Abstract
Background
This study examines whether an integrated behavioral intervention with proven efficacy in reducing psycho-behavioral risks (smoking, environmental tobacco smoke exposure (ETSE), depression, and intimate partner violence (IPV)) in African-Americans is associated with improved pregnancy outcomes
Methods
A randomized controlled trial targeting risks during pregnancy was conducted in the District of Columbia. African-American women were recruited if reporting at least one of the risks mentioned above. Randomization to intervention or usual care was site and risk specific. Sociodemographic, health risk and pregnancy outcome data were collected. Data on 819 women, and their singleton live born infants were analyzed using an intent-to-treat approach. Bivariate analyses preceded a reduced logistical model approach to elucidate the effect of the intervention on the reduction of prematurity and low birth weight.
Results
The incidence of low birthweight LBW was 12% and very low birthweight VLBW was 1.6%. Multivariate logistic regression results showed that depression was associated with LBW (OR=1.71, 95%CI=1.12–2.62). IPV was associated with PTB and VPTB (OR 1.64, 95%CI=1.07–2.51, OR=2.94, 95%CI=1.40–6.16, respectively). The occurrence of VPTB was significantly reduced in the intervention compared to the usual care group (OR=0.42, 95%CI=0.19–0.93).
Conclusions
Our study confirms the significant associations between multiple psycho-behavioral risks and poor pregnancy outcomes, including LBW and PTB. Our behavioral intervention with demonstrated efficacy in addressing multiple risk factors simultaneously reduced VPTB within an urban minority population.
Keywords: African-American, birth weight, pregnancy, gestational age
Introduction
Infant mortality continues to be an important indicator of health disparities in the United States. The relative contribution of preterm and low birth weight to infant mortality is high [1,2], especially in non-Hispanic blacks where it is more than double the rate reported in the overall U.S. population [3,4]. In general, infants at the lowest categories of weight and gestation are at highest risk for death and are the principal contributors to infant mortality [5–7]. Disparities in mortality between African-American and white infants could be partially explained by the higher rates of very low birth weight (<1500 grams) and very premature births (≤33 weeks) amongst African-Americans[8]. In 2005, the rates for very premature infants were 4.17% for non-Hispanic blacks versus 1.64% for non-Hispanic whites. Similarly for very low birth weight, the rates were 3.27% versus 1.21% [8].
While genetic and medical mediators may be associated with such disparities [9,10], there is growing evidence indicating the importance of maternal psycho-behavioral contributors to poor reproductive outcomes among African-Americans[11–13]. Despite such evidence there are few interventional models that have been tested for efficacy in reduction of these frequently co-occurring risks and their potential role in improving pregnancy outcomes. Our recent publications confirm the efficacy of cognitive behavioral interventions during pregnancy and postpartum [14,15] in urban African American women. The risk factors we chose to address (smoking, environmental tobacco smoke exposure (ETSE), depression and intimate partner violence (IPV)) have all been associated with poor pregnancy outcomes [16–19]. Mothers in the intervention group resolved some or all of these risks more frequently than those assigned to usual care[14].
Both the ACOG and AAP recognize the significance of these behavioral and mental health related issues and recommend screening for them during pregnancy [20,21]. Due to the association of such risks with underlying ecological factors, most notably poverty [22], unstable housing [23], transportation [24], the lack of cohesive social support systems [25]and stressful life events [26], it is unclear whether interventions targeting psycho-behavioral risks, identified during pregnancy, can have an impact. Furthermore, the interaction between psycho-behavioral risks and genetic predisposition [9,10] may interfere with potential intervention effects. There are only a few interventions that have demonstrated success in reducing the burden of such psycho-behavioral risks during pregnancy [13,27,28] and none have measured the effect on reproductive outcomes.
This paper addresses the effect of reducing these psycho-behavioral risks (smoking, ETSE, depression and IPV) on reduction of the rates of low and very low birth weight (LBW: <2,500 grams, VLBW: <1,500 grams) and preterm and very preterm births (PTB:<37 weeks gestation, VPTB: ≤ 33 weeks gestation). We demonstrated [14] that rates of psycho-behavioral risks are reduced in mothers randomized to a cognitive, behavioral intervention. In this study, we examine whether the intervention was also able to improve rates of LBW, VLBW, PTB, and VPTB within this population of African-American mothers.
Materials and Methods
The “NIH-DC Initiative to Reduce Infant Mortality in Minority Populations” is a congressionally mandated research collaboration between four major academic institutions in Washington, DC (Children’s National Medical Center, Georgetown University, George Washington University, and Howard University), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Minority Health and Health Disparities, and RTI International. As part of this collaboration, a randomized controlled trial was conducted to evaluate the benefits of an integrated behavioral cognitive intervention delivered during routine prenatal care (PNC) in reducing cigarette smoking, ETSE, depression, and IPV during pregnancy and improving pregnancy outcomes. These analyses used data collected from this trial.
Women presenting to six community-based clinical sites serving minority women (African-Americans and Hispanics) in the District of Columbia were screened for recruitment between July, 2001 and October, 2003. Women were deemed demographically eligible if self-identified as belonging to a minority group, being ≥ 18 years, <29 weeks of pregnant, a DC resident and English speaking. Verbal pre-screening for other characteristics was discouraged by the study protocol in order to avoid bias. Demographically eligible women were invited and consented to participate in the screening. Participants were screened for the four risk factors using an audio-computer assisted self interview (A-CASI) which also confirmed their pregnancy status and demographic eligibility. See El-Khorazaty et al. for more details on recruitment and retention activities and results [29]. After initial screening confirmed eligibility, mothers were scheduled for a telephone interview. More information on sociodemographics, reproductive history and behavioral risks was collected during a baseline interview, conducted on average at 19 weeks of gestation, nine days after A-CASI screening. Follow-up data collection by telephone interview occurred during the second and third trimesters of pregnancy (22–26 and 34–38 weeks EGA, respectively). Intervention and follow-up activities continued until July 2004.
For purposes of recruitment, gestational age (GA) was based on women’s reporting and verified by medical record abstraction. Upon delivery, infant’s GA was abstracted from the medical records prioritized in the following order: ultrasound, exam and menstrual history. Analysis for GA was based on the infants’ GA assessment. Following the baseline interview, consent to participate was obtained and women were randomized to the clinical trial.
The sample size was determined to ensure adequate statistical power to test the hypotheses that our cognitive-behavioral intervention would result in reductions in the targeted risks. Assuming a 5% level of significance, 80% power would allow the detection of 10–20% reductions in risk-specific factors among women in the intervention group from a 100% prevalence at recruitment time, a sample of 1,050 women needed to be retained at the end of the follow-up period. This number was estimated to be sufficient to detect a 25% reduction in PTB and LBW combined in the intervention group as compared to that for the usual care (estimated at 20%). Based on a declining birth rate in D.C., the recruitment period was extended four months to reach the required sample size.
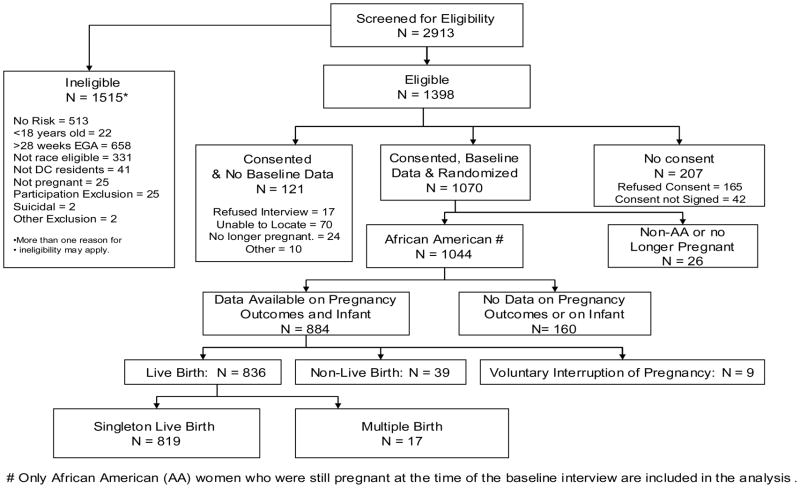
A total of 2,913 women were screened and 1,398 met eligibility criteria. There were 1,070 women, who were reached for baseline telephone interview and consented to participate in the randomized trial. This number exceeded the required sample size of 1,050 needed for 80% power and 5% level of significance. These demographically eligible pregnant women reporting one of the four risk factors were randomized to the intervention group (IG) or the usual care group (UCG) after completing the baseline interview. Of the1,070 women, 1,044 were African-American and still pregnant at the time of the interview. Pregnancy outcomes data were available on 918, 88% of these participants. Mothers with a live birth represented 95% (n=870). Only mothers of a singleton live born with infant medical abstraction data available were included in these analyses (n=819, 94% of mothers with a live birth). This final number represented 89% of all women with a known pregnancy outcome. (See Figure 1.)
Figure 1.
Profile of Project DC-HOPE Randomized Controlled Trial
The intervention of this RCT was designed for delivery during PNC. The intervention was evidence-based and specific to the designated psycho-behavioral risks [30]. The intervention for smoking and ETSE was based on Smoking Cessation or Reduction in Program Treatment (SCRIPT). This intervention is based on social cognitive theory [31] and tailored to a woman’s stage of readiness for behavioral change [32]. The intervention for depression was based on a cognitive behavioral theory program developed by Miranda and Munoz [33] and proven successful with low income minority women seeking primary care services. The original group therapy model was adapted to an individual treatment format. The intervention focused on secondary prevention of symptoms of depression and emphasized strategies for mood management as well as establishing and maintaining positive social interactions. Sessions emphasized skill development towards revising negative cognitions. A structured intervention developed by Parker and colleagues [34] and based on Dutton’s Empowerment Theory [35] which emphasized safety behaviors, was adopted for the IPV intervention. This brochure based intervention provided information about the types of abuse, the cycle of violence, a danger assessment component, in addition to the development of a safety plan. Individualized counseling sessions provided an integrated approach to multiple risks responsive to a woman’s specific risk combination. Intervention sessions were conducted privately in a room proximate to the PNC clinics and occurred immediately before or after routine PNC, for an average of 35±15 minutes. The intervention was designed to be delivered during PNC visits with eight discrete sessions. The intervention specialists were trained to apply the content of these sessions during one or more visits as needed. In order to avoid influencing the utilization patterns of PNC (an outcome compared between IG and UCG), no effort was made to facilitate or encourage participation in routine PNC in either group. Delivery of the intervention was dependent on the mother’s decision to attend a scheduled PNC visit.
Site- and risk-specific block randomization to IG or UCG was conducted. Investigators and field workers were blinded to the block size. A computer generated randomization scheme considered all possible risk combinations within each of the recruitment sites. Recruitment staff at each site called in the details of the risk profile for a new recruit, and the assignment was generated centrally by the data coordinating center.
Validated instruments were used for the A-CASI screening, and the baseline and follow-up telephone assessments of women with respect to risk factors. Telephone interviewers and their supervisors were blinded to the participants’ randomization group. At the time of recruitment and upon delivery, maternal and infant medical records were abstracted and infant and pregnancy outcomes were recorded.
To preserve the randomization, participant data were analyzed according to their care group assignment, regardless of whether they received any intervention sessions, using an intent-to-treat approach. Statistical analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). Bivariate analyses compared women with and without adverse pregnancy outcomes (LBW, VLBW, PTB, and VPTB) with respect to sociodemographic characteristics and psycho-behavioral risks. Similar analyses compared baseline characteristics and outcomes among women assigned to IG vs. UCG. Chi-square tests and t-tests were used for these analyses. Bivariate analyses using exact chi-square tests compared reduction in the number of risk factors at baseline and the last follow up interview prior to delivery for women assigned to IC vs. UCG.
To evaluate the impact of sociodemographic and psycho-behavioral risks on adverse pregnancy outcomes, we used logistic regression to model LBW, VLBW, PTB, and VPTB. Unadjusted analyses were conducted to assess the impact of care group assignment on the four outcomes. Absolute risk reduction, percent reduction in risk and number needed to treat were calculated. Adjusted logistic regression models controlled for variables including maternal age, care group, depression, IPV and illicit drug use during pregnancy. Variables were selected for inclusion in the logistic regression models if they reached significance in the bivariate analyses, and predictors significant at p<0.05 were retained in the final models.
Results
For the total sample (n=1,044), sociodemographic, psycho-behavioral and reproductive characterization at baseline showed that there were no significant differences between women randomized to the IG (n=521) or UCG (n=523), except for the number of PNC visits. In addition, there were no significant differences between women with a known infant outcome and those missing this information.
For women included in the analyses presented here (n=819), 403 were in the IG and 416 were in the UCG. Sociodemographic, psycho-behavioral and reproductive characterization at baseline are presented in Table 1 and showed no statistically significant differences between women in the two groups. On average participants in the IG received 4.4±2.7 prenatal intervention sessions. 38.5% received 0–3 visits, and 61.5% received 4 visits or more. The mean time of an intervention visit was 35 minutes. Of the 819 women analyzed in this study 13.6% reported no risks during the baseline interview despite their acknowledgment of risk during the A-CASI screening. Almost two-thirds (62.6%) of participants reported 1–2 risks at baseline and 23.8% reported 3–4 risks. There were no differences between the IG and UCG in the distribution of risks (p=0.969).
Table 1.
Characteristics of the Study Population at Baseline (N=819)
| Characteristic | Category | Intervention (n=403) | Usual Care (n=416) | P-value | Total (N=819) |
|---|---|---|---|---|---|
| Number of prenatal intervention visits | None | 39 (9.7%) | --- | --- | --- |
| 1–3 | 116 (28.8%) | --- | |||
| 4–7 | 191 (47.4%) | --- | |||
| 8+ | 57(14.1%) | --- | |||
| Maternal age | 18–22 | 189 (47.0%) | 165 (39.8%) | 0.109 | 354 (43.3%) |
| 23–27 | 118 (29.4%) | 136 (32.8%) | 254 (31.1%) | ||
| 28 + | 95 (23.6%) | 114 (27.5%) | 209 (25.6%) | ||
| Gestational age (weeks) | Mean ± SD | 19.5 ± 6.6 | 18.8 ± 6.7 | 0.121 | 19.1 ± 6.7 |
| Gestational age at PNC initiation (weeks) | Mean ± SD | 13.1 ± 6.2 | 13.2 ± 6.4 | 0.906 | 13.1 ± 6.3 |
| Education Level | < High school | 114 (28.3%) | 119 (28.6%) | 0.699 | 233 (28.4%) |
| High school graduate/GED | 202 (50.1%) | 198 (47.6%) | 400 (48.8%) | ||
| At least some college | 87 (21.6%) | 99 (23.8%) | 186 (22.7%) | ||
| Employment status | Working now | 139 (34.5%) | 156 (37.6%) | 0.351 | 295 (36.1%) |
| Not working now, worked previous to pregnancy | 149 (37.0%) | 158 (38.1%) | 307 (37.5%) | ||
| Not working now, did not work previous to pregnancy | 114 (28.3%) | 98 (23.6%) | 212 (25.9%) | ||
| Relationship status | Single/separated/widowed/divorced | 306 (75.9%) | 309 (74.3%) | 0.585 | 615 (75.1%) |
| Married or living with partner | 97 (24.1%) | 107 (25.7%) | 204 (24.9%) | ||
| Trimester of PNC initiation | 1st Trimester | 238 (60.9%) | 237 (57.8%) | 0.408 | 475 (59.3%) |
| 2nd Trimester | 143 (36.6%) | 166 (40.5%) | 309 (59.3%) | ||
| 3rd Trimester | 10 (2.6%) | 7 (1.7%) | 17 (2.1%) | ||
| Number of PNC visits | Mean ± SD | 9.3+3.4 | 8.8+3.3 | 0.053 | 9.0+3.4 |
| Household size | Mean ± SD | 3.8+1.8 | 4.0+1.9 | 0.101 | 3.9+1.8 |
| Total household income | <$2,000 per month | 204 (73.1%) | 211 (69.6%) | 0.354 | 415 (71.3%) |
| Medicaid | Yes | 321 (80.0%) | 321 (77.3%) | 0.346 | 642 (78.7%) |
| WIC | Yes | 181 (44.9%) | 187 (45.0%) | 0.991 | 368 (44.9%) |
| Food stamps/supplemental food program | Yes | 230 (57.2%) | 243 (58.4%) | 0.728 | 473 (57.8%) |
| Public assistance/TANF | Yes | 158 (39.3%) | 176 (42.4%) | 0.367 | 334 (40.9%) |
| Alcohol use during pregnancy | Yes | 86 (21.4%) | 90 (21.6%) | 0.933 | 176 (21.5%) |
| Illicit drug use during pregnancy | Yes | 56 (13.9%) | 45 (10.8%) | 0.180 | 101 (12.3%) |
| Pregnancy ‘wanted’ | Yes | 309 (76.9%) | 313 (75.6%) | 0.672 | 622 (76.2%) |
| Previous pregnancy | Yes | 330 (81.9%) | 358 (86.1%) | 0.103 | 688 (84.0%) |
| Previous live birth | Yes | 266 (66.0%) | 292 (70.2%) | 0.199 | 558 (68.1%) |
| Number of live births (women with previous live birth) | Mean ± SD | 2.1+1.4 | 2.1+1.4 | 0.591 | 2.1+1.4 |
| Previous stillbirth or miscarriage (women with previous pregnancy) | Yes | 136 (41.2%) | 143 (39.9%) | 0.735 | 279 (40.6%) |
| Active smoking | Yes | 77 (19.1%) | 70 (16.8%) | 0.395 | 147 (17.9%) |
| ETSE | Yes | 284 (71.7%) | 300 (73.5%) | 0.564 | 584 (72.6%) |
| Depression | Yes | 175 (43.4%) | 184 (44.2%) | 0.816 | 359 (43.8%) |
| IPV | Yes | 132 (32.8%) | 131 (31.5%) | 0.699 | 263 (32.1%) |
PNC: prenatal care, WIC: Women, infant, and children, ETSE: environmental tobacco smoke exposure, IPV: intimate partner violence.
For these 819 women, when comparing risk resolution between the IG and UCG, the smoking quit rate in the IG was 24.3% vs. 20.3% in the UCG (p=0.593). ETSE avoidance in the IG was 33.3% vs. 27.7% in the UCG (p=0.165) within the population of women who had reported ETSE at baseline. The recurrence of IPV in the IG was 7.9% vs. 21.6% in the UCG for women who reported IPV in the year preceding pregnancy as reported at the baseline interview (p=0.004). For women who were classified as depressed at baseline using the Hopkins scale 32.7% in the IG had resolved their symptoms and 32.5% had resolved their symptoms in the UCG (p=0.977).
Since this intervention was designed to address multiple risks simultaneously, an additional analysis was conducted to examine the interventional effect on multiple risks reported by mothers in both groups. Comparisons between IG and UCG showed a significantly greater reduction in risks in the IG compared to UCG. Table 2 reviews the effect of the intervention classified by the number of risks reported at baseline as compared to the number reported during the follow-up interview prior to delivery. There was a group of women who reported no risks at baseline in spite of acknowledging risk(s) at screening. These women may have acknowledged risks during the course of their pregnancy. Among women reporting no risks at baseline, more women in the UCG than the IG reported risks during the last follow-up interview conducted during their pregnancy (p=0.04). Women randomized to the IG reported a significant reduction in their risks if they belonged to the group of women reporting 1–2 risks at baseline (p=0.021). The intervention did not succeed in significantly reducing risks in women reporting 3–4 risks at baseline (p=0.383). Within the group of women reporting 3–4 risks at baseline, only 74.7% of the intervention group vs. 65.9% in the usual care group reported reduction to 0–2 risks at follow-up. When comparing women with 3–4 risks at baseline who did not reduce their risks to 0–2 vs. those who did, they were less likely to be employed (22% vs. 40%, p =0.02) and more likely to have used illicit drugs during pregnancy (39% vs. 18%, p=0.003). The intervention did not address illicit drug use, which could have interfered with the efficacy of the intervention on reducing other associated risks. Within this group of women, active smoking was reported at baseline in 78% of those who did not resolve risk versus 35% in those that did (p=0.001). The intervention did address active smoking, but was not successful in significantly modifying this behavior [14].
Table 2.
Distribution of the Study Population by Number of risks at Baseline and Risk Status at Last Follow-up Preceding Delivery by Care Group*
| Number of Risks | Intervention | Usual Care | Exact Pearson chi- square p-value |
|---|---|---|---|
| A. Number of Risks at Baseline | |||
| 0 | 13.9% | 13.2% | 0.968 |
| 1–2 | 62.3% | 63.0% | |
| 3–4 | 23.8% | 23.8% | |
| B. Risk Resolution/Increase at Last Follow-up Preceding Delivery | |||
| 1. For Women with 0 Risk at Baseline: | n=54 | n=50 | 0.040 |
| No change in number of risks | 61.1% | 40.0% | |
| Increase by 1–2 risks | 38.9% | 58.0% | |
| Increase by 3–4 risks | 0.0% | 2.0% | |
| 2. For Women with 1–2 Risks at Baseline: | n=221 | n=239 | 0.021 |
| Resolution of all risks | 29.0% | 18.4% | |
| No change in number of risks | 67.0% | 75.3% | |
| Increase to 3–4 risks | 4.1% | 6.3% | |
| 3. For Women with 3–4 Risks at Baseline: | N=87 | n=85 | 0.383 |
| Resolution of all risks | 8.0% | 4.7% | |
| Reduced risks to 1–2 | 66.7% | 61.2% | |
| No change in number of risks | 25.3% | 34.19% | |
among women with at least one follow-up interview preceding delivery (intervention=362 and usual care=374).
Women were classified based on four adverse pregnancy outcomes: LBW, VLBW, PTB, and VPTB. Table 3 compares the sociodemographic and psycho-behavioral characteristics of mothers delivering infants weighing < 2,500 grams versus ≥2,500 grams, and mothers delivering infants weighing <1,500 grams versus ≥1,500 grams. Illicit drug use, depression and IPV were significantly more frequent in mothers delivering LBW infants. There were no significant differences between mothers delivering infants below 1,500 grams and those delivering infants ≥1,500 grams. Table 4 presents similar comparisons of mothers delivering infants <37 weeks gestation versus ≥37 weeks and those delivering infants <34 weeks gestation versus ≥34 weeks. In both comparisons the rates for depression and IPV are significantly higher for mothers delivering infants at a lower GA. An older maternal age is associated with higher rates of PTB.
Table 3.
Demographic and Risk Characteristics at Baseline by Low Birth Weight or Very Low Birth Weight
| Characteristic | LBW (n=97) | Not LBW (n=720) | VLBW (n=13) | Not VLBW (n=804) |
|---|---|---|---|---|
| Birth weight (mean ± SD) | 2057 ± 418 | 3303 ± 440 | 1239 ± 279 | 3186 ± 545 |
| Maternal age (mean ± SD) | 25.4 ± 6.2 | 24.5 ± 5.3 | 26.8 ± 5.5 | 24.6 ± 5.5 |
| Education ≥ High school | 74% | 71% | 62% | 72% |
| Married/living with partner | 28% | 25% | 31% | 25% |
| Medicaid recipient | 79% | 79% | 77% | 79% |
| PNC initiation (weeks) (mean ± SD) | 13.0 ± 6.7 | 13.2 ± 6.2 | 13.0 ± 5.7 | 13.1 ± 6.3 |
| Number of PNC visits (mean ± SD) | 7.5 ± 3.3 | 9.3 ± 3.3 | 5.3 ± 2.0 | 9.1 ± 3.4 |
| Alcohol use during pregnancy | 24% | 21% | 23% | 22% |
| Illicit drug use during pregnancy | 20%* | 11% | 15% | 12% |
| Active smoking | 24% | 17% | 15% | 18% |
| ETSE | 72% | 73% | 54% | 73% |
| Depression | 56%** | 42% | 69% | 44% |
| IPV | 41%*** | 31% | 54% | 32% |
p=0.021,
p=0.013,
p=0.039
LBW: low birth weight, VLBW: very low birth weight, PTB: preterm births, VPTB: very preterm birth, PNC: prenatal care, ETSE: environmental tobacco smoke exposure, IPV: intimate partner violence.
Table 4.
Demographic and Risk Characteristics at Baseline by Preterm or Very Preterm Birth
| Characteristic | PTB (n=102) | Not PTB (n=716) | VPTB (n=30) | Not VPTB (n=788) |
|---|---|---|---|---|
| Maternal Age (mean ± SD) | 26.1 ± 6.6* | 24.4 ± 5.3 | 25.8 ± 6.2 | 24.5 ± 5.4 |
| Education ≥ High School | 74% | 71% | 73% | 72% |
| Married/Living with Partner | 23% | 25% | 27% | 25% |
| Medicaid Recipient | 81% | 78% | 80% | 79% |
| PNC Initiation (weeks) (mean ± SD) | 12.5 ± 6.2 | 13.2 ± 6.3 | 11.3 ± 6.1 | 13.2 ± 6.3 |
| Number of PNC visits (mean ± SD) | 7.6 ± 3.1 | 9.2 ± 3.4 | 6.1 ± 2.7 | 9.1 ± 3.4 |
| Alcohol use during pregnancy | 24% | 21% | 20% | 22% |
| Illicit drug use during pregnancy | 13% | 12% | 10% | 12% |
| Active Smoking | 23% | 17% | 20% | 18% |
| ETSE | 70% | 73% | 66% | 73% |
| Depression | 53%** | 43% | 63%† | 43% |
| IPV | 42%*** | 31% | 57%‡ | 31% |
p=0.010
p=0.046,
p=0.021,
p=0.028,
p=0.003
PTB: preterm births, VPTB: very preterm birth, PNC: prenatal care, ETSE: environmental tobacco smoke exposure, IPV: intimate partner violence.
Table 5 reviews the rates for LBW, VLBW, PTB, and VPTB by intervention group. The table also shows the absolute risk reduction (usual care rate – intervention rate) in these pregnancy outcomes and the number needed to treat (receive the intervention) to prevent one new case of the designated outcomes. The rates for the four outcomes are all higher in the UCG but the difference only reaches significance for VTPB, with 2.2% (9/402) in the IG versus 5.0% (21/416) in the UCG (OR=0.43, 95%CI=0.20–0.95). On average, 36 mothers needed to receive the intervention to prevent one VPTB. For VLBW, the rate is more than double in the UCG (2.2%=9/415) vs. that in IG (1.0%=4/402), but does not reach significance (OR=0.45, 95%CI=0.14–1.48). In addition, 83 mothers, on average, needed to receive the intervention to prevent one VLBW.
Table 5.
Effect of the Intervention on Improving Pregnancy Outcomes
| Pregnancy Outcome | Intervention (n =403) | Usual Care (n =416) | Unadjusted OR (95% Confidence Interval) | Absolute Risk Reduction (% Reduction in Risk) | Number Needed to Treat* |
|---|---|---|---|---|---|
| LBW | 10.9% (n=44) | 12.8% (n=53) | 0.84 (0.55 – 1.29) | 1.9% (15%) | 53 |
| VLBW | 1.0% (n=4) | 2.2% (n=9) | 0.45 (0.14 – 1.48) | 1.2% (55%) | 83 |
| PTB | 11.9% (n=48) | 13.0% (n=54) | 0.91 (0.60 – 1.38) | 1.1% (8%) | 91 |
| VPTB | 2.2% (n=9) | 5.0% (n=21) | 0.43 (0.20 – 0.95) | 2.8% (56%) | 36 |
LBW: low birth weight, VLBW: very low birth weight, PTB: preterm births, VPTB: very preterm birth, OR: Odds Ratio.
Both odds ratio and number needed to treat are measures of the effect size to assess clinical significance.
In the logistic regression model for LBW (Table 6), depression at baseline was associated with a significantly increased likelihood of delivering an infant <2,500 grams (OR=1.71, 95%CI=1.12–2.62). The logistic regression models for PTB and VPTB are presented in Table 6. IPV documented at baseline is associated with PTB and VPTB (OR=1.64, 95%CI=1.07–2.51, OR=2.94, 95%CI=1.40–6.16, respectively). An older maternal age is associated with an increased rate of PTB (OR=1.06, 95%CI=1.02–1.09). Assignment to the integrated intervention addressing the four psycho-behavioral risk factors was significantly associated with a reduction in VPTB rates (OR=0.42, 95%CI=0.19–0.93).
Table 6.
Logistic Regression Analysis for Low Birth Weight, Preterm Birth and Very Preterm Birth
| Characteristic | Adjusted Odds Ratio* | 95% Confidence Interval |
|---|---|---|
| Low Birth Weight Model (N=817) | ||
| Depression at baseline | 1.71 | 1.12 – 2.62 |
| Preterm Birth (N=818) | ||
| Maternal age | 1.06 | 1.02 – 1.09 |
| IPV at baseline | 1.64 | 1.07 – 2.51 |
| Very Preterm Birth (N=818) | ||
| Behavioral intervention | 0.42 | 0.19 – 0.93 |
| IPV at baseline | 2.94 | 1.40 – 6.16 |
Variables were selected for inclusion in the final logistic regression models if they reached significance in the bivariate analyses, and predictors significant at p<0.05 were retained in the final models. For low birth weight, preterm birth, and very preterm birth models, the significant variables in the bivariate analyses included: behavioral intervention, maternal age, depression, IPV, and illicit drug use during pregnancy.
Odds ratio is the preferred measure of effect size to assess clinical significance in logistic regression.
Conclusion
This study confirms findings of previous investigators showing an association between psycho-behavioral risk and poor pregnancy outcomes, including LBW and PTB. Our results emphasize the importance of testing interventions targeting multiple risks simultaneously due to their co-occurrence and potential inter-dependency [30]. We have also demonstrated the efficacy of this integrated intervention within an urban minority population exposed to many of the ecological challenges mentioned earlier. The prevalence of such risk factors within this population was strikingly high. Amongst pregnant African-American District of Columbia residents, 18+ years, at <29 weeks of gestation who were eligible for recruitment to the study, the prevalence of the targeted risk factors was 17.6% for IPV, 24.2% for depression, 10.4% for smoking and 38.8% for ETSE. (Note that this statement refers to risks, not women. Almost two-thirds (60.2%) of mothers with risk exhibited more than one risk factor concomitantly.) The population from which our sample was drawn continues to suffer from significantly high rates of poor reproductive outcomes. The disparities in LBW and PTB between blacks and whites ranged between 2.0 to 2.4 for the years 2002–2004. The disparities for VLBW and VPTB ranged from 3.9 to 4.2 during the same time period. African-Americans in the District had an infant mortality rate of 17.0/1000 live births in 2005, two times the rate reported for whites [36].
The plausibility of some causal association between psycho-behavioral risk(s) and poor pregnancy outcomes, either directly or indirectly, is high and yet no intervention so far has shown the efficacy of such risk reduction on improving infant mortality rates. Recent findings in the literature confirm the significant contribution of higher rates of preterm birth to the black white disparity in infant mortality [37]. This contribution is especially pertinent to extremely premature and VLBW groups [38]. Our results show promise in impacting infant mortality rates through reduction of birth rates within the highest risk categories (VLBW and extremely premature). Prenatal health care providers may be reluctant to screen for pyschobehavioral risk due to the lack of evidence to support the value of intervention. Our results underscore the importance of psycho-social and behavioral therapy resource availability as an integral component of comprehensive prenatal care. Funding and reimbursement for such services should become a health policy priority.
Limitations included that this study was not powered to test efficacy of the intervention with respect to pregnancy outcomes, but rather resolution of psycho-behavioral risks. Another limitation was the inability to reach 9.7% of women in the intervention group (n=39). Whether intervention delivery would be further compromised when tested for effectiveness under non-experimental conditions remains to be seen. This would be unlikely since the intervention was not intended nor did it interfere with the mother’s choice to attend a particular routine PNC visit. Our results on reduction of risk were based on mothers’ self-report and therefore represent a limitation in the accuracy of measuring outcomes. Whether including fathers could have improved intervention efficacy is unknown, especially when studying biological outcomes. The intervention effect(s) we found may apply only to high risk minority pregnant women. It would be difficult to speculate whether the unique distribution of risk factors within this population influenced the results positively or negatively. Conclusions on effectiveness can only be reached after applying the intervention to the general population.
The results of our study show that the intervention, after controlling for confounding variables, reduced VPTB rates, i.e., increased gestational ages at delivery significantly and showed a trend toward reduction in VLBW rates, in spite of the fact that the study was not powered for these outcomes. Post-hoc calculations show the need for 1,878 enrollees to reach significant differences in the VLBW category. It would be important to test this intervention in other racial or sociodemographic groups or in a similar, but larger population to show whether results can be generalized. Our results also validate the association between psycho-behavioral risk and LBW, PTB and VPTB. In bivariate analysis, depression and IPV are statistically associated with a higher incidence in the three birth outcomes. When entered into a logistic regression model with other predictors, depression retains a statistical association with LBW, while IPV is only significantly associated with PTB and VPTB. A recent study with a similar population used a home visiting model for high risk pregnant women focusing on social support, health education and access to services, significantly lowered the women’s risk of delivering a LBW infant [39]. An earlier paper showed that in comparison with usual care women in the intervention group more frequently resolved some or all of their risks than did women in the usual care group (odds ratio=1.61; 95% confidence interval=1.08–2.39;p=0.02)[14]. The notable finding in this study is that this randomized integrated cognitive behavioral intervention can also show improvement in pregnancy outcome.
It is also worthy of note that the behavioral intervention had a stronger impact on reducing the more preterm and lower birth weight rates. One could speculate that the targeted psychosocial and behavioral risks are either more directly involved in the triggering of labor in the earlier gestational period or could be mediating other health behaviors or responses associated with earlier preterm labor. Nonetheless, the efficacy of targeting the risks we chose appears to improve pregnancy outcomes directly or indirectly in an urban minority population. The significant reduction of VPTB implies increases in gestational ages and birth weight in infants born to women receiving cognitive behavioral interventions during pregnancy, promises to impact on infant mortality reduction, but larger studies will be required to confirm such an impact.
Acknowledgments
The authors wish to thank the field work staff, the interviewers, and data management staff. We wish to thank the participants who welcomed us into their lives in hopes of helping themselves and their children.
Funding/Support: This study was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD03919; 5U18HD036104, Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities
Footnotes
Financial disclosure: This study was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD031919; 5U18HD036104, National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Human subjects protection: This study was reviewed and approved by the institutional review boards of the participating institutions, RTI International, and NICHD.
References
- 1.Mathews TJ, MacDorman MF. National vital statistics reports. 2. Vol. 57. Hyattsville, MD: National Center for Health Statistics; 2008. Infant mortality statistics from the 2005 period linked birth/infant death data set. [PubMed] [Google Scholar]
- 2.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lakritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clinical Obstetrics and Gynecology. 2008;51:360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 4.Reagan PB, Salsberry PJ. Race and ethnic differences of preterm birth in the USA: Broadening the social context. Social Science & Medicine. 2005;60:2217–2228. doi: 10.1016/j.socscimed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Serenius F, Ewald U, Farooqi A, Holmgren PA, Hakansson S, Sedin G. Short-term outcome after active perinatal management at 23–25 weeks of gestation. A study from two Swedish tertiary care centres. Part 1: maternal and obstetric factors. Acta Paediatrica. 2004;93(7):945–953. [PubMed] [Google Scholar]
- 6.Serenius F, Ewald U, Farooqi A, Holmgren PA, Hakansson S, Sedin G. Short-term outcome after active perinatal management at 23–25 weeks of gestation. A study from two Swedish tertiary care centres. Part 1: infant survival. Acta Paediatrica. 2004;93(8):1081–1089. [PubMed] [Google Scholar]
- 7.Shankaran S, Fanaroff AA, Wright LL, Stevenson DK, Donovan EF, Ehrenkranz RA, et al. Risk factors for early death among extremely low-birth-weight infants. American Journal of Obstetrics and Gynecology. 2002;186(4):796–802. doi: 10.1067/mob.2002.121652. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. National vital statistics reports. 6. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Births: Final data for 2005. [PubMed] [Google Scholar]
- 9.Nguyen DP, Genc M, Vardhana S, Babula O, Onderdonk A, Witkin SS. Ethnic differences of polymorphisms in cytokine and innate immune system genes in pregnant women. Obstetrics and Gynecology. 2004;104(2):293–300. doi: 10.1097/01.AOG.0000133486.85400.5e. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Journal of the American Medical Association. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Calvo J, Jackson J, Hansford C, Woodman C. Psychosocial factors and birth outcome: African American women in case management. Journal of Health Care for the Poor and Underserved. 1998;9:395–419. doi: 10.1353/hpu.2010.0409. [DOI] [PubMed] [Google Scholar]
- 12.Ricketts SA, Murray EK, Schwalberg R. Reducing low birthweight by resolving risks: results from Colorado’s Prenatal Plus Program. American Journal of Public Health. 2005;95:1952–1957. doi: 10.2105/AJPH.2004.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmer-Gembeck MJ, Helfand M. Low birthweight in a public prenatal care program: Behavioral and psychosocial risk factors and psychosocial intervention. Social Science & Medicine. 1996;43:187–197. doi: 10.1016/0277-9536(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 14.Joseph JG, El-Mohandes AAE, Kiely M, El-Khorazaty MN, Gantz MG, Johnson AA, Katz KS, Blake SM, Rossi MW, Subramanian S. Results of a randomized clinical Trial in high-risk pregnant African-American women to reduce psychosocial and behavioral risk factors in pregnancy. American Journal of Public Health. 2009;99(6):1053–1061. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Mohandes AE, Kiely M, Joseph JG, Subramanian S, Johnson AA, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to improve postpartum outcomes in African American mothers: A randomized controlled trial. Obstetrics and Gynecology. 2008;112(3):611–620. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depression and Anxiety. 2003;17(3):140–151. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 17.Neggers Y, Goldenberg R, Cliver S, Hauth J. Effects of domestic violence on preterm birth and low birth weight. Acta Obstetrica Gynecologica Scandanavica. 2004;83(5):455–460. doi: 10.1111/j.0001-6349.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- 18.Phares TM, Morrow B, Lansky A, Barfield WD, Prince CB, Marchi KS, et al. Surveillance for disparities in maternal health-related behaviors selected states, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000–2001. Morbidity and Mortality Weekly Report. 2004;53(4):1–13. [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 20.American College of Obstetricians and Gynecologists. Psychosocial risk factors: perinatal screening and intervention. International Journal of Gynaecology and Obstetrics. 2000;69:195–200. [PubMed] [Google Scholar]
- 21.Durant T, Colley Gilbert B, Saltzman LE, Johnson CH. Opportunities for intervention: discussing physical abuse during prenatal care visits. American Journal of Preventive Medicine. 2000;19:238–244. doi: 10.1016/s0749-3797(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 22.Berkowitz GS. An epidemiologic study of preterm delivery. American Journal of Epidemiology. 1981;113:81–92. doi: 10.1093/oxfordjournals.aje.a113068. [DOI] [PubMed] [Google Scholar]
- 23.Culhane J, Elo IT. Neighborhood context and reproductive health. American Journal of Obstetrics and Gynecology. 2005;192(Suppl 1):S22–S29. doi: 10.1016/j.ajog.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 24.Wingate MS, Swaminathan S, Alexander GR. The influence of maternal mobility on birth outcomes of non-Hispanic Blacks. Maternal and Child Health Journal. 2009;13:48–55. doi: 10.1007/s10995-007-0290-4. [DOI] [PubMed] [Google Scholar]
- 25.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiological Review. 1993;15:414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead N. RTI Press publication No. RR-0003-0809. Research Triangle Park, NC: RTI International; 2008. The relationship between individual life events and preterm delivery. Retrieved 02/06/09 from http://www.rti.org/rtipress. [Google Scholar]
- 27.Lightwood JM, Phibbs CS, Glantz SA. Short-term and Economic benefits of smoking cessation: Low birth weight. Pediatrics. 1999;104(6):1312–1320. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- 28.Norbeck JS, DeJoseph JF, Smith RT. A randomized trial of an empirically-derived social support intervention to prevent low birthweight among African American women. Social Science & Medicine. 1996;43(6):947–954. doi: 10.1016/0277-9536(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 29.EI-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AAE, Subramanian S, Laryea HA, Murray KB, Thornberry JS, Joseph JG. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. Biomedical Central Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, Rossi MA, Murray KB. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. Biomedical Central Pregnancy and Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 32.DiClemente CC, Prochaska J, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 33.Miranda J, Munoz R. Intervention for minor depression in primary care patients. Psychosomatic Medicine. 1994;56(2):136–141. doi: 10.1097/00006842-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Parker B, McFarlane J, Soeken K, Silva C, Reel S. Testing an intervention to prevent further abuse to pregnant women. Research in Nursing and Health. 1999;22:59–66. doi: 10.1002/(sici)1098-240x(199902)22:1<59::aid-nur7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Dutton MA. Empowering and Healing Battered Women. New York: Springer; 1992. [Google Scholar]
- 36.Kung H-C, Hoyert DL, Xu J, Murphy SL. Deaths: Final data for 2005. National Vital Statistics Reports. 2008;56(10) [PubMed] [Google Scholar]
- 37.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the black white infant mortality gap, 1990 and 2000. American Journal of Public Health. 2007;97:1255–1260. doi: 10.2105/AJPH.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadeau L, Tessier R, Boivin M, Lefebvre F, Robaey P. Extremely premature and very low birthweight infants: A double hazard population? Social Development. 2003;12(2):235–248. [Google Scholar]
- 39.Lee E, Mitchell-Herzfeld SD, Lowenfels AA, Greene R, Dorabawila V, DuMont KA. Reducing low birth weight through home visitation: A randomized controlled trial. American Journal of Preventive Medicine. 2009;36(2):154–160. doi: 10.1016/j.amepre.2008.09.029. [DOI] [PubMed] [Google Scholar]