Abstract
Background
Many people experiment with alcohol and other drugs of abuse during their teenage years. Epidemiological evidence suggests that younger initiates into drug taking are more likely to develop problematic drug seeking behavior, including binge and other high-intake behaviors. The level of drug intake for any individual depends on the balance of rewarding and aversive effects of the drug in that individual. Multiple rodent studies have demonstrated that aversive effects of drugs of abuse are reduced in adolescent compared to adult animals. In the present study we addressed two key questions: First, do reduced aversive effects of ethanol in younger rats correlate with increased ethanol consumption? Second, are the reduced aversive effects in adolescents attributable to reduced sensitivity to ethanol's physiological effects?
Methods
Adolescent and adult rats were tested for ethanol conditioned taste aversion followed by a voluntary drinking period, including post-deprivation consumption. Multivariate regression was used to assess correlations. In separate experiments, adolescent and adult rats were tested for their sensitivity to the hypothermic and sedative effects of ethanol, and for blood ethanol concentrations (BECs).
Results
We observed that in adolescent rats but not adults, taste aversion was inversely correlated with post-deprivation consumption. Adolescents also exhibited a greater increase in consumption after deprivation than adults. Furthermore, the age difference in ethanol conditioned taste aversion was not attributable to differences in hypothermia, sedation, or BECs.
Conclusions
These results suggest that during adolescence, individuals that are insensitive to aversive effects are most likely to develop problem drinking behaviors. These results underscore the importance of the interaction between developmental stage and individual variation in sensitivity to alcohol.
Introduction
Ethanol is one of the most popular drugs of abuse among adolescents. In 2007, according to SAMHSA, 4 % of 12-13 year-olds, 15% of 14-15 year olds, 29% of 16-17 year olds, and 51% of 18-20 year olds were current consumers of alcohol (SAMHSA, 2008). Binge drinking is also high among this age group; 9.7% of youth ages 12-17 participated in binge-drinking (SAMHSA, 2008). It is clear, therefore, that most alcohol use begins in adolescence (Chen and Kandel, 1995). It is unclear, however, what effect alcohol consumption during adolescence has on subsequent development of alcoholism and alcohol abuse.
Alcohol intake is determined by various factors related to the individual, his peer group, and the effect of alcohol itself. Alcohol itself has both use-promoting and use-limiting effects which can occur during and after the drinking episode. During a drinking episode, dizziness, motor incoordination and sedation can acutely limit intake. After a drinking episode, hangover can reduce future intake. Humans who are less sensitive to these effects are more likely to develop alcoholism (Schuckit et al., 2006; Schuckit et al., 2004; Schuckit et al., 1996). In rodents, motor incoordination and sedation can be measured directly, and a hangover-like reaction can be assessed as an increase in anxiety-like behavior and/or a decrease in exploratory or social behavior (Doremus-Fitzwater and Spear, 2007; Varlinskaya and Spear, 2004). All of these effects are reduced in adolescent rats compared to adults (Doremus-Fitzwater and Spear, 2007; Little et al., 1996; Varlinskaya and Spear, 2004). However, the impact of ethanol's subjective effects on ethanol consumption has not been compared in adolescents and adults.
Conditioned taste aversion (CTA) is a behavioral paradigm which is sensitive to use-limiting effects that originate from multiple mechanisms, including physiologic and subjective effects. By pairing a potentially aversive experience with something the animal would naturally approach, one can measure the ability of the aversive experience to discourage future approach. Previously, we and others have demonstrated that conditioned aversive effects of many commonly abused substances are reduced in adolescent compared to adult rodents. This has been shown for cocaine, nicotine, THC, and ethanol (Philpot et al., 2003; Schramm-Sapyta et al., 2007; Schramm-Sapyta et al., 2006; Shram et al., 2006; Wilmouth and Spear, 2004).
Reduced conditioned aversive effects of ethanol are known to be related to increased voluntary ethanol intake in genetically-selected adult rodents. One study of inbred mouse and rat strains showed a consistent negative relationship between CTA and voluntary drinking, suggesting that rodent strains which found ethanol less aversive also consumed more ethanol in voluntary consumption testing (Green and Grahame, 2008). For example, in experiments comparing group averages, alcohol-preferring P rats and Sardinian alcohol-preferring sP rats both exhibit reduced taste aversion compared to their non-preferring counterparts (Brunetti et al., 2002; Froehlich et al., 1988). We wanted to assess whether this relationship occurs in individual rats. We therefore used an outbred strain of rats to examine whether individual variation in sensitivity to conditioned aversion was correlated with individual variation in voluntary drinking. We also determined whether this relationship is affected by age.
We also wanted to examine potential mechanisms for the reduced aversive effects of ethanol that are reported in adolescents. Reduced aversion could result from either reduced ethanol effects, reduced perception of the effects as negative, or reduced ability to learn from the effects. In the present study we evaluated the first of these possibilities. We tested two potentially aversive physiological effects (hypothermia and sedation) in adolescent vs. adult rats to assess whether they might contribute to the differing CTA outcomes we observed.
Hypothermia at least partially contributes to alcohol's aversive effects. When hypothermic effects of ethanol are reduced by pairing ethanol injection with high (32°C) ambient temperature, subsequent taste and place aversion are reduced (Cunningham and Niehus, 1993; Cunningham et al., 1992). Conversely, pairing of ethanol injection with low (5°C) ambient temperature exacerbates taste aversion (Cunningham et al., 1992). We therefore tested whether there is an age difference in ethanol's hypothermic effect that might explain the age difference in taste aversion.
Insensitivity to ethanol-induced sedation is also a major factor in increased ethanol intake in humans (Hinckers et al., 2006; Schuckit et al., 2006; Schuckit et al., 2004; Schuckit et al., 1997; Schuckit et al., 1996; Schuckit et al., 2005; Wilhelmsen et al., 2003). In rodents, selective breeding for high alcohol consumption can also result in strains which are insensitive to its sedating effects (Froehlich and Wand, 1997; Morzorati and Kubek, 1993). Both of these observations indicate that sedation is a use-limiting effect. Sedative effects of ethanol differ between adolescents and adults (Little et al., 1996; White et al., 2002), so we hypothesized that sedation could partially account for the aversive effects of ethanol, and that age differences in sedation could be related to age differences in aversion.
We also examined the role of ethanol pharmacokinetics in sensitivity to conditioned taste aversion. Some researchers have reported differences between adolescent and adult rodents in the rate of ethanol clearance (Brasser and Spear, 2002; Doremus et al., 2003) though not in ethanol uptake (Varlinskaya and Spear, 2006). However, pharmacokinetics do not account for age differences in ethanol-induced sleep time (Little et al., 1996). We therefore examined blood ethanol levels (BECs) to test whether they might account for differing taste aversions between the two ages.
We report here that the relationship between taste aversion and voluntary consumption is age-dependent. In adolescent rats, aversion is negatively correlated with consumption. In adult rats, in contrast, the two measures were not significantly correlated. Furthermore, the age difference in conditioned taste aversion to ethanol is not attributable to hypothermic effects, sedative effects, or ethanol pharmacokinetics.
Materials and Methods
Animals
Male CD rats (an outbred Sprague-Dawley derived strain) were obtained from Charles River Laboratories, Raleigh, NC. Adolescent rats were received at postnatal day 21 and adult rats were received at postnatal day 58 and allowed to acclimate to our housing facility for one week before the start of testing. Rats were maintained in a temperature and humidity-controlled vivarium on a standard 12:12 light-dark cycle during all experiments (lights on at 7am). Food and water were available ad libitum except as indicated below. All procedures were approved by Duke University's Animal Care and Use committee.
Conditioned Taste Aversion
A total of 94 rats were used for this experiment. Rats were water deprived for 24 hours and then allowed 15-minute access to distilled water in cages containing only pre-weighed water bottles and bedding. They were then returned to the home cage with ad libitum access to water for 24 hours. The next day, they were water deprived for 24 hours, and then given 15 minute access to 0.2% saccharin solution. After the 15-minute access, they were injected intraperitoneally with the indicated dose of ethanol (20% v/v in saline) or saline and returned to their home cages with ad libitum access to drinking water for 24 hours. On the next day, the rats were water deprived for 24 hours before being given 15 minute access to two bottles—one containing water and the other containing 0.2% saccharin solution. The water bottle was always placed on the left side of the cage and saccharin on the right, to generate both a place and taste association. The intake of all fluids was measured by weighing the bottles before and after each session. The dependent measure of interest is the percent saccharin consumption on the test day, expressed as:
Rats that consumed less than 1mL of total solution on either the water day, saccharin day, or test day were excluded from further analysis.
Measured Ethanol Drinking
A total of 66 rats were used for this experiment; 11 were excluded from analysis due to failure to consume at least 1 mL of solution in the CTA phase of the experiment. Ethanol consumption was measured in a paradigm we have previously used to sequentially determine ethanol consumption in an ethanol-only environment, a choice environment, and after alcohol deprivation (Schramm-Sapyta et al., 2008). Beginning at 35 days of age, rats were placed individually in cages equipped with bottles at each end from 1600 hrs each night until 0800 the next morning for 16 consecutive days. During the day (0800 until 1600) rats were pair-housed. Food was always available ad libitum. Water was always available in the home cage, and liquids were available in the individual cages as described below. Bottles were filled and weighed each night, and reweighed in the morning to determine the mass of each liquid consumed. Mass consumed was converted to volume and then g/kg based on the density of the fluids. On the first three nights, rats were placed in the cages with water in both bottles (water, W, consumption period). The following 3 nights (Ethanol-only (EO) Consumption Period), rats were exposed to a solution of 10% ethanol (v/v) in both bottles. We chose a relatively high ethanol concentration during this phase to be sure that the rats would overcome any neophobia for the taste of ethanol, and to acquaint them with its pharmacological effects. For the next 10 nights (Choice Consumption Period), rats were exposed to one bottle of 8% ethanol (v/v) and one bottle of water. We used the lower concentration during this phase to increase the volume consumed and potentially increase interindividual variability during the choice phase. To avoid placement preference, bottle position was alternated in the drinking cage each night. After 10 days of choice consumption, rats were deprived of ethanol for two nights, and then allowed to choose between 8% ethanol and water to assess alcohol deprivation-stimulated drinking.
Assessment of Hypothermic Effects
A total of 44 rats were used for this experiment. Core body temperature was assessed using a tympanic infrared thermometer (Vet-Temp, Advanced Monitors Corp.). These devices have been validated to determine core temperature accurately in a variety of species including rats (Sharp et al., 1993) and have been used specifically to assess drug-induced changes in core temperature in rats (Hargreaves et al., 2007; Louw et al., 2000). Measurements were taken immediately prior to injection (baseline), and 15, 30, 45 and 60 minutes after injection. Adolescents (28-30 days of age) and adults (65-67 days of age) were treated with either saline or 1 g/kg ethanol i.p. During the monitoring of body temperatures, the rats were placed in individual cages without food or water.
Assessment of Sleep Time
A total of 69 rats were used for this experiment. Sleep time was assessed based on reappearance of righting reflex after a sleep-inducing dose of ethanol. Immediately after injection of the indicated doses of ethanol or saline, adolescent (28-30 days of age) and adult (65-67 days of age) rats were placed in individual cages containing corn-cob bedding. An observer recorded the time post-injection at which each rat initially lost its righting reflex: when the rat appeared to fall asleep, the investigator turned it onto its back. If the rat did not attempt to regain sternal recumbency, it was considered to have lost its righting reflex. Recovery of righting reflex was recorded as the time at which each rat regained sternal recumbency, and then turned upright a second time when the experimenter attempted to turn it onto its back.
Assessment of Blood Alcohol Levels
A total of 16 rats were used for this experiment. Adolescent and adult males were injected with either saline or 1 g/kg ethanol (20% v/v solution), and then placed back in their home cages with their cagemate for 15 minutes. This time point is based on (Varlinskaya and Spear, 2006) and should reflect near peak blood alcohol levels. The rats were then anesthetized with isoflurane and decapitated. The trunk blood from each rat was collected on ice. Serum was separated via centrifugation at 3000 × g for 15 minutes. Alcohol level in the serum was determined using the alcohol assay kit from Pointe Science Inc. Spectrophotometric readings were collected on a Bio-Tek Power Wave XS microtiter plate reader.
Statistics
Age-group effects were tested using ANOVA and repeated measures ANOVA where appropriate. Fisher's PLSD was used for post-hoc analysis as indicated. Standard linear regression was used to assess the relationship between CTA and voluntary consumption, with a cutoff of p<0.05 for significance testing. Statview and JMP statistical software packages were used.
Results
Adolescent rats exhibit reduced conditioned taste aversion compared to adults
As shown in figure 1, the pairing of saccharin with an injection of ethanol decreased subsequent consumption of saccharin in a dose-dependent manner (ANOVA, dose effect: F(3,83)=9.86; p<0.0001). Adults decreased intake more; adolescents consumed more saccharin than adults (ANOVA, age effect, F(1,83)=5.65, p= 0.02). Post hoc analysis showed that adolescents and adults differed after saccharin pairing with an injection of 0.5 or 1 g/kg but not after pairing with saline or 3.5 g/kg ethanol. At the dose of 0.5 g/kg, the age difference reflects a slight increase in adolescent saccharin intake in combination with a slight decrease in adult saccharin intake, although neither age group differed significantly from their age-matched saline controls. At 1 g/kg, adolescents treated with ethanol did not significantly differ from age-matched controls, whereas adults treated with 1 g/kg exhibited a significant decrease in saccharin intake relative to age-matched controls. At the high dose, both age groups maximally avoided ethanol-paired saccharin. This result was confirmed in a separate group of rats which were treated with 1 g/kg ethanol or saline prior to assessment of their drinking behavior (Adults, saline: Mean ± SEM = 0.50 ± 0.07; Adults, 1 g/kg ethanol: 0.15 ± 0.05; Adolescents, saline: 0.58 ± 0.07; Adolescents, 1 g/kg ethanol: 0.51 ± 0.07; ANOVA, dose effect: F(1,51)=9.00; p=0.0042; age effect: F(1,51)=9.48; p=0.0033; age × dose interaction: F(1,51)=4.10; p=0.048, data not shown).
Figure 1.
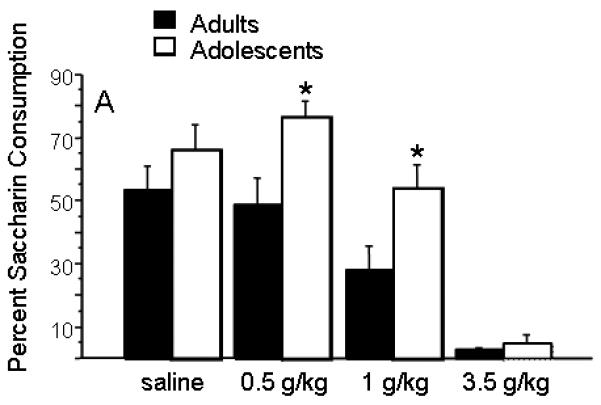
Reduced CTA to ethanol in adolescent rats. Percent saccharin consumption for rats exposed to the indicated doses of ethanol (20% v/v, i.p.). (N=12-15 for doses 0-1; N=3 rats at 3.5 g/kg) *, significantly different from adults at same dose.
Effect of Age on Measured Ethanol Consumption
After assessment of CTA, rats were entered into assessment of fluid consumption as previously described (Schramm-Sapyta et al., 2008). During the W phase, adolescents consumed more g/kg water than adults (RMANOVA, age effect, F(1,50)=29.98; p<0.0001; figure 2a). During the EO phase, adolescents also consumed more g/kg ethanol than adults (RMANOVA, age effect, F(1,51)=110.8; p<0.0001; figure 2b). The adolescent rats' intake of ethanol declined across the EO phase, whereas the adult rats' intake remained stable (RMANOVA, age × day interaction: F(1,102)=9.61; p=0.0002). During the choice phase, both age groups consumed similar amounts of ethanol (Figure 3a). After 2 days of deprivation, adolescent rats exhibited a marked increase in consumption, whereas adults did not (RMANOVA, age × day interaction: F(1,51)=8.20, p=0.006; Figure 3b).
Figure 2.
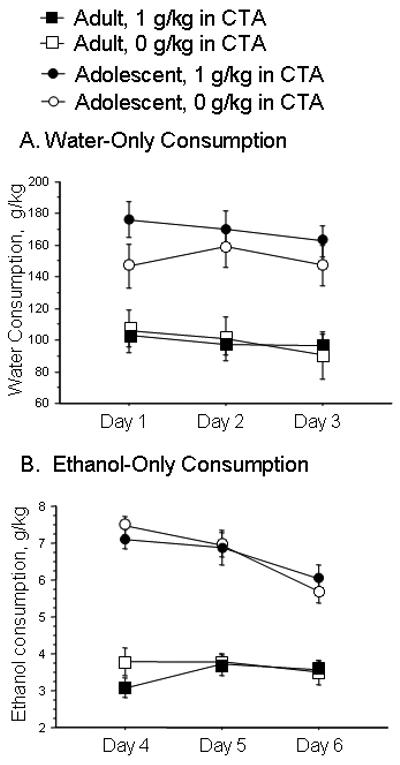
Fluid consumption during the two single-fluid phases A. Water consumption during the first 3 days, in which water was the only available fluid. B. ethanol consumption during days 4-6, in which 10% ethanol was the only available fluid. (N=17-19 per group)
Figure 3.
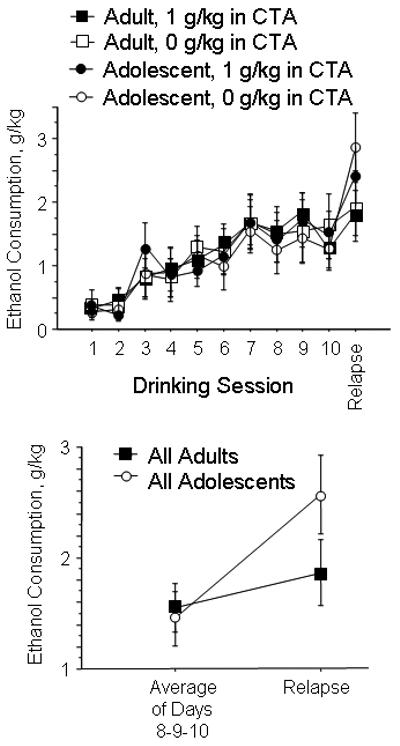
Ethanol consumption during the choice period. A. g/kg consumed by adolescent and adult rats during the 10 consecutive days during which 8% ethanol and water were available and the 1 day post-deprivation (relapse). B. Ethanol intake averaged from days 8-9-10 of the choice phase, and on relapse day. Significant age × phase interaction, see text for details. (N=17-19 per group)
Consumption at the end of the choice phase (averaging days 8-9-10) was highly correlated with post-deprivation consumption (p<0.0001; R2=0.67) as previously reported (Schramm-Sapyta et al., 2008). Ethanol consumption on the third night of the EO phase was significantly correlated with post-deprivation consumption (p=0.011; R2=0.15); this correlation is mostly attributable to higher adolescent consumption at both phases. This experiment extends those previously published by examining adult-onset consumption alongside adolescent-onset consumption.
Relationship of conditioned taste aversion to post-deprivation consumption
We observed an age-specific relationship between the conditioned aversive effects of ethanol and post-deprivation consumption, as shown in Figure 4 (age × CTA score interaction effect, p=0.035). Adolescent rats that exhibited less ethanol aversion in the CTA task consumed more ethanol on the post-deprivation day (R2=0.26; p=0.024). In adult rats, the relationship between CTA score and post-deprivation consumption was non-significant (R2=0.07; p=0.31).
Figure 4.
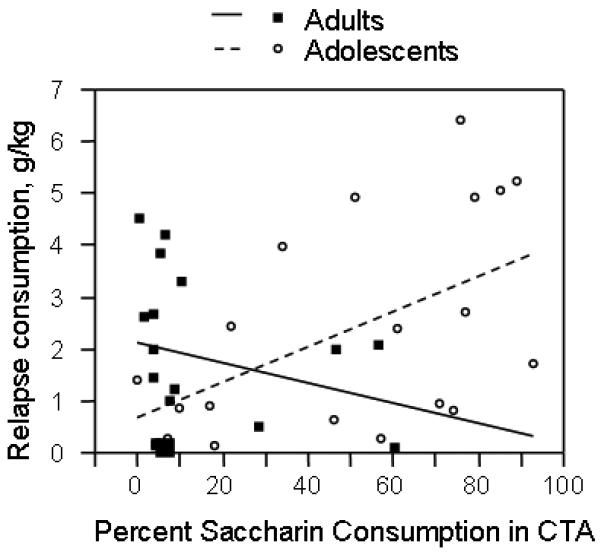
Relationship between ethanol taste aversion and post-deprivation intake. Adolescent (open circles, dotted line) and adult (black squares, solid line) rats. Significant age × CTA score interaction, see text for details.
We also observed a significant interaction between age and CTA score in predicting choice consumption at the end of the choice period (averaging consumption on days 8, 9, and 10) (p=0.029), but the analysis of each age group separately yielded non-significant correlations. There was no correlation between CTA and consumption when ethanol was the only available fluid, and no interaction with age. There was no correlation between CTA score and post-deprivation consumption when saline-treated animals were examined, and no age × CTA score interaction. There was also no effect of prior exposure to ethanol in the CTA task on consumption in any of these phases, as shown in figures 2 and 3.
Age differences in taste aversion are not attributable to differing hypothermic effects
We tested whether the age difference we observed in ethanol's aversive effects might be attributable to age differences in hypothermic effects. However, at the dose (1 g/kg) at which we saw the most robust age effect on conditioned aversion, we observed no alcohol-induced hypothermia (figure 5). Adolescents consistently exhibited lower temperatures than adults (age effect: F(1, 160)=17.7; p<0.0001).
Figure 5.
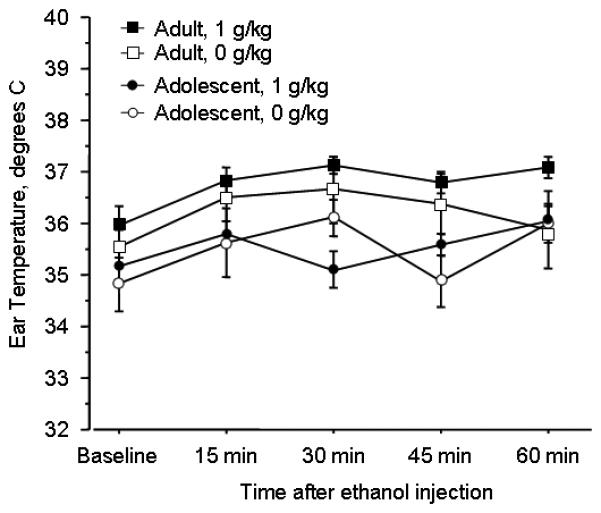
Age differences in ethanol CTA are not attributable to differing hypothermic effects. 1g/kg ethanol did not elicit hypothermia in either adolescent or adult rats, although adults had a higher average temperature than adolescents. (N=7-15 per group)
Age differences in taste aversion are not attributable to different sedative effects
We hypothesized that another explanation for age differences in ethanol's aversive effects might be related to age differences in ethanol's sedative effects. As shown in figure 6, there are robust age differences in ethanol's sedative effects. Adult rats consistently lost their righting reflex after a dose of 5 g/kg (6 out of 7 rats slept) and sleep for approximately 7 hours. Adolescents, in contrast, did not consistently lose their righting reflex after 5 g/kg (4 out of 8 rats) and slept for an average of approximately 1 hour. Including rats of both ages which did and did not sleep, the sleep time at 5 g/kg is significantly different between adolescents and adults (F(1,13)=19.7; p=0.0007). At 6 g/kg, 5 out of 8 adolescent rats slept for an average of approximately 1.5 hours. Adolescent sleep time was not examined at lower doses, based on previous studies (Little et al., 1996).
Figure 6.
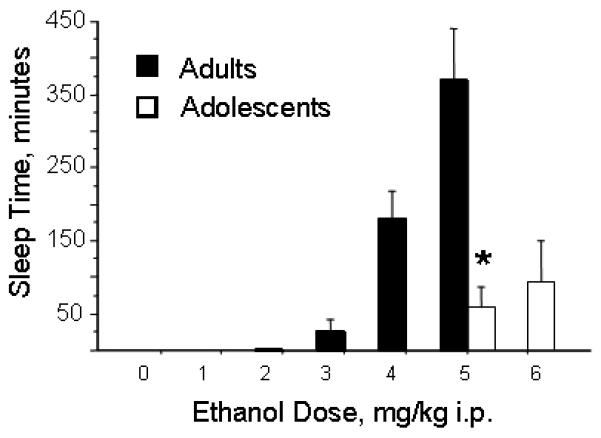
Effect of age and dose on ethanol sleep time. Adult rats were treated with saline, or 1, 2, 3, 4, or 5 g/kg ethanol (N=3-10 per group). Adolescent rats were treated with saline or 5 or 6 g/kg of ethanol (N=8-11 per group). *, significantly different from adults at the same dose.
Age differences in taste aversion are not attributable to pharmacokinetics
Age differences in pharmacokinetics of ethanol could explain the age difference we observed in both taste aversion and sedation. Higher blood levels would suggest greater sedation and aversion. However, we obtained the opposite result: Adolescents had blood alcohol levels that were slightly but not significantly higher than adults at 15 min post injection (ANOVA, age effect, p=0.0529, see fig 7). This suggests that the age differences are not the result of differing drug metabolism.
Figure 7.
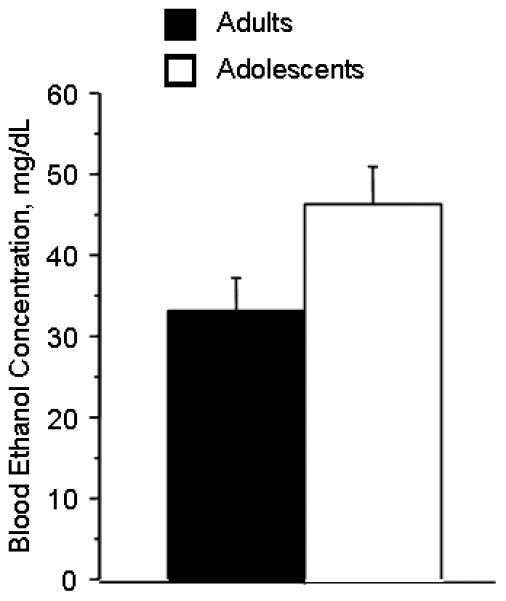
Blood alcohol level at 15 minutes after injection of 1 g/kg ethanol. (N=8 per group, age effect, NS, p=0.0529)
Discussion
These experiments reveal three key findings. First, the conditioned aversive effects of ethanol predict post-deprivation ethanol consumption in an age-specific manner. In adolescent rats, ethanol aversion is inversely correlated with post-deprivation consumption, whereas there is no such effect in adult rats at the dose we examined. Second, age affects ethanol consumption. Adolescent rats consumed more ethanol than adult rats in two phases of our experiment: when it was the only available fluid, and after 2 days of deprivation. Third, reduced sensitivity to conditioned taste aversion in adolescent rats as a group is not attributable to differences in the pharmacokinetic or physiological effects we examined: at the dose (1 g/kg) at which ethanol was differentially aversive in the 2 ages, we observed no difference in sedation, hypothermia, or blood alcohol levels.
The most important finding of this paper is that age is a major factor in determining a rat's behavior toward a conditioned stimulus associated with ethanol, and in its consumption of ethanol itself. In rats initially exposed to ethanol during adolescence, the approach/avoidance response to ethanol-paired saccharin parallels the approach/avoidance response to ethanol itself. However, in adults, there was no such correlation. There is some evidence in the literature for developmental alteration in the circuitry of aversion learning (Manrique et al., 2009; Raineki et al., 2009). Although these studies did not directly examine the time period and conditions we examined, they do raise the possibility that the neural circuitry underlying aversion learning is dynamic, and that our finding may result from developing neural circuitry.
Research from genetically selected and genetically modified mice and rats demonstrates that strains which voluntarily consume high amounts of ethanol are generally less sensitive to conditioned aversive effects than their non-high-drinking counterparts (Green and Grahame, 2008). However, in a comparison of adult rats of strains which were not selected for high alcohol drinking, conditioned aversion does not always parallel voluntary drinking across strains (Cailhol and Mormede, 2002). By examining individual animals, rather than making comparisons across strains, our results suggest that both genetics and developmental stage contribute to this relationship. Lack of sensitivity to conditioned aversive effects may facilitate increased drinking in both genetically susceptible individuals and in adolescents as a group.
The lack of correlation between conditioned aversion to ethanol and ethanol consumption that we observed in adult animals could be the result of lack of variance in CTA in the adults. If we had treated adults with a lower dose of ethanol, we might have observed more variance in CTA and thereby been able to see a correlation with consumption. At odds with this prediction, however, is the observation (as seen in Figure 3) that there are a few adults with low levels of CTA to ethanol (in the range of 0.3-0.6 on the x-axis) that consume low amounts of ethanol (in the range of 0-2 on the y-axis). These data suggest to us that caution should be exercised when using conditioned aversion as an index of use-limiting effects of ethanol in adults.
Some argue that conditioned taste aversion to addictive drugs is a manifestation of rewarding effects, not aversive effects (Gomez, 2002; Grigson, 2008; Grigson and Freet, 2000; Grigson and Twining, 2002; Liu et al., 2009; Parker, 1995; Parker and Gillies, 1995). These authors suggest that the avoidance of sweet-flavored solution after pairing with an addictive drug is a result of anticipatory avoidance: The rats avoid drinking sweet-flavored solution because they know that it will soon be followed by a “better” reward, the addictive drug. For adolescent rats, our results are clearly inconsistent with this hypothesis.
Our finding that 1 g/kg did not cause hypothermia in either age group after a dose of 1 g/kg ethanol is consistent with previously-published reports (Myers, 1981; Rezvani and Levin, 2004; Rezvani et al., 1986) that suggest that hypothermic effects occur at doses of 2.0 g/kg and above. Thus, we conclude that age differences in ethanol's aversive effects are not attributable to age differences in hypothermia. Other investigators have observed age differences in hypothermia after dosing with 2.0 and 4.0 g/kg (Brasser and Spear, 2002; Ristuccia et al., 2007). This result is highly dependent upon handling and stress (Ristuccia et al., 2007). In pre-handled animals, which are habituated to the stress of injection, adolescents exhibit a hypothermic response to 2 g/kg ethanol, whereas adults do not. In non-handled animals, both age groups exhibit hypothermia, with adults exhibiting a greater response. We, therefore, cannot rule out the possibility that subtle differences in hypothermia might have contributed to the age differences we observed in CTA. Similarly, sedation did not occur in either age group at 1 g/kg, but an age difference was apparent at higher doses. At sleep-inducing doses, CTA was maximal in both ages. Therefore, we cannot entirely rule out the possibility that subtle differences in sedation affected our CTA results.
The reduced conditioned taste aversion to ethanol in adolescents observed in the present study parallels earlier reports in which adolescent rodents exhibit reduced CTA for many drugs of abuse, including cocaine, nicotine, THC, and amphetamine (Infurna and Spear, 1979; Quinn et al., 2008; Schramm-Sapyta et al., 2007; Schramm-Sapyta et al., 2006; Shram et al., 2006; Wilmouth and Spear, 2004). These reports, coupled with the present finding that hypothermia and sedation do not differ between adolescents and adults at 1 g/kg suggests that reduced aversion results from reduced cognitive processing of the aversive effects. This speculation is consistent with neuroanatomical studies showing that neural connections between the amygdala, which is critical for the affective component of conditioned taste aversion (Welzl et al., 2001; Yamamoto, 2007) and the cortex, which may be most important for regulating the behavioral response (Welzl et al., 2001; Yamamoto, 2007) matures across adolescents in rodents (Cunningham et al. 2002; 2008).
Another finding in this study is that adolescent rats exhibited greater post-deprivation consumption than adults. After deprivation, rats consume significantly more alcohol than before it (Sinclair and Senter, 1968). Post-deprivation consumption is sometimes used as a model of alcohol craving and relapse (Spanagel and Holter, 1999). This behavior may model that of human alcoholics who abstain and then relapse to consume higher levels than they had before abstinence (Rodd et al., 2004). Our result implies that, regardless of choice consumption level, adolescents may be more prone to alcoholism-like behavior than adults. Similar results have been published for stress-induced reinstatement of alcohol drinking (Fullgrabe et al., 2007; Siegmund et al., 2005). The enhanced deprivation-induced consumption observed in adolescents implies that adolescent-onset drinkers may be particularly vulnerable to alcoholism.
The increased drinking we observed in the post-deprivation phase could alternatively result from the higher drinking levels we had observed in the ethanol-only phase. The younger rats drank more during the EO phase, and the added exposure to ethanol could have elicited neuroadaptations that later increased relapse drinking. For example, tolerance to a single injection of ethanol can vary developmentally (Lumeng and Li, 1986), and can persist for 7-14 days (Gatto et al., 1987). Alternatively, increased post-deprivation drinking could result from increased susceptibility, in the younger rats, to the short-term effects of deprivation-induced withdrawal. Future experiments will be required to differentiate between these 2 possibilities.
Rats that are less sensitive to alcohol initially are also more likely to develop tolerance (Kurtz et al., 1996). This phenomenon could explain some of our findings. First, adolescents as a group are less sensitive to CTA, and consume higher amounts of ethanol during the ethanol-only and post-deprivation phases. Second, the individual adolescents that were least sensitive to CTA consumed the most post-deprivation. Therefore, on both a group and individual level, tolerance in the least-sensitive rats could facilitate greater consumption.
These studies suggest two points in the progression of drinking behavior at which adolescents might be particularly vulnerable to alcoholism, and a potential mechanism of this vulnerability. Adolescents found ethanol less aversive and initially consumed higher levels than adults. Second, after repeated consumption and forced abstinence, adolescents exhibited greater relapse-like behavior. This paper replicates and extends previous work from our lab in which voluntary consumption during regular nightly drinking is highly correlated to post-deprivation consumption. We now show that this relationship exists in both adolescent-onset and adult-onset drinkers. If these findings can be extrapolated to humans, then adolescent-onset drinking could be particularly detrimental, because adolescents, lacking the sensitivity to aversive use-limiting effects, could consume greater amounts ethanol and thereby experience greater neuroadaptation leading to alcoholism. In summary, these results in a rodent model suggest that young drinkers, especially those with a high level of intake, are particularly prone to alcoholism-like behavior.
Acknowledegments
This work was supported by NIDA, DA020729, to NLSS.
References
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Brunetti G, Carai MA, Lobina C, Melis S, Serra S, Vacca G, Gessa GL, Colombo G. Differences in ethanol-induced conditioned taste aversion in Sardinian alcohol-preferring and Sardinian alcohol-nonpreferring rats. Alcohol. 2002;26:167–172. doi: 10.1016/s0741-8329(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–99. [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS. Drug-induced hypothermia and conditioned place aversion. Behav Neurosci. 1993;107:468–479. doi: 10.1037//0735-7044.107.3.468. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF. Ambient temperature effects on taste aversion conditioned by ethanol: contribution of ethanol-induced hypothermia. Alcohol Clin Exp Res. 1992;16:1117–1124. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Wand GS. Adenylyl cyclase signal transduction and alcohol-induced sedation. Pharmacol Biochem Behav. 1997;58:1021–1030. doi: 10.1016/s0091-3057(97)00305-5. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li TK. Persistence of tolerance to a single dose of ethanol in the selectively-bred alcohol-preferring P rat. Pharmacol Biochem Behav. 1987;28:105–110. doi: 10.1016/0091-3057(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Gomez F. Conditioned saccharin aversion induced by self-administered cocaine negatively correlates with the rate of cocaine self-administration in rats. Brain Res. 2002;946:214–220. doi: 10.1016/s0006-8993(02)02886-x. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. The state of the reward comparison hypothesis: theoretical comment on Huang and Hsiao (2008) Behav Neurosci. 2008;122:1383–1390. doi: 10.1037/a0013968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114:353–363. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Hargreaves GA, Hunt GE, Cornish JL, McGregor IS. High ambient temperature increases 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”)-induced Fos expression in a region-specific manner. Neuroscience. 2007;145:764–774. doi: 10.1016/j.neuroscience.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry. 2006;60:282–287. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- I Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Showalter J, Grigson PS. Ethanol-induced conditioned taste avoidance: reward or aversion? Alcohol Clin Exp Res. 2009;33:522–530. doi: 10.1111/j.1530-0277.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw DF, Yang FW, Sutherland GR. The effect of delta-9-tetrahydrocannabinol on forebrain ischemia in rat. Brain Res. 2000;857:183–187. doi: 10.1016/s0006-8993(99)02422-1. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Manrique T, Gamiz F, Moron I, Ballesteros MA, Gallo M. Peculiar modulation of taste aversion learning by the time of day in developing rats. Dev Psychobiol. 2009;51:147–157. doi: 10.1002/dev.20354. [DOI] [PubMed] [Google Scholar]
- Morzorati S, Kubek MJ. The effect of TRH on ethanol-induced sedation in alcohol-preferring and -nonpreferring rats. Neuropeptides. 1993;25:283–287. doi: 10.1016/0143-4179(93)90045-c. [DOI] [PubMed] [Google Scholar]
- Myers RD. Alcohol's effect on body temperature: hypothermia, hyperthermia or poikilothermia? Brain Res Bull. 1981;7:209–220. doi: 10.1016/0361-9230(81)90085-x. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci. 1995;109:71–78. doi: 10.1037//0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. Int J Dev Neurosci. 2004;22:349–354. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Mack CM, Crovi SI, Myers RD. Central Ca++-channel blockade reverses ethanol-induced poikilothermia in the rat. Alcohol. 1986;3:273–279. doi: 10.1016/0741-8329(86)90037-6. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Hernandez M, Wilmouth CE, Spear LP. Differential expression of ethanol-induced hypothermia in adolescent and adult rats induced by pretest familiarization to the handling/injection procedure. Alcohol Clin Exp Res. 2007;31:575–581. doi: 10.1111/j.1530-0277.2007.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- SAMHSA National Survey on Drug Use and Health. 2008 doi: 10.1016/j.jpain.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res. 2008;32:754–762. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M, Smith T, Pierson J, Danko G, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk--a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr. Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Wilhelmsen K, Smith TL, Feiler HS, Lind P, Lange LA, Kalmijn J. Autosomal linkage analysis for the level of response to alcohol. Alcohol Clin Exp Res. 2005;29:1976–1982. doi: 10.1097/01.alc.0000187598.82921.27. [DOI] [PubMed] [Google Scholar]
- Sharp P, Sanow C, Oteham C, Suckow M, Gridesby C. Use of the Thermoscan thermometer in determining body temperature in laboratory rabbits, rodents, dogs, and cats. Contemp Top Lab Anim Sci. 1993;32:38. [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzl H, D'Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, Kalmijn J. The search for genes related to a low-level response to alcohol determined by alcohol challenges. Alcohol Clin Exp Res. 2003;27:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem Senses. 2007;32:105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]


