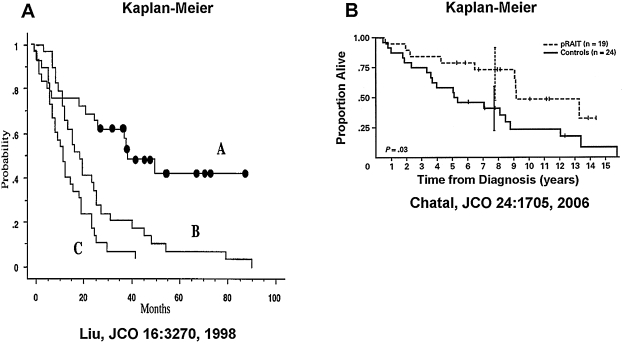
(A) Kaplan-Meier graphs showing progression-free survival after 131I-tositumomab therapy in non-Hodgkin's lymphoma patients (A), compared with best results from other therapies (B) and chemotherapies (C). (B) Overall survival in patients with high-risk radioresistant medullary thyroid cancer after pretargeted radioimmunotherapy. Vertical bars correspond to a 95% confidence interval for survival rate at the median follow-up (reproduced with permission from Liu et al. 41 and Chatal et al. 24 ).
Small-Molecule Carriers
Whereas intact mAbs recognize malignant cells specifically, size limits their value. As size decreases, blood clearance and tissue penetration increase. mAb fragments have been used to improve the therapeutic index (tumor–blood ratio) and to serve as building blocks for more complicated molecules of interest. 67 Although smaller than an intact mAb, and generally having the preferred pharmacokinetics for RIT, mAb fragments still are appreciably larger than chemotherapeutic drugs. Peptides are very small radionuclide carriers. A notable group are those that bind to the somatostatin and related growth-factor receptors. They have been successfully translated to the clinic.68–74 Aptamers, 75 affibodies, 76 and selective high-affinity ligands (SHALs)77–79 are promising classes of molecules of very small size, high affinity, and specificity. All of these molecules can be generated using combinatorial libraries as well as conventional synthetic methods.
SHALs
The class II major histocompatibility, human leukocyte antigens (HLAs) serve as signaling receptors and in the immune mechanism. These proteins are abundantly present on the surface and inside malignant B-lymphocytes. Based on predictions from in silico modeling and from empiric testing, small organic ligands have been selected to bind to docking sites within the Lym-1 mAb epitopic region of HLA-DR10. Covalently linking sets of these ligands, SHALs, have been generated for B-cell-derived lymphomas and leukemias. 80 These novel nanomolecules mimic the binding of mAbs because of contacts between multiple residues on the surface of the SHAL and its target protein, and have had rapid uptake by NHL xenografts and fast clearance from normal tissues (Fig. 7). 81 Although the SHALs differed with respect to the number and nature of ligands, polyethylene glycol monomers, and lysines, all SHALs were neutral, readily diffused into cells, and bound selectively to HLA-DR10 protein and expressing cells. Histochemical analyses of NHL tissues showed that the SHALs bind to lymphoma from patients. SHALs having a Ct (3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid) ligand had additional properties of importance (Fig. 8). These SHALs residualized inside HLA-DR10-expressing cells and showed antilymphoma potency against live human lymphoma cells and xenografts in mice (Figs. 9 and 10). 81 Readily conjugated to a radionuclide, SHALs also serve as carriers for selective cell level radiation.
Figure 7.
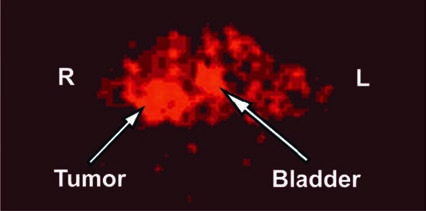
Micro–positron emission tomography tomographic molecular imaging of a mouse obtained 2 hours after intravenous 64Cu-labeled SHAL (7.4 MBq; 4 μg). Transverse section shows increased radioactivity in the Raji human non-Hodgkin's lymphoma, but not in the HBT 3477 human breast cancer xenograft, on the right and left lower abdomen, respectively. Raji xenografts express, whereas HBT 3477 xenografts do not express human leukocyte antigen (HLA)-DR10. The xenografts provided positive (Raji) and negative (HBT 3477) controls for HLA-DR10, with which the SHALs were intended to bind (modified and reproduced with permission from DeNardo et al. (DeNardo GL, Natarajan A, Hok S, et al. Pharmacokinetic characterization in xenografted mice of a series of first-generation mimics for HLA-DR antibody, Lym-1, as carrier molecules to image and treat lymphoma. J Nucl Med 2007;48:1338.)
Figure 8.
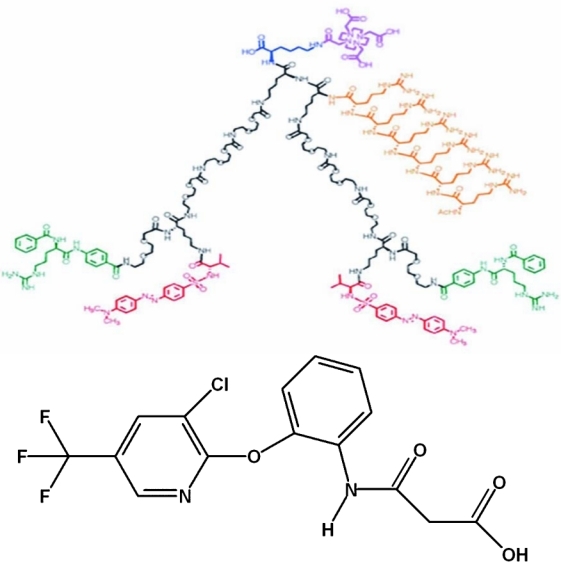
Chemical structure in 2 dimensions for dimeric selected high-affinity ligand, (DvLPBaPPP)2(Arg)6LLDo, with an Arg6 nuclear localization sequence (upper). SHALs having the Ct ligand, 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid (lower), selectively residualized and were cytotoxic. (Analog-biotinylated SHALs differ only with respect to the substitution of biotin for the macrocycle chelate, to the upper right of SHAL.)
Figure 9.
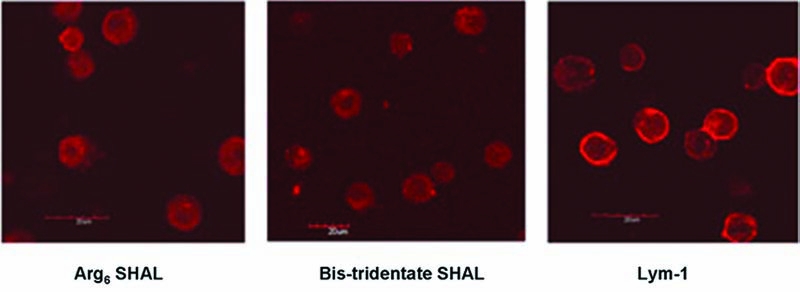
Confocal micrographs of washed live Raji (expressing) cells after incubation with Arg6 selected high-affinity ligand (SHAL) or dimeric (bis), tridentate SHAL having the ((DvPLLCtPCbPPP)2LLDo)Ct ligand or ChLym-1 (left to right). ChLym-1 (right) showed cell-surface membrane binding, whereas the SHAL with Arg6 (left) and the SHAL with Ct (middle) showed intracellular binding to Raji cells. There was no binding to Jurkat's cells (not shown). AlexaFlor (Invitrogen, Eugene, OR) (red) was used to locate the SHALs and ChLym-1.
Figure 10.
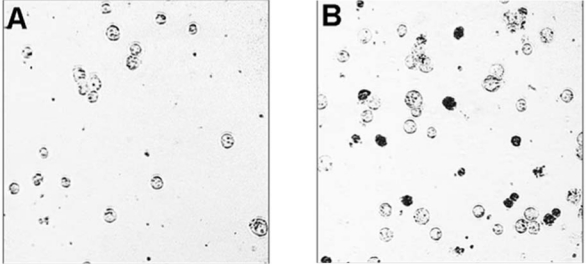
Photomicrographs of Raji-cell viability in an untreated sample (A) and a sample (B) treated with selected high-affinity ligand (SHAL) (DvPLLCtPCbL) at 24 hours. Intracellular staining (trypan blue dye) of dead Raji cells illustrates the effects induced by SHALs containing the Ct ligand.
Delivery of carrier molecules to intracellular sites, including nuclear localization, has been demonstrated for mAbs and other proteins, using natural and synthetic cationic peptides as transporters. 82 To improve SHAL uptake and residence in NHL cells, hexa-arginine was conjugated to a SHAL (Fig. 8). The hexa-arginine sequence enhanced the SHAL's internalization by HLA-DR10-expressing NHL cells (Fig. 9).
SHALs have great potential as novel small molecules for targeting lymphoma and leukemia for molecular therapy and imaging. SHAL-based therapeutics to transport and residualize a variety of agents near critical sites inside malignant cells can be developed. The SHAL production platform is efficient, flexible, and permits rapid synthesis and modifications, leading to SHALs with highly improved properties. Unlike their biologic counterparts, these chemicals are inexpensive and easy to produce with consistency.
Conclusions
In summary, where are we with MTRT? Conventional RIT for patients with NHL is attractive as both first-line and salvage therapy, whether as a single agent or combined with other drugs, and has an attractive adverse-event profile. Because of the radiosensitivity of NHL, modest dose intensification, using existing strategies and drugs, should cure most of these patients. RIT should be given greater consideration for first-line therapy because of its remarkable response and safety profile. In solid malignancies, there is evidence for biologic activity, but it has been insufficient to establish the role of MTRT in the management of these patients. Dose intensification in meaningful phase II and III trials is required here. Dose intensification can be achieved by using stem cell transplantation SCT and/or multiple dosing strategies. Strategies using small molecules to deliver the radionuclide, whether in pretargeted RIT or in a direct mode, seem particularly attractive for dose intensification. Pretargeted RIT is ready now for widespread patient trials while awaiting more extensive preclinical trials with small molecules that can be used in direct strategies.
Acknowledgments
This work was supported by National Cancer Institute Grant PO1-CA47829 and Lawrence Livermore National Laboratory Awards 01-ERD-111, 01-ERD-046, and 01-SI-012. Lawrence Livermore National Laboratory was operated by Lawrence Livermore National Security, LLC, for the U.S. Department of Energy, National Nuclear Security Administration, under Contract DEAC52-07NA27344. This work was presented, in part, at the “Nuclear Medicine Tomorrow Conference,” March 30–April 2, 2008 in Nantes, France. We wish to thank B. Petitt for manuscript preparation.
About the Authors

Gerald L. DeNardo received his medical degree in 1957 from the University of California School of Medicine in San Francisco. Following internship and resident training in Internal Medicine and military service, he began his career in academic medicine at the University of Colorado in 1961 and at Stanford in 1965. He joined the Department of Radiology and Medicine at the University of California, Davis School of Medicine in 1970. Dr. DeNardo specializes in systemic radiotherapy for the treatment of lymphoma, leukemia, and metastatic breast and prostate cancers, currently focusing on novel, small-molecule carriers (SHALs). He has been the recipient of the Cassen Prize for 2000, presented at the 47th Annual Meeting of the Society of Nuclear Medicine, St. Louis, Missouri, and the Berson-Yallow Award in 1978 and 1984. Dr. DeNardo is currently Professor Emeritus in Internal Medicine, Radiology and Pathology at the University of California, Davis School of Medicine and Cancer Center.

Sally J. DeNardo received her medical degree in 1965 from the University of Chicago, Pritzer School of Medicine, and her postdoctoral training in Internal Medicine, Hematology/Oncology and Nuclear Medicine at Stanford from 1965 to 1971. She then joined the faculty of the University of California Davis, School of Medicine. Her primary clinical and research focus has been nuclear oncology: basic science development and clinical evaluation of new tumor targeting antibody or recombinant fragment radiopharmaceuticals for cancer therapy. She serves as a current member of the American Board of Nuclear Medicine, and on the editorial boards of several journals. Dr. DeNardo is a senior professor in the Departments of Internal Medicine (Hematology/Oncology) and Radiology (Nuclear Medicine) at the University of California Davis, School of Medicine and Cancer Center.

Rod Balhorn received his BS in chemistry in 1969 and his PhD in biochemistry in 1972, both degrees from the University of Iowa. He completed a 2-year Postdoctoral in Roger Chalkley's laboratory at the University of Iowa and, in 1974, joined the Biology and Biotechnology Research Program at Lawrence Livermore National Laboratory (LLNL) in Livermore, California. Dr. Balhorn's areas of research at LLNL include chromatin organization; protein-DNA interactions; molecular recognition; single-molecule studies of protein and DNA function; and the development of selective high-affinity ligands that bind to specific sites on the surface of proteins. Dr. Balhorn is the Group Leader for Molecular Toxicology, in the Biosciences and Biotechnology Division at Lawrence Livermore National Laboratory.
References
- 1. Wiseman GA. Kornmehl E. Leigh B, et al. Radiation dosimetry results and safety correlations from 90Y-ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory non-Hodgkin's lymphoma: Combined data from 4 clinical trials. J Nucl Med. 2003;44:465. [PubMed] [Google Scholar]
- 2. Wahl RL. Tositumomab 131I therapy in non-Hodgkin's lymphoma. J Nucl Med. 2004;46:128s. [PubMed] [Google Scholar]
- 3. Jacobs SA. Harrison A. Swerdlow S, et al. Radioisotopic localization of 90yttrium-ibritumomab tiuxetan in patients with CD20+ non-Hodgkin's lymphoma. Mol Imaging Biol. 2008 doi: 10.1007/s11307-008-0170-3. (in press). [DOI] [PubMed] [Google Scholar]
- 4. Witzig TE. Flinn IW. Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:3262. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 5. Witzig TE. Gordon LI. Cabanillas F, et al. Randomized, controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 6. Wiseman G. Gordon LI. Multani PS, et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin's lymphoma and mild thrombocytopenia: A phase II multicenter trial. Blood. 2002;99:4336. doi: 10.1182/blood.v99.12.4336. [DOI] [PubMed] [Google Scholar]
- 7. Gordon LI. Molina A. Witzig TE, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: Long-term followup of a phase I–II study. Blood. 2004;103:4429. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 8. Wiseman GA. Witzig TE. Yttrium-90 (90Y) ibritumomab tiuxetan (Zevalin) induces long-term durable responses in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Cancer Biother Radiopharm. 2005;20:185. doi: 10.1089/cbr.2005.20.185. [DOI] [PubMed] [Google Scholar]
- 9. Fisher RI. Kaminski MS. Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:7565. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 10. Kashyap A. Zelenetz A. Vose J, et al. Tositumomab and iodine-131-tositumomab produce a meaningful therapeutic benefit for patients with relapsed, refractory, and transformed low-grade (LG) NHL: Summary of the long-term response population (LTRP) Proc Am Soc Clin Oncol. 2003;22:576. [Google Scholar]
- 11. Kaminski MS. Zelenetz AD. Press OW, et al. Pivotal study of iodine I-131-tositumomab for chemotherapy-refractory, low-grade, or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 12. Kaminski MS. Estes J. Zasadny KR, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed refractory B-cell non-Hodgkin lymphoma: Update results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259. [PubMed] [Google Scholar]
- 13. DeNardo GL. Sysko VV. DeNardo SJ. Cure of incurable lymphoma. Int J Radiat Oncol Biol Phys. 2006;66:s46. doi: 10.1016/j.ijrobp.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 14. Kaminski MS. Tuck M. Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 15. Liersch T. Meller J. Kulle B, et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-Labetuzumab after salvage resection of colorectal metastases in the liver: Five-year safety and efficacy results. J Clin Oncol. 2005;23:6763. doi: 10.1200/JCO.2005.18.622. [DOI] [PubMed] [Google Scholar]
- 16. Meredith RF. Alvarez RD. Partridge EE, et al. Intraperitoneal radioimmunochemotherapy of ovarian cancer: A phase I study. Cancer Biother Radiopharm. 2001;16:305. doi: 10.1089/108497801753131381. [DOI] [PubMed] [Google Scholar]
- 17. Zalutsky MR. Targeted alpha-particle therapy of microscopic disease: Providing a further rationale for clinical investigation. J Nucl Med. 2006;47:1238. [PubMed] [Google Scholar]
- 18. Emami B. Lyman J. Brown A, et al. Tolerance of normal tissue to therapeutic radiation. Int J Radiat Oncol Biol Phys. 1991;21:109. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 19. Schrier DM. Stemmer SM. Johnson T, et al. High-dose 90Y Mx-diethylenetriaminepentaacetic acid (DTPA)-BrE-3 and autologous hematopoietic stem cell support (AHSCS) for the treatment of advanced breast cancer: A phase I trial. Cancer Res. 1995;55:5921s. [PubMed] [Google Scholar]
- 20. Cagnoni PJ. Ceriani RL. Cole WC, et al. Phase I study of high-dose radioimmunotherapy with 90-Y-hu-BrE-3 followed by autologous stem cell support (ASCS) in patients with metastatic breast cancer. Cancer Biother Radiopharm. 1998;13:328. [Google Scholar]
- 21. Wong JYC. Somlo G. Odom-Maryon T, et al. Initial results of a phase I trial evaluating 90yttrium (90Y)-chimeric T84.66 (cT84.66) anti-CEA antibody and autologous stem cell support in CEA-producing metastatic breast cancer. Cancer Biother Radiopharm. 1998;13:314. [Google Scholar]
- 22. Richman CM. DeNardo SJ. O'Donnell RT, et al. Dosimetry-based therapy in metastatic breast cancer patients using 90Y monoclonal antibodies 170H.82 with autologous stem cell support and cyclosporin A. Clin Cancer Res. 1999;5:3243s. [PubMed] [Google Scholar]
- 23. Macey DJ. Grant EJ. Kasi LP, et al. Effect of recombinant alpha-interferon on pharmacokinetics, biodistribution, toxicity, and efficacy of 131I-labeled monoclonal antibody CC49 in breast cancer: A phase II trial. Clin Cancer Res. 1997;3:1547. [PubMed] [Google Scholar]
- 24. Chatal JF. Campion L. Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: A collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 25. Wong JYC. Somlo G. Odom-Maryon T, et al. Initial clinical experience evaluating yttrium-90-chimeric T84.66 anticarcinoembryonic antigen antibody and autologous hematopoietic stem cell support in patients with carcinoembryonic antigen-producing metastatic breast cancer. Clin Cancer Res. 1999;5:3224s. [PubMed] [Google Scholar]
- 26. Davis TA. Kaminski MS. Leonard JP, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10:7792. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 27. DeNardo GL. Concepts in radioimmunotherapy and immunotherapy: Radioimmunotherapy from a Lym-1 perspective. Sem Oncol. 2005;32:27. doi: 10.1053/j.seminoncol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28. DeNardo GL. Tobin E. Chan K, et al. Direct antilymphoma effects on human lymphoma cells of monotherapy and combination therapy with CD20 and HLA-DR antibodies and yttrium-90 HLA-DR antibodies. Clin Cancer Res. 2005;11:7075. doi: 10.1158/1078-0432.CCR-1004-0008. [DOI] [PubMed] [Google Scholar]
- 29. Goel A. Augustine S. Baranowska-Kortylewicz J, et al. Single-dose versus fractionated radioimmunotherapy of human colon carcinoma xenografts using 131I-labeled multivalent CC49 single-chain Fvs1. Clin Cancer Res. 2001;7:175. [PubMed] [Google Scholar]
- 30. DeNardo SJ. Mirick GR. Kroger LA, et al. The biologic window for chimeric L6 radioimmunotherapy. Cancer. 1994;73(Suppl):1023. doi: 10.1002/1097-0142(19940201)73:3+<1023::aid-cncr2820731341>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31. DeNardo SJ. Kroger LA. Lamborn KR, et al. Importance of temporal relationships in combined modality radioimmunotherapy of breast carcinoma. Cancer. 1997;80(Suppl):2583. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2583::aid-cncr34>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32. DeNardo SJ. Kukis DL. Kroger LA, et al. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: Efficacy and toxicity in breast cancer xenografts. Proc Nat Acad Sci US Am. 1997;94:4000. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graves SS. Dearstyne E. Lin Y, et al. Combination therapy with pretarget CC49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin Cancer Res. 2003;9:3712. [PubMed] [Google Scholar]
- 34. Grunwald V. Hidalgo M. The epidermal growth-factor receptor: A new target for anticancer therapy. Curr Probl Cancer. 2002;26:109. doi: 10.1067/mcn.2002.125874. [DOI] [PubMed] [Google Scholar]
- 35. Salomon DS. Brandt R. Ciardiello F, et al. Epidermal growth-factor-related peptides and their receptors in human malignancies. Crit Rev Oncol/Hematol. 1995;19:183. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 36. Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689. doi: 10.1677/erc.1.00600. [DOI] [PubMed] [Google Scholar]
- 37. Kapadia NS. Engles JM. Wahl RL. In vitro evaluation of radioprotective and radiosensitizing effects of rituximab. J Nucl Med. 2008;49:674. doi: 10.2967/jnumed.107.043752. [DOI] [PubMed] [Google Scholar]
- 38. Skvortsova I. Popper BA. Skvortsov S. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin's lymphoma cells. J Radiat Res. 2005;46:241. doi: 10.1269/jrr.46.241. [DOI] [PubMed] [Google Scholar]
- 39. Golay J. Clark EA. Beverley PC. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. J Immunol. 1985;135:3795. [PubMed] [Google Scholar]
- 40. Press OW. Eary JF. Appelbaum FR, et al. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet. 1995;346:336. doi: 10.1016/s0140-6736(95)92225-3. [DOI] [PubMed] [Google Scholar]
- 41. Liu SY. Eary JF. Petersdorf SH, et al. Follow-up of relapsed B-cell lymphoma patients treated with iodine-131-labeled anti-CD20 antibody and autologous stem-cell rescue. J Clin Oncol. 1998;16:3270. doi: 10.1200/JCO.1998.16.10.3270. [DOI] [PubMed] [Google Scholar]
- 42. Press OW. Eary JF. Gooley T, et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934. [PubMed] [Google Scholar]
- 43. Gopal AK. Rajendran JG. Petersdorf SH. High-dose chemoradioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99:3158. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 44. Nademanee A. Molina A. Dagis A, et al. Autologous stem-cell transplantation for poor-risk and relapsed intermediate- and high-grade non-Hodgkin's lymphoma. Clin Lymphoma. 2000;1:46. doi: 10.3816/clm.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 45. Gopal AK. Gooley TA. Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin's lymphoma: A multivariable cohort analysis. Blood. 2003;102:2351. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 46. Press OW. Unger JM. Braziel RM, et al. A phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131-tositumomab for previously untreated follicular non-Hodgkin's lymphoma: Five-year follow-up of Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 47. Meredith RF. Khazaeli MB. Liu T, et al. Dose fractionation of radiolabeled antibodies in patients with metastatic colon cancer. J Nucl Med. 1992;33:1648. [PubMed] [Google Scholar]
- 48. Schlom J. Molinolo A. Simpson JF, et al. Advantage of dose fractionation in monoclonal antibody-targeted radioimmunotherapy. J Nat Cancer Inst. 1990;82:763. doi: 10.1093/jnci/82.9.763. [DOI] [PubMed] [Google Scholar]
- 49. DeNardo GL. Schlom J. Buchsbaum DJ, et al. Rationales, evidence, and design considerations in fractionated radioimmunotherapy. Cancer. 2002;94:1332. doi: 10.1002/cncr.10304. [DOI] [PubMed] [Google Scholar]
- 50. DeNardo SJ. Kramer EL. O'Donnell RT, et al. Radioimmunotherapy for breast cancer using indium-111/yttrium-90 BrE-3: Results of a phase I clinical trial. J Nucl Med. 1997;38:1180. [PubMed] [Google Scholar]
- 51. DeNardo GL. DeNardo SJ. Lamborn KR, et al. Low-dose fractionated radioimmunotherapy for B-cell malignancies using 131I-Lym-1 antibody. Cancer Biother Radiopharm. 1998;13:239. doi: 10.1089/cbr.1998.13.239. [DOI] [PubMed] [Google Scholar]
- 52. DeNardo GL. DeNardo SJ. Goldstein DS, et al. Maximum tolerated dose, toxicity, and efficacy of 131I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin's lymphoma. J Clin Oncol. 1998;16:3246. doi: 10.1200/JCO.1998.16.10.3246. [DOI] [PubMed] [Google Scholar]
- 53. Boerman OC. van Schaijk FG. Oyen WJG, et al. Pretargeted radioimmunotherapy of cancer: A progress step by step. J Nucl Med. 2003;44:400. [PubMed] [Google Scholar]
- 54. Cremonesi M. Ferrari M. Chinol M, et al. Three-step radioimmunotherapy with yttrium-90 biotin: Dosimetry and pharmacokinetics in cancer patients. Eur J Nucl Med. 1999;26:110. doi: 10.1007/s002590050366. [DOI] [PubMed] [Google Scholar]
- 55. Goldenberg DM. Chatal JF. Barbet J, et al. Cancer imaging and therapy with bispecific antibody pretargeting. Update Cancer Ther. 2007;2:19. doi: 10.1016/j.uct.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pagel JM. Hedin N. Subbiah K, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 57. Pagel JM. Lin Y. Hedin N, et al. Comparison of a tetravalent single-chain antibody-streptavidin fusion protein and an antibody-streptavidin chemical conjugate for pretargeted anti-CD20 radioimmunotherapy of B-cell lymphomas. Blood. 2006;108:328. doi: 10.1182/blood-2005-11-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Press OW. Corcoran M. Subbiah K, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 59. Weiden PL. Breitz HB. Press OW, et al. Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin's lymphoma (NHL): Initial phase I/II study results. Cancer Biother Radiopharm. 2000;15:15. doi: 10.1089/cbr.2000.15.15. [DOI] [PubMed] [Google Scholar]
- 60. Pagel JM. Hedin N. Drouet L, et al. Eradication of disseminated leukemia in a syngeneic murine leukemia model using pretargeted anti-CD45 radioimmunotherapy. Blood. 2008;111:2261. doi: 10.1182/blood-2007-06-097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sharkey RM. Karacay H. Cardillo TM, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 62. Forero A. Weiden PL. Vose JM, et al. A phase I trial of a novel anti-CD20 fusion protein in pretargeting radioimmunotherapy for B-cell non-Hodgkin's lymphoma. Blood. 2004;104:227. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 63. Axworthy DB. Reno JM. Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Nat Acad Sci US Am. 2000;97:1802. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldenberg DM. Rossi EA. Sharkey RM, et al. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 65. Rossi EA. Goldenberg DM. Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. PNAS. 2006;103:6841. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goldenberg DM. Sharkey RM. Paganelli G, et al. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 67. Wu AM. Engineering multivalent antibody fragments for in vivo targeting. Meth Mol Biol. 2004;248:209. doi: 10.1385/1-59259-666-5:209. [DOI] [PubMed] [Google Scholar]
- 68. Krenning EP. Teunissen JJ. Valkema R. Molecular radiotherapy with somatostatin analogs for (neuro) endocrine tumors. J Endocrinal Invest. 2005;28:146. [PubMed] [Google Scholar]
- 69. Breeman WA. de Jong M. de Blois E. Radiolabeling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005;32:478. doi: 10.1007/s00259-004-1702-y. [DOI] [PubMed] [Google Scholar]
- 70. Reubi JC. Macke HR. Krenning EP. Candidates for peptide receptor radiotherapy today and in the future. J Nucl Med. 2005;46:67S. [PubMed] [Google Scholar]
- 71. Valkema R. Pauwels S. Kvols LK. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanded gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 72. de Jong M. Breeman WA. Valkema R, et al. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med. 2005;46:13S. [PubMed] [Google Scholar]
- 73. Anderson CJ. Dehdashti F. Cutler PD, et al. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213. [PubMed] [Google Scholar]
- 74. Van Den Bossche B. Van de Wiele C. Receptor imaging in oncology by means of nuclear medicine: Current status. J Clin Oncol. 2004;22:3593. doi: 10.1200/JCO.2004.10.216. [DOI] [PubMed] [Google Scholar]
- 75. Missailidis S. Perkins A. Aptamers as novel radiopharmaceuticals: Their applications and future prospects in diagnosis and therapy. Cancer Biother Radiopharm. 2007;22:453. doi: 10.1089/cbr.2007.357. [DOI] [PubMed] [Google Scholar]
- 76. Orlova A. Feldwisch J. Abrahmsen L, et al. Affibody molecules for molecular imaging and therapy for cancer. Cancer Biother Radiopharm. 2007;22:573. doi: 10.1089/cbr.2006.004-U. [DOI] [PubMed] [Google Scholar]
- 77. Balhorn R. Hok S. Burke P, et al. Selective high-affinity ligand antibody mimics for cancer diagnosis and therapy: Initial application to lymphoma/leukemia. Clin Cancer Res. 2007;13:5621s. doi: 10.1158/1078-0432.CCR-07-1128. [DOI] [PubMed] [Google Scholar]
- 78. West J. Perkins J. Hok S, et al. Direct antilymphoma activity of novel, first-generation “antibody mimics” that bind HLA-DR10-positive non-Hodgkin's lymphoma cells. Cancer Biother Radiopharm. 2006;21:645. doi: 10.1089/cbr.2006.21.645. [DOI] [PubMed] [Google Scholar]
- 79. DeNardo GL. Hok S. Natarajan A, et al. Characteristics of dimeric (bis) bidentate selective high-affinity ligands as HLA-DR10 beta antibody mimics targeting non-Hodgkin's lymphoma. Int J Oncol. 2007;31:729. [PubMed] [Google Scholar]
- 80. Hok S. Natarajan A. Balhorn R, et al. Synthesis and radiolabeling of selective high-affinity ligands designed to target non-Hodgkin's lymphoma and leukemia. Bioconj Chem. 2007;18:912. doi: 10.1021/bc060305o. [DOI] [PubMed] [Google Scholar]
- 81. DeNardo GL. Natarajan A. Hok S, et al. Novel nanomolecular HLA-DR10 antibody mimics provide a potent system for molecular therapy and imaging. J Nucl Med. 2008;49:319P. doi: 10.1089/cbr.2008.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Costantini DL. Chan C. Cai Z, et al. 111In-labeled trastuzumab (herceptin) modified with nuclear localization sequences (NLS): An Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]