Abstract
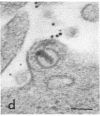
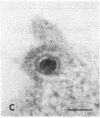
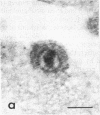
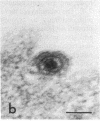
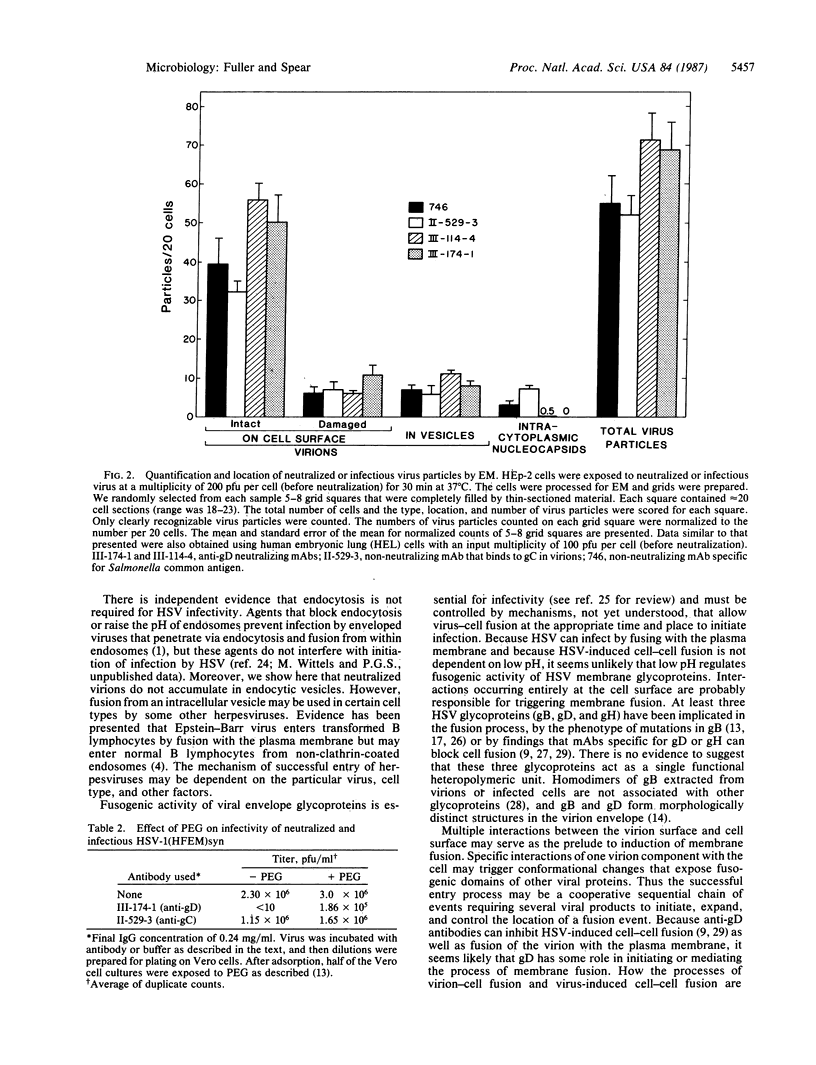
Certain monoclonal antibodies specific for glycoprotein D of herpes simplex virus have potent neutralizing activity but fail to block attachment of virus to cells. Here we have investigated the fate of neutralized and infectious virus after attachment to primate cells. Infectious virions fused with the cell surface such that naked nucleocapsids were detectable in the cytoplasm near or just under the plasma membrane. Neutralized virions did not fuse with the cell. They remained attached to the cell surface and could be rendered infectious by treatment with polyethylene glycol. We conclude that some anti-glycoprotein D neutralizing antibodies can inhibit the penetration of herpes simplex virus by blocking fusion of the virion envelope with the plasma membrane. These results identify a pathway of entry that initiates successful herpes simplex virus infection and a step in this pathway that is highly sensitive to neutralizing antibodies. A role for glycoprotein D in virion-cell fusion is indicated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Spear P. G. Oligomerization of herpes simplex virus glycoprotein B. J Virol. 1986 Nov;60(2):803–806. doi: 10.1128/jvi.60.2.803-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D., Person S., Snipes W. Early events in herpes simplex virus type 1 infection: photosensitivity of fluorescein isothiocyanate-treated virions. Proc Natl Acad Sci U S A. 1981 Feb;78(2):912–916. doi: 10.1073/pnas.78.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., HUMMELER K., BERKALOFF A. THE ENTRY AND DISTRIBUTION OF HERPES VIRUS AND COLLOIDAL GOLD IN HELA CELLS AFTER CONTACT IN SUSPENSION. J Exp Med. 1964 Feb 1;119:291–302. doi: 10.1084/jem.119.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature. 1986 May 15;321(6067):244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Lippe P. A., Pauza K. J., Spear P. G., Pereira L., Tevethia S. S. Kinetics of expression of herpes simplex virus type 1-specific glycoprotein species on the surfaces of infected murine, simian, and human cells: flow cytometric analysis. J Virol. 1987 Jan;61(1):104–112. doi: 10.1128/jvi.61.1.104-112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A. H., Uchida T. Inhibition of multiplication of herpes simplex virus type 1 by ammonium chloride and chloroquine. Virology. 1984 Oct 30;138(2):332–335. doi: 10.1016/0042-6822(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984 Jan 15;132(1):186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Honda E., Minetoma T., Kumagai T. Mechanisms of neutralization by monoclonal antibodies to different antigenic sites on the bovine herpesvirus type 1 glycoproteins. Virology. 1986 Apr 15;150(1):260–264. doi: 10.1016/0042-6822(86)90285-0. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., de Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. II. An ultrastructural study of viral penetration. J Virol. 1974 Oct;14(4):945–956. doi: 10.1128/jvi.14.4.945-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard L. M., Fuller A. O., Spear P. G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987 Mar;68(Pt 3):715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]