Abstract
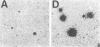
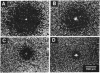
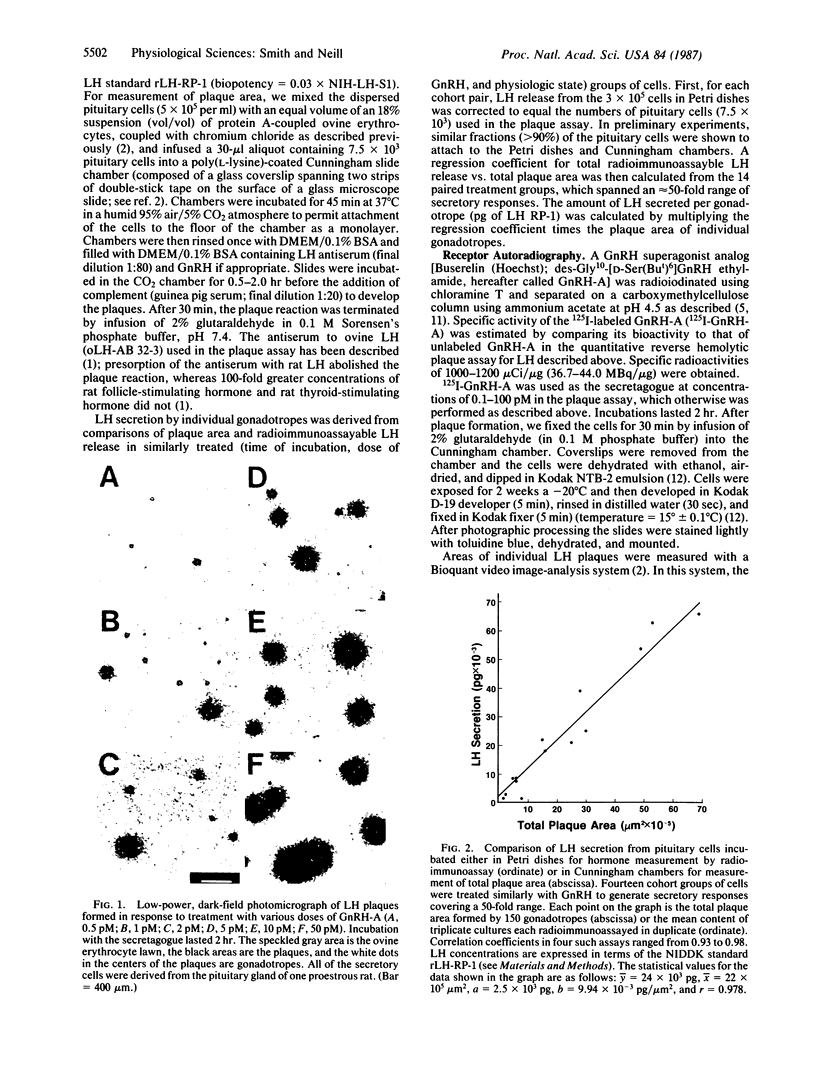
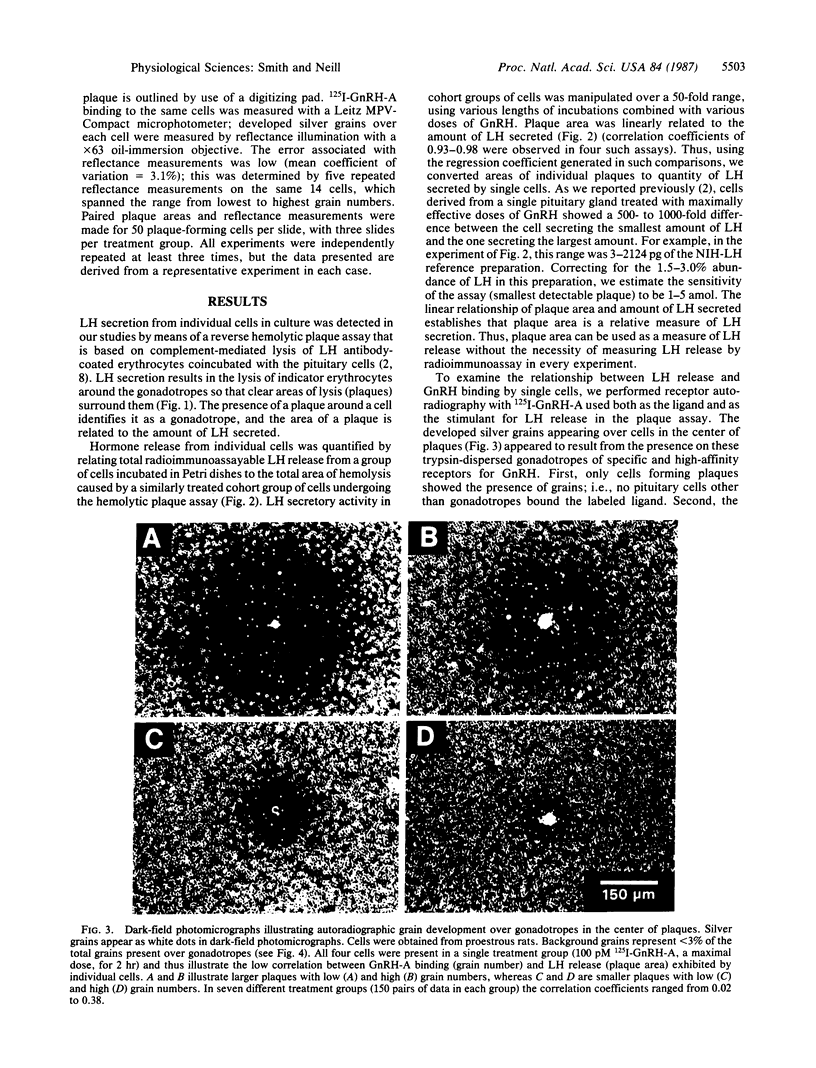
The quantitative relationship between receptor binding and hormone secretion at the single-cell level was investigated in the present study by combining a reverse hemolytic plaque assay for measurement of luteinizing hormone (LH) secretion from individual pituitary cells with an autoradiographic assay of 125I-labeled gonadotropin-releasing hormone (GnRH) agonist binding to the same cells. In the plaque assay, LH secretion induces complement-mediated lysis of the LH-antibody-coated erythrocytes around the gonadotropes, resulting in areas of lysis (plaques). LH release from individual gonadotropes was quantified by comparing radioimmunoassayable LH release to hemolytic area in similarly treated cohort groups of cells; plaque area was linearly related to the amount of LH secreted. Receptor autoradiography was performed using 125I-labeled GnRH-A (a superagonist analog of GnRH) both as the ligand and as the stimulant for LH release in the plaque assay; the developed silver grains appearing over cells in the center of plaques were measured microscopically. The grains appeared to represent specific and high-affinity receptors for GnRH because no pituitary cells other than gonadotropes bound the labeled ligand and grain development was progressively inhibited by coincubation with increasing doses of unlabeled GnRH-A. Despite high correlations between mean grain number and mean plaque area in dose-response curves, the correlation coefficients for these parameters were low (range 0.02-0.38) in the individual cells comprising these groups. We conclude that GnRH receptor number for any individual gonadotrope is a weak determinant of the amount of LH it can secrete; nevertheless, full occupancy of all its GnRH receptors is required for any gonadotrope to reach its full LH-secretory capacity. Apparently the levels of other factors comprising the steps along the secretory pathway determine the secretory capacity of an individual cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton R. N., Catt K. J. Gonadotropin-releasing hormone receptors: characterization, physiological regulation, and relationship to reproductive function. Endocr Rev. 1981 Spring;2(2):186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- Clayton R. N., Shakespear R. A., Duncan J. A., Marshall J. C., Munson P. J., Rodbard D. Radioiodinated nondegradable gonadotropin-releasing hormone analogs: new probes for the investigation of pituitary gonadotropin-releasing hormone receptors. Endocrinology. 1979 Dec;105(6):1369–1376. doi: 10.1210/endo-105-6-1369. [DOI] [PubMed] [Google Scholar]
- Clayton R. N., Solano A. R., Garcia-Vela A., Dufau M. L., Catt K. J. Regulation of pituitary receptors for gonadotropin-releasing hormone during the rat estrous cycle. Endocrinology. 1980 Sep;107(3):699–706. doi: 10.1210/endo-107-3-699. [DOI] [PubMed] [Google Scholar]
- Fink G. Feedback actions of target hormones on hypothalamus and pituitary with special reference to gonadal steroids. Annu Rev Physiol. 1979;41:571–585. doi: 10.1146/annurev.ph.41.030179.003035. [DOI] [PubMed] [Google Scholar]
- Frawley L. S., Neill J. D. Biphasic effects of estrogen on gonadotropin-releasing hormone-induced luteinizing hormone release in monolayer cultures of rat and monkey pituitary cells. Endocrinology. 1984 Feb;114(2):659–663. doi: 10.1210/endo-114-2-659. [DOI] [PubMed] [Google Scholar]
- Hazum E., Keinan D. Photoaffinity labeling of pituitary gonadotropin releasing hormone receptors during the rat estrous cycle. Biochem Biophys Res Commun. 1982 Jul 30;107(2):695–698. doi: 10.1016/0006-291x(82)91546-7. [DOI] [PubMed] [Google Scholar]
- Hymer W. C., Hatfield J. M. Separation of cells from the rat anterior pituitary gland. Methods Enzymol. 1983;103:257–287. doi: 10.1016/s0076-6879(83)03017-7. [DOI] [PubMed] [Google Scholar]
- Iwashita M., Catt K. J. Photoaffinity labeling of pituitary and gonadal receptors for gonadotropin-releasing hormone. Endocrinology. 1985 Aug;117(2):738–746. doi: 10.1210/endo-117-2-738. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Marshall J. C., Odell W. D. Preparation of biologically active 125-I LH-RH suitable membrane-binding studies. Proc Soc Exp Biol Med. 1975 Jun;149(2):351–355. doi: 10.3181/00379727-149-38806. [DOI] [PubMed] [Google Scholar]
- McCann S. M. Physiology and pharmacology of LHRH and somatostatin. Annu Rev Pharmacol Toxicol. 1982;22:491–515. doi: 10.1146/annurev.pa.22.040182.002423. [DOI] [PubMed] [Google Scholar]
- Mulchahey J. J., Neill J. D., Dion L. D., Bost K. L., Blalock J. E. Antibodies to the binding site of the receptor for luteinizing hormone-releasing hormone (LHRH): generation with a synthetic decapeptide encoded by an RNA complementary to LHRH mRNA. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9714–9718. doi: 10.1073/pnas.83.24.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z., Clayton R. N., Catt K. J. Characterization of gonadotropin-releasing hormone receptors in cultured rat pituitary cells. Endocrinology. 1980 Oct;107(4):1144–1152. doi: 10.1210/endo-107-4-1144. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Frawley L. S. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology. 1983 Mar;112(3):1135–1137. doi: 10.1210/endo-112-3-1135. [DOI] [PubMed] [Google Scholar]
- Pickering A. J., Fink G. Variation in size of the 'readily releasable pool' of luteinizing hormone during the oestrous cycle of the rat. J Endocrinol. 1979 Oct;83(1):53–59. [PubMed] [Google Scholar]
- Savoy-Moore R. T., Schwartz N. B., Duncan J. A., Marshall J. C. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science. 1980 Aug 22;209(4459):942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Bothwell M. A. Nerve growth factor receptors on PC12 cells: evidence for two receptor classes with differing cytoskeletal association. Cell. 1981 Jun;24(3):867–874. doi: 10.1016/0092-8674(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Frawley L. S., Neill J. D. Detection of LH release from individual pituitary cells by the reverse hemolytic plaque assay: estrogen increases the fraction of gonadotropes responding to GnRH. Endocrinology. 1984 Dec;115(6):2484–2486. doi: 10.1210/endo-115-6-2484. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Luque E. H., Neill J. D. Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol. 1986;124:443–465. doi: 10.1016/0076-6879(86)24034-3. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Ignatius M. J., Shooter E. M. Association of nerve growth factor receptors with the triton X-100 cytoskeleton of PC12 cells. J Neurosci. 1985 Oct;5(10):2762–2770. doi: 10.1523/JNEUROSCI.05-10-02762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring D. W., Turgeon J. L. Luteinizing hormone-releasing hormone-induced luteinizing hormone secretion in vitro: cyclic changes in responsiveness and self-priming. Endocrinology. 1980 May;106(5):1430–1436. doi: 10.1210/endo-106-5-1430. [DOI] [PubMed] [Google Scholar]