Abstract
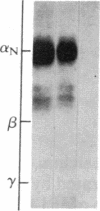
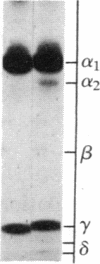
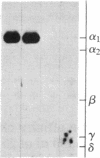
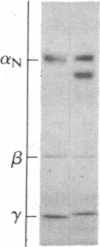
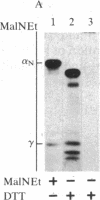
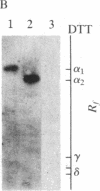
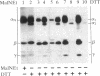
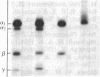
Purified dihydropyridine-sensitive calcium channels from rabbit transverse-tubule membranes consist of three noncovalently associated classes of subunits: alpha (167 kDa), beta (54 kDa), and gamma (30 kDa). Cleavage of disulfide bonds reveals two distinct alpha polypeptides and an additional component, delta. The alpha 1 subunit, a 175-kDa polypeptide that is not N-glycosylated, contains the dihydropyridine binding site, cAMP-dependent protein kinase phosphorylation site(s), and substantial hydrophobic domain(s). alpha 2, a 143-kDa glycoprotein, has none of the properties characteristic of alpha 1 but binds lectins and contains about 25% N-linked carbohydrate. alpha 2 is disulfide-linked to delta, a 24- to 27-kDa glycopeptide. beta (54 kDa) contains a cAMP-dependent phosphorylation site but is not N-glycosylated and does not have a hydrophobic domain. gamma (30 kDa) has a carbohydrate content of about 30% and extensive hydrophobic domain(s). Precipitation with affinity-purified anti-alpha 1 antibodies or alpha 2-specific lentil lectin-agarose demonstrated that alpha 1 alpha 2 beta gamma delta behaves as a complex in the presence of digitonin or 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, whereas the alpha 2 delta complex dissociates from alpha 1 beta gamma in the presence of Triton X-100. A model for subunit interaction and membrane insertion is proposed on the basis of these observations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsotto M., Barhanin J., Fosset M., Lazdunski M. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca2+ channel. Purification and subunit composition. J Biol Chem. 1985 Nov 15;260(26):14255–14263. [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Reconstitution of the voltage-sensitive calcium channel purified from skeletal muscle transverse tubules. Biochemistry. 1986 Jun 3;25(11):3077–3083. doi: 10.1021/bi00359a002. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Solubilization of the calcium antagonist receptor from rat brain. J Biol Chem. 1983 Jun 25;258(12):7280–7283. [PubMed] [Google Scholar]
- Ferry D. R., Rombush M., Goll A., Glossmann H. Photoaffinity labelling of Ca2+ channels with [3H]azidopine. FEBS Lett. 1984 Apr 9;169(1):112–118. doi: 10.1016/0014-5793(84)80299-9. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F. Purification of a functional receptor for calcium-channel blockers from rabbit skeletal-muscle microsomes. Eur J Biochem. 1986 Nov 17;161(1):217–224. doi: 10.1111/j.1432-1033.1986.tb10145.x. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Solubilization and partial purification of putative calcium channels labelled with [3H]-nimodipine. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):279–291. doi: 10.1007/BF00512465. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne W. A., Weiland G. A., Oswald R. E. Solubilization and hydrodynamic characterization of the dihydropyridine receptor from rat ventricular muscle. J Biol Chem. 1986 Mar 15;261(8):3588–3594. [PubMed] [Google Scholar]
- Hosey M. M., Borsotto M., Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Kirley T. L., Vaghy P. L., McKenna E., Schwartz A. Purification of putative Ca2+ channel protein from rabbit skeletal muscle. Determination of the amino-terminal sequence. J Biol Chem. 1987 May 15;262(14):6572–6576. [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M., Hidalgo C., Vergara C., Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1981 Aug 10;256(15):8140–8148. [PubMed] [Google Scholar]
- Schmid A., Barhanin J., Coppola T., Borsotto M., Lazdunski M. Immunochemical analysis of subunit structures of 1,4-dihydropyridine receptors associated with voltage-dependent Ca2+ channels in skeletal, cardiac, and smooth muscles. Biochemistry. 1986 Jun 17;25(12):3492–3495. doi: 10.1021/bi00360a002. [DOI] [PubMed] [Google Scholar]
- Seagar M. J., Labbé-Jullié C., Granier C., Goll A., Glossmann H., Van Rietschoten J., Couraud F. Molecular structure of rat brain apamin receptor: differential photoaffinity labeling of putative K+ channel subunits and target size analysis. Biochemistry. 1986 Jul 15;25(14):4051–4057. doi: 10.1021/bi00362a010. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Knaus H. G., Grabner M., Moosburger K., Seitz W., Lietz H., Glossmann H. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett. 1987 Feb 23;212(2):247–253. doi: 10.1016/0014-5793(87)81354-6. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Moosburger K., Goll A., Ferry D. R., Glossmann H. Stereoselective photoaffinity labelling of the purified 1,4-dihydropyridine receptor of the voltage-dependent calcium channel. Eur J Biochem. 1986 Dec 15;161(3):603–609. doi: 10.1111/j.1432-1033.1986.tb10484.x. [DOI] [PubMed] [Google Scholar]
- Tung A. S. Production of large amounts of antibodies, nonspecific immunoglobulins, and other serum proteins in ascitic fluids of individual mice and guinea pigs. Methods Enzymol. 1983;93:12–23. doi: 10.1016/s0076-6879(83)93032-x. [DOI] [PubMed] [Google Scholar]