Abstract
Background
Surveillance cultures may be helpful in identifying patients at increased risk of developing invasive candidiasis. However, only scant information exists on the effect of Candida colonization on serum levels of diagnostic biomarkers. This prospective surveillance study determined the extent of Candida colonization among pediatric cancer patients and its possible impact on serum levels of (1-3)-β-D-glucan (BDG), Candida mannan and Candida DNA.
Methods
A total of 1075 swabs originating from oropharynx (n = 294), nostrils (n = 600), rectum (n = 28), groin (n = 50), ear (n = 54), and axilla (n = 49) of 63 pediatric cancer patients were cultured for the isolation of Candida spp. Patients yielding Candida spp. from any sites were considered as colonized. Serum samples were collected from patients at the time of first surveillance culture for detection of BDG by Fungitell kit and Candida mannan by Platelia Candida Ag. Candida DNA was detected by using panfungal primers and identification was carried out by using species-specific primers and DNA sequencing.
Results
Seventy-five (7.6%) swab cultures from 35 (55.5%) patients yielded Candida spp. These isolates included C. albicans (n = 62), C. dubliniensis (n = 8), C. glabrata and C. tropicalis (n = 2 each) and C. krusei (n = 1). Eleven patients were colonized at three or more sites. Eight of 36 serum samples from 6 colonized patients yielded BDG values higher than the currently recommended cut-off value of ≥80 pg/ml. However, none of the serum samples yielded Candida mannan levels ≥0.5 ng/ml and PCR test for Candida DNA was also negative in all the serum samples of colonized patients. During the study period, only two colonized patients subsequently developed candidemia due to C. tropicalis. Besides positive blood cultures, C. tropicalis DNA, BDG and Candida mannan were also detected in serum samples of both the patients.
Conclusions
The present study demonstrates that while mucosal colonization with Candida species in pediatric cancer patients is common, it does not give rise to diagnostically significant levels of Candida mannan or Candida DNA in serum specimens. However, BDG values may be higher than the cut-off value in some pediatric patients without clinical evidence of invasive Candida infection. The study suggests the utility of Candida mannan or Candida DNA in the diagnosis of invasive candidiasis, however, the BDG levels in pediatric cancer subjects should be interpreted with caution.
Background
The incidence of fungal infections among cancer patients has shown a steady increase in recent years [1-3]. This may partly be attributed to the use of more aggressive chemotherapeutic regimens, resulting in more prolonged survival of these immunosuppressed patients while they continue to remain vulnerable to invasive fungal infections [4,5]. Although establishing an early diagnosis for invasive mycoses is ideal for timely administration of specific antifungal therapy, it invariably gets delayed due to want of culture or histopathologic evidence [5]. Strategies are now being evolved to identify a subgroup of high-risk patients where prophylactic or empirical therapeutic approach could be used for preventing development of invasive fungal infections [6]. Recently, Maertens et al. [7] proposed a preemptive approach based on radiologic and other surrogate markers for the early diagnosis of invasive mycoses in high-risk patients. Surveillance cultures for determining the Candida colonization index in high-risk patients may be helpful in identifying patients at increased risk of invasion and hematogenous dissemination [8-11]. However, only scant information is available on the effect of Candida colonization on the serum levels of BDG, Candida mannan or Candida DNA [12-14]. In the present communication, we report results of Candida colonization among hospitalized pediatric cancer patients and its possible impact on serum levels of BDG, Candida mannan, and Candida DNA.
Methods
Study population
The study was carried out in a tertiary care Pediatric Cancer Ward, Al-Sabah Hospital, Kuwait between July 1, 2007 to December 31, 2008. Sixty-three cancer patients, 57 (90%) with acute lymphoblastic leukemia (ALL) and 6 (10%) with acute myeloid leukemia (AML) were followed-up by weekly surveillance cultures for varying periods for assessing the extent of Candida colonization. Forty-five patients were males. Their age ranged from 1 to 16 years. A child was considered as colonized if Candida sp. was isolated from one or more anatomic sites. A patient yielding Candida sp. on repeat cultures at least from one site was considered as persistently colonized [15]. The study was approved by the Ethics Committees of the Faculty of Medicine, Kuwait University and Ministry of Health, Kuwait. Informed consent of the patients was obtained before collecting the clinical samples.
Isolation and identification
A total of 1075 swabs originating from oropharynx (n = 294), nasal (n = 600), rectum (n = 28), groin (n = 50), ear (n = 54), and axilla (n = 49) of 63 pediatric cancer patients were cultured on Sabouraud dextrose agar supplemented with chloramphenicol (Table 1). The germ tube test was performed on all the Candida spp. isolates for the presumptive identification of C. albicans or C. dubliniensis. Subsequently, Candida isolates were also identified by Vitek2 yeast identification system (BioMerieux, France). The identification of Candida spp. isolates was also confirmed by species-specific amplification and/or sequencing of internally transcribed spacer (ITS) region of rDNA.
Table 1.
Surveillance cultures for yeast species in pediatric cancer patients
| Sample site | No. positive/No. samples (%) |
|---|---|
| Oropharyngeal |
53/294 (18) |
| Nasal |
6/600 (1) |
| Rectal |
10/28 (35.7) |
| Groin |
5/50 (10) |
| Ear |
1/54 (1.9) |
| Axilla |
0/49 (0) |
| Total | 75/1075 (7) |
Collection of serum samples
Five ml of blood was collected in sterile BDG-free clotting tubes and serum was separated for the detection of (1-3)-β-D-glucan (BDG), Candida mannan and species-specific Candida DNA at the time of surveillance culture. The serum was kept frozen at -20°C until used. Thirty-six serum samples from 20 colonized patients and 11 serum samples from nine non-colonized patients were tested.
(1-3)-β-D-glucan detection in serum
The BDG levels in serum samples were determined using a Fungitell kit (Associates of Cape Cod Inc., East Falmouth, MA, USA) according to the procedure described by the manufacturer. BDG levels were assayed against a purified Pachyman standard, which included a five-point two-fold curve ranging from 31 pg/ml to 500 pg/ml. In brief, 5 μl of serum was dispensed per well in duplicate and pretreated with 20 μl of 0.25 M KOH and 1.2 M KCl for 10 min at 37°C. This step inactivated protease and other inhibitors present in human serum. The Fungitell BG reagent was then reconstituted and dispensed according to the instructions supplied by the manufacturer of the Fungitell kit. A Microplate Spectrphotometer (Bio-Tek Instruments, Inc., Winooski, VT, USA) with Gen5™ software onboard was used to accomplish kinetic analysis of the microtiter plate. The BDG value of ≥80 pg/ml was considered as positive and a value between 60-79 pg/ml as intermediate.
Candida mannan detection in serum
Mannan antigen was measured by Platelia Candida Ag (BioRad, Marnes La Coquette, France). The test was performed according to the instructions of the manufacturer. Briefly, each test serum (300 μl) was mixed with 100 μl of treatment solution and placed in a boiling water bath for 3 minutes. After centrifugation, the supernatant was used for further testing. Fifty-μl of the conjugate and an equal amount of the treated serum supernatant was introduced into micro-titer plate wells pre-coated with anti-mannan monoclonal antibody. After incubation at 37°C for 90 min and 5 washing steps, 200 μl of the substrate buffer was added to each well, and the plates were incubated for 30 min at room temperature. The enzymatic reaction was terminated by adding stopping solution and the optical density was read at 450 nm using a Microplate Spectrphotometer (Bio-Tek Instruments, Inc.). The reactions were performed in duplicates and each experiment included positive and negative controls as well as a calibration curve. The calibration curve was made with a pool of normal human serum supplemented with known concentrations of mannan ranging from 0.1 to 2 ng/ml. A value of ≥0.5 ng/ml was taken as positive and ≥0.25 ng/ml but ≤0.5 ng/ml as doubtful.
Candida DNA detection by PCR
DNA from cultured Candida spp. was isolated as described in detail previously [16,17]. DNA from serum was extracted using the QIAamp DNA kit (QIAGEN, Hilden, Germany) by following the instructions supplied by the manufacturer. DNA sequences of pan-fungal and species-specific forward and reverse primers and DNA amplification protocol were same as described previously [16,18]. Cultures of C. albicans ATCC 90029, C. parapsilosis ATCC 10233, C. tropicalis ATCC 750, C. glabrata ATCC 90030 and C. dubliniensis CBS 7987/CD36 were used as reference for amplification of specific products. The amplified DNA fragments were detected by agarose gel electrophoresis using 2% agarose gels as described previously [19].
The DNA isolated from selected isolates was also subjected to direct DNA sequencing of ITS region of rDNA (containing the ITS-1, 5.8S rRNA and ITS-2) to confirm species-specific identification by PCR. The ITS region was amplified by using ITS1 and ITS4 primers and both strands of amplified DNA were sequenced as described previously [20,21]. The sequencing primers, in addition to the amplification primers, included ITS1FS, ITS2, ITS3 and ITS4RS and the sequences were assembled as described previously [22]. GenBank basic local alignment search tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi?) were performed for species identification
Statistical analysis
Mann-Whitney 2-tailed test was applied to determine the significance of differences that existed between BDG or Candida mannan levels between different patient groups who were colonized with Candida spp. on single occasion or persistently and non-colonized subjects. The Spearman correlation test was performed to determine the correlation between BDG and Candida mannan. A P value of <0.05 was considered as significant.
Results
Surveillance cultures
The results of the surveillance cultures for Candida spp. are presented in Tables 1-2. Of 1075 swabs cultured from different anatomic sites of 63 pediatric cancer patients, 75 (7.8%) were positive for Candida spp. These isolates included C. albicans (n = 62), C. dubliniensis (n = 8), C. glabrata and C. tropicalis (n = 2 each) and C. krusei (n = 1) (Tables 1-2). Apart from phenotypic identification, all Candida spp. isolates were also identified by species-specific amplification of ITS region of rDNA (data not shown). The identity of six selected isolates was further confirmed by direct DNA sequencing of ITS region of rDNA (EMBL Accession Nos. FN652297, FN652298, FN652301 to FN652304). The distribution of anatomic sites yielding Candida spp. in culture was as follows: rectum, 36%; oropharynx, 18%; groin, 10%; ear, 2% and nasal, 1% (Table 1). Seventeen patients were colonized at one site and 18 at two or more sites. None of the swabs taken from axilla were positive for Candida spp. Of the 8 C. dubliniensis isolates, 5 came from oropharynx, and one each from nose, groin and rectum of six patients. Two patients yielded C. glabrata (nose and rectum) and two others yielded C. tropicalis (oropharynx and rectal). A single isolate of C. krusei was recovered from the oropharynx (Table 2).
Table 2.
Species spectrum of Candida species isolated from different anatomic sites of pediatric cancer patients
| Site | C. albicans | C. dubliniensis | C. glabrata | C. tropicalis | C. krusei |
|---|---|---|---|---|---|
| Oropharyngeal |
46 |
5 |
0 |
1 |
1 |
| Nasal |
3 |
1 |
1 |
0 |
0 |
| Rectal |
8 |
1 |
1 |
1 |
0 |
| Groin |
4 |
1 |
0 |
0 |
0 |
| Ear |
1 |
0 |
0 |
0 |
0 |
| Total | 62 | 8 | 2 | 2 | 1 |
In colonized patients, the mean Candida mannan value was 0.16 ± 0.044 ng/ml, which was not significantly different from those that were not colonized (p = 0.660) (Table 3). There was also no significant difference in the mean Candida mannan values between patients colonized at one anatomic site or two or more sites (p = 0.665) or between those that were colonized once or those yielding Candida spp. repeatedly (p = 0.474) (Table 3). Although mean BDG values in colonized patients were nearly same as non-colonized patients (46.98 pg/ml vs. 36.77 pg/ml), 8 serum samples from 6 colonized patients were positive for BDG (range 82 pg/ml to 141 pg/ml, mean = 98.3 pg/ml). Three of these serum samples were obtained from the same patient within a span of 40 days and the BDG levels varied between 85 pg/ml to 115 pg/ml. However, none of these 8 serum samples yielded mannan levels >0.25 ng/ml. Additionally, 5 serum samples from two colonized patients yielded BDG values in the intermediate range (Table 3). There was no significant difference in the mean BDG values between patients that were colonized once or those that yielded Candida spp. persistently (p = 1.0) (Table 3). The mean and standard deviation of BDG and Candida mannan levels in serum samples collected from the patients at the time of surveillance samples were 42.74 ± 30.18 pg/ml and 0.173 ± 0.03 ng/ml, respectively. No correlation was observed between BDG and Candida mannan values among patients that were colonized with Candida species by Spearman correlation test (p = 0.531, R = 0.108). None of the serum sample from the colonized patients was positive for the detection of Candida DNA by PCR.
Table 3.
(1-3)-β-D-glucan and Candida mannan levels in serum samples of different groups of patients
| Colonization Status | No. of patients | No. of samples | BDG (pg/μl) | GM ± SD | Mannan (ng/ml) | GM ± SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
≥80 |
<80 - ≥60 |
<60 |
≥0.5 |
<0.5 - ≥0.25 |
<0.25 |
|||||
|
Colonized |
20 |
36 |
8 |
5 |
23 |
46.98 ± 30.22 |
- |
2 |
34 |
0.166 ± 0.044 |
|
Persistently colonized |
10 |
20 |
3 |
5 |
12 |
46.60 ± 29.30 |
- |
1 |
19 |
0.169 ± 0.046 |
|
One-time colonized |
10 |
16 |
5 |
- |
11 |
47.45 ± 32.30 |
- |
1 |
15 |
0.164 ± 0.044 |
| Non-colonized | 9 | 11 | - | - | 11 | 36.77 ± 9.96 | - | 1 | 10 | 0.161 ± 0.06 |
GM, Geometric mean; SD, Standard deviation
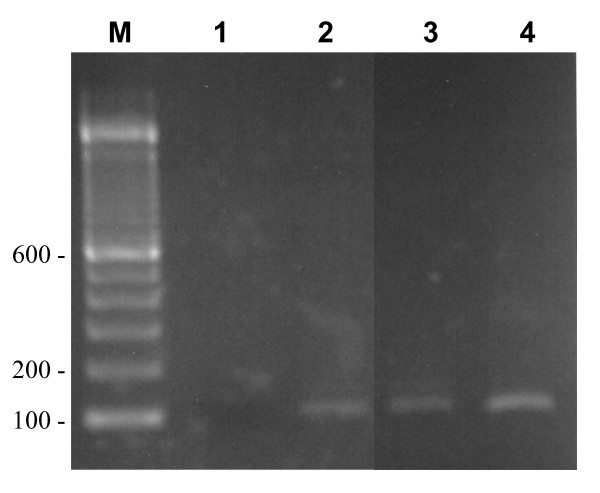
Two patients whose oropharyngeal or rectal samples yielded C. tropicalis (in addition to C. dubliniensis in one and C. albicans in the other) developed candidemia due to C. tropicalis. Besides blood culture positivity, both these patients showed presence of C. tropicalis DNA in serum by species-specific PCR amplification (Fig. 1) and also showed elevated levels of BDG (238.6 and 406 pg/ml), whereas Candida mannan was positive in one (0.77 ng/ml) and border line (0.28 ng/ml) in the other.
Figure 1.
Agarose gel showing amplification of a DNA fragment of ~106 bp by seminested PCR with DNA isolated from serum from patient 1 (lane 2) and patient 2 (lane 3). An amplicon of the same size was also obtained with genomic DNA isolated from reference strain of C. tropicalis (lane 4) while no amplicon was obtained in the reagent control tube in which water instead of DNA was added (lane 1). Lane M is 100 bp DNA marker and the positions of migration of 100 bp, 200 bp and 600 bp fragments are marked.
Discussion
Infections caused by Candida spp. are the major cause of morbidity and mortality among seriously ill patients. Prior colonization with Candida spp. has been regarded as an essential step for the development of invasive disease [10,23]. The colonization index could be helpful in predicting risk of developing systemic infection in critically ill patients, and thus, may offer opportunities for early therapeutic or prophylactic interventions [6,10]. In the present study, although 11 of 35 (31%) patients were colonized with Candida spp. At ≥3 sites, none of them, despite being leukemic, developed candidemia or invasive candidiasis. Two patients with ALL who subsequently developed candidemia due to C. tropicalis were colonized at two sites with two different Candida spp. (C. albicans with C. tropicalis or C. dubliniensis with C. tropicalis) and their serum samples also yielded positive results for C. tropicalis DNA, mannan and BDG. Recently, Leon et al. [9] conducted a prospective observational study in a cohort of non-neutropenic patients to assess the value of "Candida score" for the probability of developing invasive candidiasis. Since invasive candidiasis occurred only in <5% of patients who had Candida colonization score of <3, the likelihood of developing invasive candidiasis in such patients was considered very low.
Since early diagnosis of invasive candidiasis is challenging, the role of surrogate markers, such as Candida species-specific DNA, mannan, and BDG in predicting the onset of invasive candidiasis has attracted considerable attention [13,24]. None of the colonized patients in the present study were found positive for Candida mannan or Candida DNA. However, BDG levels were positive in eight serum samples from six patients with values ranging from 82 pg/ml to 141 pg/ml. These observations are generally in agreement with previous studies showing that patients colonized at single or multiple sites yield BDG levels below the cut-off value recommended by the manufacturer [12,25-27]. The BDG levels above the cut-off values (80 pg/ml) in 6 of 20 (30%) colonized pediatric cancer patients in our study may either result from absorption of BDG through the gut due to mucositis [24] or due to contamination with cellulose [28], gauze [29], bacterial sepsis [30,31] or intravenous therapy with amoxicillin-calvulanic acid in these subjects [32]. Despite the above limitations of the test, several studies have used BDG monitoring to identify patients at risk of developing invasive candidiasis to improve therapeutic outcome [25,33,34]. A wide range of sensitivities and specificities have been obtained in different study populations [25,34,35], probably due to use of different cut-off values for a positive BDG test or due to use of different brand of kits that may react differently to BDG present in the clinical samples [36,37]. Unlike BDG, Candida mannan levels in serum seem to be less susceptible to the extent of Candida colonization. As stated above, none of our colonized patient was found to have positive serum levels (>0.5 ng/ml) for Candida mannan (mean 0.16 ± 0.04 ng/ml). In two patients who had Candida mannan in the intermediate range (0.308 and 0.287 ng/ml), sera were negative for BDG as well as Candida DNA. These findings are in conformity with the results of previous studies [12,14].
The normal BDG values in healthy pediatric population are not yet established. In a preliminary study, Brian Smith et al. [38] estimated BDG levels in serum samples from 120 non-immunocompromised children (0.5 to 18 year-old) and found higher levels (mean 68 ± 128 pg/ml) than those reported earlier in adult population [25,35]. A noteworthy observation of this study was that 18 of 120 (15%) children had BDG levels >80 pg/ml and 8 of 120 (7%) had BDG levels between 60-79 pg/ml [38]. Thus, higher BDG levels in a minority (6 of 20, 30%) of pediatric cancer patients in our study is consistent with the BDG data reported by Brian Smith et al. [38]. These observations warrant further studies in pediatric population for establishing BDG cut-off values for a positive test to validate the diagnostic utility of this marker for invasive fungal infections.
Conclusions
This prospective surveillance study revealed that while nearly half (55%) of the pediatric cancer patients were colonized with Candida spp. at one or more anatomic sites, the serum levels for Candida mannan remained significantly less than the cut-off value recommended by the manufacturer for a positive test. Also, Candida DNA was not detected in the serum samples of the colonized patients by the semi-nested PCR used. However, BDG levels were elevated in a minority of colonized pediatric patients suggesting that additional evidence of infection may be needed for the diagnosis of invasive candidiasis in this patient population.
Abbreviations
BDG: (1-3)-β-D-glucan; PCR: Polymerase Chain Reaction; DNA: Deoxyribonucleic Acid; ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloblastic leukemia; KOH: Potassium Hydroxide; KCl: Potassium Chloride; ITS: Internally Transcribed Spacer; CBS: Centraalbureau voor Schimmeculture
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EM, MHB, and ZUK: Conceived and supervised the study. SA supervised the molecular part of the study. All the authors contributed to the writing and finalizing the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Eiman Mokaddas, Email: e.mokaddas@hsc.edu.kw.
Mona HA Burhamah, Email: bufahad86@hotmail.com.
Zia U Khan, Email: zkhan@hsc.edu.kw.
Suhail Ahmad, Email: suhail_ah@hsc.edu.kw.
Acknowledgements
Excellent technical support from Ajmal Theyyathel and Josephin Jhonson is thankfully acknowledged. The study was supported by KFAS grant No. 2005-130-205.
References
- Kobayashi R, Kaneda M, Sato T, Ichikawa M, Suzuki D, Ariga T. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol. 2008;30:886–890. doi: 10.1097/MPH.0b013e3181864a80. [DOI] [PubMed] [Google Scholar]
- Lockhart SR, Diekemas DJ, Pfaller MA. In: Clinical Mycology. 2. Anaissie EJ, McGinnis MR, Pfaller MA, editor. Churchill-Livingstone, Elsevier; 2009. The epidemiology of fungal infections; pp. 1–14. full_text. [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TA, Hajjeh RA. Recent trends in the epidemiology of invasive mycoses. Curr Opin Infect Dis. 2002;15:569–574. doi: 10.1097/00001432-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Michallet M, Ito JI. Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J Clin Oncol. 2009;27:3398–3409. doi: 10.1200/JCO.2008.20.1178. [DOI] [PubMed] [Google Scholar]
- Charles PE. Multifocal Candida species colonization as a trigger for early antifungal therapy in critically ill patients: what about other risk factors for fungal infection? Crit Care Med. 2006;34:913–914. doi: 10.1097/01.CCM.0000202435.98240.ED. [DOI] [PubMed] [Google Scholar]
- Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, Wilmer A, Verhaegen J, Boogaerts M, Van Eldere J. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–1250. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]
- Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/S1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, Balasini C, Utande-Vázquez A, González de Molina FJ, Blasco-Navalproto MA, López MJ, Charles PE, Martín E, Hernández-Viera MA. Cava Study Group. Usefulness of the "Candida score" for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–1633. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751–758. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford GR, Merz WG, Wingard JR, Charache P, Saral R. The value of fungal surveillance cultures as predictors of systemic fungal infections. J Infect Dis. 1980;142:503–509. doi: 10.1093/infdis/142.4.503. [DOI] [PubMed] [Google Scholar]
- Alam FF, Mustafa AS, Khan ZU. Comparative evaluation of (1, 3)-β-D-glucan, mannan and anti-mannan antibodies, and Candida species-specific snPCR in patients with candidemia. BMC Infect Dis. 2007;7:103–112. doi: 10.1186/1471-2334-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M, Al-Ramadi B, Bernsen R, Kristensen J, Alizadeh H, Hedstrom U. Prospective evaluation of mannan and anti-mannan antibodies for diagnosis of invasive Candida infections in patients with neutropenic fever. J Med Microbiol. 2009;58:606–615. doi: 10.1099/jmm.0.006452-0. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Ishikawa N, Suzuki H. Diagnosis of invasive candidiasis by detection of mannan antigen by using the avidin-biotin enzyme immunoassay. J Clin Microbiol. 1991;29:2363–2367. doi: 10.1128/jcm.29.11.2363-2367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderwick SA, Lyons MJ, Liu M, Vazquez JA, Kauffman CA. Epidemiology of yeast colonization in the intensive care unit. Eur J Clin Microbiol Infect Dis. 2000;19:663–670. doi: 10.1007/s100960000348. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Khan Z, Mustafa AS, Khan ZU. Seminested PCR in the diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for identification. J Clin Microbiol. 2002;40:2483–2489. doi: 10.1128/JCM.40.7.2483-2489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Mustafa AS, Khan Z, Rifaiy A, Khan ZU. PCR-enzyme immunoassay for rDNA in the diagnosis of candidemia and comparison with amplicon detection by agarose gel electrophoresis. Int J Med Microbiol. 2004;294:45–51. doi: 10.1016/j.ijmm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Khan Z, Mokaddas E, Khan ZU. Isolation and molecular identification of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Kuwait. J Med Microbiol. 2004;53:633–637. doi: 10.1099/jmm.0.05315-0. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mustafa AS. Detection of Candida species by polymerase chain reaction (PCR) in blood samples of experimentally infected mice and patients with suspected candidemia. Microbiol Res. 2001;156:95–102. doi: 10.1078/0944-5013-00072. [DOI] [PubMed] [Google Scholar]
- Al-Sweih N, Ahmad S, Khan ZU, Khan S, Chandy R. Prevalence of Candida dubliniensis among germ tube-positive Candida isolates in a maternity hospital in Kuwait. Mycoses. 2005;48:347–351. doi: 10.1111/j.1439-0507.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Al-Mahmeed M, Khan ZU. Characterization of Trichosporon species isolated from clinical specimens in Kuwait. J Med Microbiol. 2005;54:639–646. doi: 10.1099/jmm.0.45972-0. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Ahmad S, Mokaddas E, Chandy R, Cano J, Guarro J. Actinomucor elegans var. kuwaitiensis isolated from the wound of a diabetic patient. Antonie van Leeuwenhoek. 2008;94:343–352. doi: 10.1007/s10482-008-9251-1. [DOI] [PubMed] [Google Scholar]
- Lai YY, Bao L, Lu XJ, Lu J, Yu J, Li RY, Huang XJ. Candida colonization and invasive fungal infection in hospitalized patients with hematological malignancies. Zhonghua Yi Xue Za Zhi. 2009;89:239–242. [PubMed] [Google Scholar]
- Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L. Assessment of the clinical utility of serial β-D-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol. 2008;57:287–295. doi: 10.1099/jmm.0.47479-0. [DOI] [PubMed] [Google Scholar]
- Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. β-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, Yamaguchi H, Shimada K, Kawai Tl. Plasma (13)-β-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/S0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- Pazos C, Pontón J, Del Palacio A. Contribution of (1->3)-β-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol. 2005;43:299–305. doi: 10.1128/JCM.43.1.299-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Takita T, Furuhashi M, Takahashi T, Maruyama Y, Hishida A. Elevation of blood (13)- β-D-glucan concentrations in hemodialysis patients. Nephron. 2001;89:15–19. doi: 10.1159/000046037. [DOI] [PubMed] [Google Scholar]
- Nakao A, Yasui M, Kawagoe T, Tamura H, Tanaka S, Takagi H. False-positive endotoxemia derives from gauze glucan after hepatectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology. 1997;44:1413–1418. [PubMed] [Google Scholar]
- Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol. 2003;10:882–885. doi: 10.1128/CDLI.10.5.882-885.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a (1->3)- β-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennink-Kersten MA, Warris A, Verweij PE. 1,3-β-D-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med. 2006;354:2834–2835. doi: 10.1056/NEJMc053340. [DOI] [PubMed] [Google Scholar]
- Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M. Preemptive treatment of fungal infection based on plasma (1-3)- β-D-glucan levels after liver transplantation. Infection. 2007;35:346–351. doi: 10.1007/s15010-007-6240-7. [DOI] [PubMed] [Google Scholar]
- Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, Matsuura S, Duvoisin B, Bille J, Calandra T, Marchetti O. 1,3-β-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis. 2008;46:878–885. doi: 10.1086/527382. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH. Multicenter clinical evaluation of the (13)-β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- Marty FM, Koo S. Role of (13)-β-D-glucan in the diagnosis of invasive aspergillosis. Med Mycol. 2009;47(Suppl 1):233–240. doi: 10.1080/13693780802308454. [DOI] [PubMed] [Google Scholar]
- Wheat LJ. Approach to the diagnosis of invasive aspergillosis and candidiasis. Clin Chest Med. 2009;30:367–377. doi: 10.1016/j.ccm.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Smith PB, Benjamin DK Jr, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ. Quantification of 1,3-β-D-glucan levels in children: preliminary data for diagnostic use of the beta-glucan assay in a pediatric setting. Clin Vaccine Immunol. 2007;14:924–925. doi: 10.1128/CVI.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]