Abstract
The inflammatory status of the brain in patients as well as animal models of Alzheimer's disease (AD) has been extensively studied. Accumulation of activated microglia producing TNF-α and MCP-1 contribute to the pathology of the disease. However, little is known about the changes in the spleen and associated peripheral immunity that might contribute to AD pathology. The goal of this study was to characterize phenotypic and functional changes in spleen, blood and brain cell populations that contribute to development of an AD-like disease in a triple transgenic (3xTg-AD) mouse model. The 3xTg-AD mice had increased percentages of brain Gr-1+ granulocytes, dendritic cells and macrophages, spleen and blood derived CD8+Ly6C+ memory T cells and CCR6+ B cells, as well as increased levels of secreted IL-6. Brain tissue from older 12 month old symptomatic 3xTg-AD female mice exhibited highly elevated mRNA expression of CCR6 compared to WT mice. Importantly, this pronounced increase in expression of CCR6 was also detected in brain and spleen tissue from pre-symptomatic 5-6 month old 3xTg-AD females and males. Our data demonstrate increased expression of CCR6 in the brain and peripheral immune organs of both pre-symptomatic and symptomatic 3xTg-AD mice, strongly suggesting an ongoing inflammatory process that precedes onset of clinical AD-like disease.
Keywords: Alzheimer's disease, CCR6, 3xTg-AD mice, inflammation
Introduction
Alzheimer's disease (AD) is the leading cause of dementia in elderly populations throughout the world with more than 35 million people worldwide having AD. AD is characterized by a deterioration of memory and other cognitive domains. Epidemiological studies indicate increased risk for AD in women [1], although the contributing factors remain unknown. AD is associated with the deposition in brain tissue of misfolded β-amyloid (Aβ) originated from proteolysis of the amyloid precursor protein (APP) by several enzymes, including presenilin-1. Pathologic forms of Aβ include soluble oligomers and insoluble Aβ plaques which are surrounded by activated microglia, reactive astrocytes, and dystrophic neurites. In addition, neurons located mainly in the cerebral cortex and subcortical nuclei accumulate neurofibrillary tangles made of paired helical filaments derived from the cytoskeletal protein tau (for review see [2]). Inflammation associated with glial activation and synaptic and neuronal loss is also characteristic of AD. Chronically activated microglia release IL-1, IL-6, and TNF-α [3, 4] and express receptors for Aβ oligomers triggering the release of cytokines, glutamate, and nitric oxide [5, 6]. Also, chemokines promote the migration of monocytes from the peripheral blood into plaque-bearing brain, compromising the blood–brain barrier in AD patients [7].
Epidemiologic studies showing a lower risk of developing AD in users of non-steroidal anti-inflammatory drugs (NSAIDs) provide further evidence that inflammatory mechanisms may play a role in AD pathogenesis. However, trials of NSAIDs in established and prodromal AD have failed to modify the clinical outcomes. If inflammation is a pathogenic factor in AD, the critical inflammatory events (and the window for effective intervention) are likely to occur very early in the disease process, long before clinical signs are evident. If interventions are to be tested in asymptomatic human subjects, it will be necessary to identify accessible biomarkers for identification of appropriate subjects. Some investigators have attempted to do this in human subjects using proteomic approaches [8], but these studies are fraught with confounders and do not permit comparison of brain versus peripheral markers. With an eye towards the identification of AD-associated biomarkers, we examined brain and peripheral mediators of inflammation in older (12-15 month old) transgenic mice which express AD-like pathology in order to establish target “markers of interest” that could be evaluated more precisely in younger (5-6 month old) pre-symptomatic mice.
The inflammatory response in the brains of older symptomatic mice with AD-like disease has been investigated extensively [9, 10]. Much less is known about the inflammatory response in the periphery of pre-symptomatic younger mice versus the older AD-like mice. To investigate this question, we evaluated the distribution of leukocyte subsets in spleen, blood and brain, and cytokine and gene expression profiles in a triple transgenic model of AD (3xTg-AD) in which the mice over-express three mutant genes - APP, presenilin-1, and tau - that phenocopy the critical aspects of AD neuropathology [11]. Our current study demonstrates the presence of inflammatory factors in the brain and periphery of both younger and older 3xTg-AD mice. Moreover, brain tissue from older symptomatic 3xTg-AD female mice exhibited highly elevated mRNA expression of CCR6 compared to age-matched wild type (WT) control mice without AD-like disease. Importantly, this pronounced increase in expression of CCR6 was also detected in brain and spleen tissue from pre-symptomatic 3xTg-AD females and males vs. WT controls, strongly suggesting an ongoing inflammatory process that precedes onset of clinical AD-like disease.
Materials and Methods
Mice
WT and 3xTg-AD (12-15 and 5-6 month old) mice were generated from breeding pairs generously provided by Dr. Frank LaFerla (UC Irvine). Mice were maintained in a climate-controlled environment with a 12-hr light/12-hr dark cycle, and fed AIN-93M Purified Rodent Diet (Dyets Inc, Bethlehem, PA). Diet and water were supplied ad libitum. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional Animal Care and Use Committee of the Portland VA Medical Center. We used 3 each of the 12 month old female WT and 3xTg-AD mice and 9 each of the 5-6 month old WT and 3xTg-AD male and female mice for all analyses. Twelve month old male 3xTg-AD mice were not available for this study.
Isolation of mononuclear cells from spleen, blood and brain
Spleen and brain were isolated from WT and 3xTg-AD mice. Single cell suspensions were prepared by passing the tissue through a 100μm nylon mesh screen. Spleen cells were washed using RPMI medium and red cells were lysed using 1X red cell lysis buffer (eBiosciences, San Diego, CA). The cells were washed twice, counted and resuspended in stimulation medium containing 10% fetal bovine serum (FBS). Central nervous system (CNS) mononuclear cells were isolated by Percoll gradient centrifugation as described [12]. Cardiac blood was collected in EDTA, and the cells were pelleted after lysis of red cells, washed, counted and resuspended in stimulation medium containing 10% FBS.
Analysis of cell populations by FACS
All antibodies were purchased from BD Biosciences, San Jose, CA or eBiosciences, Inc., San Diego, CA. Four-color (FITC, PE, PI, allophycocyanin) fluorescence flow cytometric analyses were performed to determine the phenotypes of spleen, brain and blood mononuclear cells. Cells were washed with staining medium and stained with a combination of the following mAbs: CD3e (145-2C11), CD4 (L3T4), CD8 (Ly-2), CD11b (M1/70), CD11c (HL-3), CD19 (1D3), DX5, Ly6C, Gr-1(IA8) and CD45 for 10min at 4°C. After incubation with mAb, cells were acquired with a FACSCalibur (BD Biosciences). Forward and side scatter parameters were chosen to identify lymphocytes. Dead cells were gated out using propidium iodide discrimination. Data were analyzed using FCS express software (De Novo).
Cytokine detection by Luminex bead array
Single-cell suspensions of spleens and blood mononuclear cells from WT and 3xTg-AD mice were cultured in the presence of plate bound anti-CD3 (5μg) and anti-CD28 (1μg) mAb for 24h. Culture supernatants were assessed for cytokine levels using a Luminex Bio-Plex cytokine assay kit (Bio-Rad, Richmond, CA) following the manufacturer's instructions. The following cytokines were determined in a single assay in three separate experiments: IL-2, IL-6, IL-10, IL-13, IL-17, IFN-γ, MCP-1 and TNF-α.
RNA Isolation and Reverse transcription-Polymerase Chain Reaction
Total RNA was isolated from spleen and brain using the RNeasy mini kit protocol (Qiagen, Valencia, CA, USA) and converted into cDNA using oligo-dT, random hexamers, and Superscript RT II (Invitrogen, Grand Island, NY, USA). Reverse transcription-PCR was performed using TaqMan PCR master mix (Applied Biosystems, Foster City, CA, USA) and primers. Reactions were conducted on the ABI Prism 7000 Sequence Detection System (Applied Biosystems) to detect mRNA quantified as relative units compared with the β-Actin reference gene. Pre-designed Taqman primers for ICAM-1, VCAM-1, IL-1β, IL-2, IL-6, IL-10, IL-17α, TNF-α, dysferlin, Foxp3, CCL20, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, and CCR8 were obtained from Applied Biosystems (Foster City, CA).
Statistical analyses
Statistical differences between groups were determined by Student's t test. A p value ≤ 0.05 was considered significant.
Results
Distribution of cell subsets in spleen, blood and brain of Tg mice
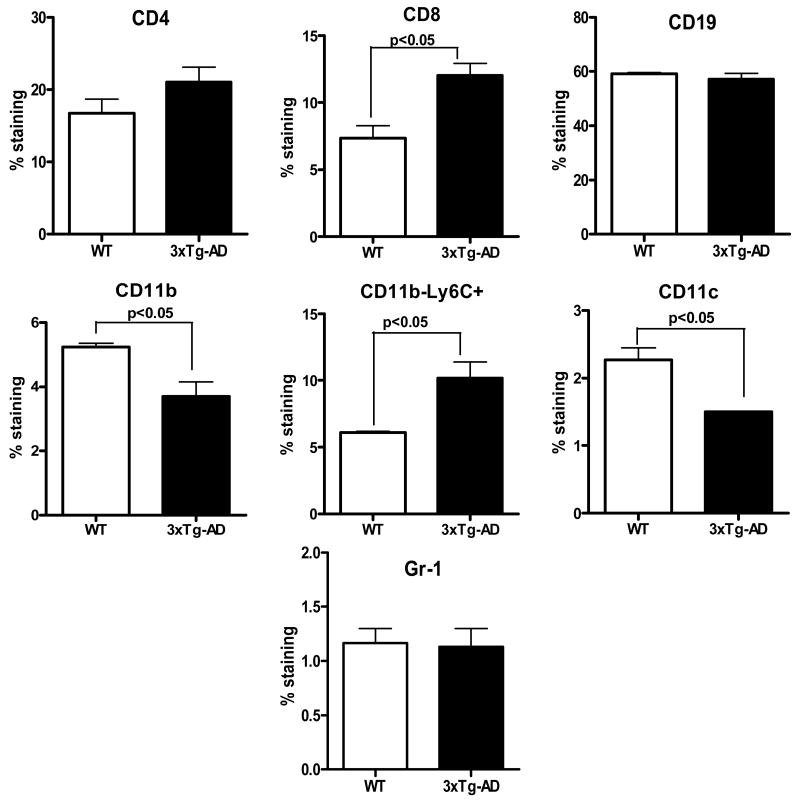
Although brain pathology and associated changes have been extensively studied in the 3xTg-AD mouse model, the impact of these changes on inflammatory processes in the periphery, including the spleen and blood, have been overlooked. The goal of the present study is to address this aspect of the disease. In order to determine if 3xTg-AD mice show an altered cellular profile compared to their WT counterparts, splenocytes from 12-15 month-old female mice were evaluated by FACS staining to identify T and B cells (CD4+, CD8+ and CD19+), macrophages (CD11b+), dendritic cells (DC, CD11c+) and granulocytes (Gr-1+). The 3xTg-AD mice showed a marked increase in CD8+ T cells (Fig. 1) and a small but significant decrease in macrophages and DCs in spleen (Fig. 1). The 3xTg-AD splenocytes were also increased in Ly6C+ CD11b- cells (Fig. 1) that were likely CD8+ T cells expressing this memory marker [13, 14].
Figure 1. Distribution of cell subtypes in spleens from 12-15 month old WT and 3xTg-AD female mice.
Changes in lymphoid and myeloid markers in spleens of 12-15 month old female 3xTg-AD vs. WT mice. Splenic leukocytes isolated from WT and 3xTg-AD female mice were stained for expression of indicated cell specific markers. Data are presented as mean ± SD of 3 mice in each group.
The distribution of cellular subtypes was also evaluated in spleen, blood and brain of younger (5-6 month old) male and female mice. In spleens of the 3xTg-AD mice, there was a significant decrease in CD4+ T cells in both males and females and a decrease in macrophages in female mice similar to that observed in 12 month female 3xTg-AD mice (Fig. 2). Moreover, there was a significant increase in the expression of Ly6C on CD8+ T cells in female and male mice, although total CD8+ cells remained unchanged. Two additional significant changes observed in splenocytes from younger female 3xTg-AD mice were an increase in the chemokine receptor, CCR6, on CD19+ B cells and a decrease in Foxp3+ Treg cells (Fig. 2)
Figure 2. Distribution of cell subsets in spleens of 5-6 month old WT and 3xTg-AD mice.

Changes in lymphoid and myeloid markers in spleens of 5-6 month old male and female 3xTg-AD vs. WT mice. Splenic leukocytes isolated from WT and 3xTg-AD mice were stained for expression of indicated cell specific markers. Data are presented as mean ± SD of one of three replicated experiments involving a total of 7-9 mice.
Blood cells from younger 3xTg-AD mice only partially reflected the cellular distribution pattern observed in spleen. As seen in spleen, there was a significant reduction in blood CD4+ T cells and a significant increase in the expression of Ly6C on CD8+ cells in both females (p<0.001) and males (p<0.01)(Supplemental Fig. 1). However, CCR6 expression on blood B cells was up-regulated only in male mice (along with an overall increase in the percentage of B cells), with only a trend towards increased CCR6 on B cells in females.
In the brains of 5-6 month old 3xTg-AD mice, the most striking change was an increase in the Gr1+ granulocyte population, paralleled by a smaller change in CD11c+ DC observed in females (Fig. 3) but not males (not shown). There was also an increase in the CD45hi CD11b+ cells (non-microglial fraction, not shown) in females which could possibly be accounted for by the change in the granulocytes. No changes were found in T cells or B cells (data not shown).
Figure 3. Distribution of cell subsets in brains of 5-6 month old WT and 3xTg-AD mice.
Brains of 5-6 month old 3xTg-AD mice have higher percentages of Gr-1+ granuloctyes (upper right quadrant) and CD11c dendritic cells (lower panel) than age-matched WT mice. Brains were pooled and mononuclear cells were isolated on a Percoll gradient from WT and 3xTg-AD female and male mice and stained for T cells, B cells, macrophages, microglia, dendritic cells and granulocytes. Data are presented as mean ± SD of 2 experiments, each with pooled brains from 3 WT and 4 3xTg-AD mice.
Cytokine production in peripheral tissues of 3xTg-AD mice
It is of interest to determine if changes in the cellular distribution resulted in an altered cytokine secretion pattern induced in supernatants after stimulation of T cells with plate bound anti-CD3 and CD28 mAbs. In splenocytes from the older 12-15 month-old 3xTg-AD mice, secretion of IL-2 and IL-6 was significantly enhanced (p<0.01) whereas secretion of IL-10 was reduced 3-fold compared to WT mice (p<0.01, Fig. 4A). TNF-α levels were also increased although not significantly (p=0.051) in the older 3xTg-AD mice. Other cytokines tested did not show notable differences between the 2 groups. In younger 3xTg-AD mice, there was a significant increase in secreted IL-6 in anti-CD3/CD28 stimulated splenocytes from both females (Fig. 4B) and males (Fig. 4C) that was also observed in stimulated PBMC (females only)(Supplemental Figs. 2A and 2B). Of note, there was a significant reduction in IL-10 in the younger 3xTg-AD females not seen in males, and a significant increase in IL-13 levels in younger 3xTg-AD males not seen in females.
Figure 4. Cytokine production in spleens of old and young WT and 3xTg-AD mice after 24h stimulation with anti-CD3/CD28 mAb.
Changes in cytokine levels in activated splenocytes from 12-15 month old and 5-6 month old WT and 3xTg-AD mice. Splenocytes from WT and 3xTg-AD mice were cultured in the presence of plate-bound anti-CD3 (5μg) and anti-CD28 (1μg) mAb for 24h and supernatants were assayed for cytokine levels by Luminex assay as described in Materials and Methods. Note a significant increase in IL-6 levels in old and young 3xTg-AD females and in young 3xTg-AD males, decreased IL-10 levels in both old and young 3xTg-AD females, and increased IL-13 levels in young 3xTg-AD males compared to WT controls. Data are presented as mean ± SD of 7-9 mice in each group. ND = not detectable.
Gene expression in 3xTg-AD brain and spleen tissue
To establish an inflammatory AD profile, mRNA levels were assessed in brain and spleen tissue for expression of adhesion molecules (ICAM-1, VCAM-1, dysferlin), cytokines (IL-1β, IL-2, IL-6, IL-10, IL-17α, TNF-α), chemokines and receptors (CCL20, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8), and the Treg marker, Foxp3. As shown in Fig. 5A, brain tissue from older symptomatic 3xTg-AD female mice exhibited highly elevated expression of CCR6 (p<0.001) and VCAM (p<0.01) compared to brain tissue from age-matched WT mice. This pronounced increase in expression of CCR6 (but not VCAM) was also detected in brain tissue from pre-symptomatic 3xTg-AD females (p<0.001, Fig. 5B) and males (p<0.001, Fig. 5C), indicating an ongoing inflammatory process in the CNS that preceded onset of AD-like signs.
Figure 5. Gene expression in brains of old and young WT and 3xTg-AD female and male mice.
Gene expression in brain tissue from old and young WT and 3xTg-AD mice. Brains were collected from 12-15 month old (Old) WT and 3xTg-AD females (A), 5-6 month old (Young) WT and 3xTg-AD females (B) and 5-6 month old (Young) WT and 3xTg-AD males. mRNA was prepared and analyzed by reverse transcription–PCR in triplicate for relative expression (R.E.) of various factors compared with the housekeeping gene, β-actin. Data are presented as mean ±SD of N=3/group for 12-15 month old mice and 3-4/group for younger female and male WT and 3xTg-AD mice
To determine if changes in CCR6 gene expression in the brains of old and young 3xTg-AD mice might also be detected systemically, mRNA levels were assessed in spleens from the same mice. As shown in Figure 6, highly significant increases in splenic CCR6 expression were observed from the 12-15 month old symptomatic females (p<0.001, Fig. 6A) as well as the 5-6 month old pre-symptomatic females (p<0.001, Fig. 6B) and males (p<0.001, Fig. 6C) compared to WT controls. These data indicate that increases in CCR6 occur systemically prior to onset of AD-like symptoms, implicating CCR6 as a possible biomarker for development of AD-like disease. Other changes in gene expression were also detected in spleen but not brain of both older and younger 3xTg-AD mice, including reduced levels of mRNA for CCR5 and Foxp3, and significant or directionally reduced levels of VCAM and ICAM (Fig. 6). Changes in mRNA expression for IL-1β, IL-2, IL-6, IL-10, IL-17α, TNF-α, CCR2, CCR3, CCR4, CCR7, CCR8, CCL20 and dysferlin were not detected (data not shown).
Figure 6. Gene expression in spleens of old and young WT and 3xTg-AD female and male mice.
Gene expression in spleen tissue from old and young WT and 3xTg mice. Spleens were collected from 12-15 month old (Old) WT and 3xTg-AD females (A), 5-6 month old (Young) WT and 3xTg-AD females (B) and 5-6 month old (Young) WT and 3xTg-AD males. mRNA was prepared and analyzed by reverse transcription–PCR in triplicate for relative expression (R.E.) of various factors compared with the housekeeping gene, β-actin. Data are presented as mean ± SD of N=3/group for 12-15 month old mice and 7-9/group for younger female and male WT and 3xTg-AD mice.
Discussion
The results presented above clearly demonstrate: 1) a decline in peripheral macrophages and an increase in brain granulocytes and macrophages/microglia in 3xTg-AD mice that precedes significant brain pathology; 2) an increase in peripheral (but not CNS) CD8+Ly6C memory T cells and IL-6 production which also precedes significant brain pathology; and 3) up-regulation of CCR6 in the brain and spleen of older symptomatic 3xTg-AD mice that was also present in both the brain and spleen of younger pre-symptomatic 3xTg-AD mice. Brain-induced inflammation has been described previously by us and others in CNS injuries such as stroke, and is consistent with a growing body of evidence indicating that the immune system plays an active role in controlling pathologic brain Aβ in AD. The observation that the immune system is mobilized at such an early age in pre-symptomatic mice suggests that immune surveillance for pathologic Aβ may begin well before the appearance of brain pathology. The early immune system changes may serve as a marker of subjects at high risk for AD, perhaps even before cerebral Aβ deposition is evident. The identification of pre-symptomatic markers of AD risk is critical for the development of AD prevention strategies.
CNS injury models such as stroke have been found to have a profound impact on the peripheral immune system [15, 16]. In animal models as well as in patients with AD there is strong evidence suggesting the presence of inflammatory mediators in the brain [17, 18]. Our study demonstrates for the first time that peripheral immunological pathways in the spleen could synergize with neural networks that might determine the outcome in neurodegenerative diseases like AD. Triple transgenic mice develop plaques and tangles with increased accumulation of Aβ [11, 19], with females developing more aggressive Aβ pathology [20]. Younger triple transgenic mice (6 months old) exhibit impaired synaptic transmission and intracellular β-amyloid deposits but not plaques or tau reactivity. In contrast, older mice (12+ months) have progressive β-amyloid deposition in the hippocampus along with extensive tau pathology. Our studies demonstrate that immunological changes in the periphery start early in the triple transgenic mice and persist as the mice age. Our major new finding is the systemic increase in expression of CCR6 (in brain and spleen) found in both 5-6 month old females and males and 12 month old female 3xTg-AD mice. This finding was supported in some instances by increased levels of CD19+CCR6+ B cells in spleen and blood in younger 3xTg-AD mice compared to age-matched WT congenic controls. Other potential disease-associated immunological changes observed consistently in the spleen but not the brain of both younger and older 3xTg-AD female and male mice included reduced expression of CCR5 and Foxp3. Additionally, there were readily apparent gender-specific immunological changes, specifically reduced levels of IL-10 in spleens from younger and older females and an increased level of IL-13 in spleens from younger males. These differences in anti-inflammatory cytokines conceivably could contribute to more severe AD-like disease in female 3xTg-AD mice, and will be further studied.
At present, however, the role of CCR6 in the pathogenesis of AD has not been clearly defined. CCR6 is normally found on immature dendritic cells, B cells, effector/memory T cells and T regulatory cells [21]. Studies have identified CCR6 as contributing to the pathology of inflammatory conditions such as asthma [22] and autoimmune disorders such as rheumatoid arthritis [23, 24]. In brain, CCR6 is up-regulated in hippocampal neurons in 12 month old amyloid precursor protein (APP) transgenic mice [25]. Although CCL20 is the natural ligand for CCR6 [26], we did not detect CCL20 in brains or spleens of older or younger 3xTg-AD mice, even though mRNA for CCR6 was detectable in all mice tested. Although the increased presence of CCR6 in the periphery may serve as an early biomarker for detecting risk of AD, additional studies are needed to implicate this chemokine receptor in an AD or an AD-like pathogenic mechanism.
We also found a reduction in cells of the myeloid lineage in spleens of both older and younger transgenic mice. In further analyzing this lineage, we found that CD11c+ DC were markedly less abundant in 3xTg-AD mice, with no changes in the granulocyte population compared to their WT counterparts. It is likely that this decrease in spleen indicates re-direction of these cells to the site of inflammation i.e. CNS, where we observed elevated levels of activated macrophages, DC and granulocytes. Data from AD patients as well as transgenic models show evidence of accumulation of activated microglia at sites of β-amyloid deposition [27-29], leading to the production of pro-inflammatory cytokines like IL-6, TNF-α and IL-1β [3, 4] that likely contribute to AD–associated pathology. In addition, improvement in cognitive function has been associated with a reduction in the number of microglia [30, 31]. Further examination of this cell population revealed that granulocytes comprised a majority of the CD45hi CD11b+ cell fraction. These cells are among the first to arrive at the site of injury following ischemic insult [32] and lack of CD11b reduces neutrophil infiltration and decreases infarction volume [33].
Several reports have documented the presence of T cells in Alzheimer's lesions [34, 35] with elevated levels of memory CD8+ T cells being detected in blood of AD patients as well as mouse models of AD [36]. There is a strong correlation between age and levels of memory T cells. Older mice demonstrate higher levels of memory CD8+ T cell that produce soluble mediators independent of antigen and can have characteristics that are different from their younger counterparts [37, 38]. These cells can also impede immune responses in the aged host upon antigenic challenge [39]. In this study, we report an appreciable increase in the memory CD8+ T cell pool in both younger and older triple transgenic mice. Our findings in spleen corroborate the changes identified in blood by others [36]. Memory T cells exhibit stronger survival and respond robustly to recall responses without the need for strong co-stimulation [40]. Up-regulation of Ly6C on CD8 cells in the lymphoid organs is also suggestive of its homing status and adherence to endothelial cells [41, 42]. A surprising finding was the consistently reduced expression of Foxp3 in spleens from both young and old 3xTg-AD mice in concert with a reduced percentage of splenic CD4+FoxP3+ Treg cells in younger female triple transgenic mice. Regulatory T cells mediate immune tolerance and suppress clinical signs of autoimmune disease [43, 44], and it is conceivable that the observed reduction in Foxp3 expression might indicate reduced regulatory activity that could contribute to enhanced systemic inflammation. Further investigation of the possible role of Foxp3+Treg cells in controlling inflammatory responses in 3xTg-AD mice is currently in progress.
IL-6 and TNF-α have been well documented as driving a pro-inflammatory response in several disease conditions including Alzheimer's [17, 45, 46]. Elevated levels of IL-6 across the tested age groups in both blood and spleen suggest a strong mechanistic role for this cytokine in the disease pathology. Rosenberg et al suggest that IL-6 levels in plasma can be used as a predictor of AD severity and neuropsychiatric outcomes [47]. Furthermore, release of IL-6 by activated microglia [48] up-regulates adhesion molecule expression in an inflammatory milieu [49], perhaps accounting for the observed increased expression of VCAM and ICAM in older and younger 3xTg-AD females, respectively. Our consistent detection of increased IL-6 levels in younger and older 3xTg-AD mice without concomitant changes in TNF-α levels provides direction for future studies. Vaccination therapy using Aβ-specific T cells improves cognitive performance together with decreases in peripheral levels of inflammatory cytokines [30] implicating these soluble factors in the AD pathology. It was further identified that Th2 but not Th1 β-amyloid specific cells were capable of reversing cognitive decline [31], and our observation that both younger and older 3xTg-AD female mice have reduced splenic IL-10 levels may be a reflection of a Th1 dominated inflammatory response.
In conclusion, our studies demonstrate significant changes in cellular distribution and expression of inflammatory factors, notably CCR6, in the peripheral immune system and brain of pre-symptomatic AD-prone mice that precede similar changes in symptomatic AD-like mice. The cellularity and cytokine milieu of the spleen has a profound impact on outcomes of neurological disorders and understanding these mechanisms might carve the path to more effective and targeted therapies. Moreover, identifying markers such as CCR6 for early detection in asymptomatic individuals might allow for early intervention strategies and more favorable outcomes for subjects developing AD.
Supplementary Material
Supplementary Figure 1. Distribution of cell subsets among PBMCs in 5-6 month old female and male 3xTg-AD vs. WT mice. Blood mononuclear cells isolated from individual WT and 3xTg-AD mice were stained for T cells, B cells, macrophages, dendritic cells and granulocytes as well as for expression of CCR6 and Ly6C markers. Data are presented as mean ± SD with n=8-9 mice/group.
Supplementary Figure 2. Changes in cytokine levels in activated PBMC from 5-6 month old WT and 3xTg-AD female and male mice. PBMC from WT and 3xTg-AD mice were cultured in the presence of plate-bound anti-CD3 (5μg) and anti-CD28 (1μg) mAb for 24h and supernatants were assayed for cytokine levels by Luminex assay as described in Materials and Methods. Note increases in IL-6 and IFN-γ levels and a decrease in IL-2 levels in young 3xTg-AD females but not males compared to WT controls. Data are presented as mean ± SD with n=7-8 mice/group. ND = not detectable.
Acknowledgments
This work was supported in part by a Department of Veterans' Affairs Merit Review Grant (Joseph Quinn); National Institutes of Health Grants NS45445 (Halina Offner) and NS47661 (Arthur A. Vandenbark); and The Biomedical Laboratory R&D Service, Department of Veterans Affairs. The authors wish to thank Ms. Eva K. Niehaus for assistance in manuscript preparation.
References
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Mechanisms of disease: Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Meda L, Baron P, Prat E, Scarpini E, Scarlato G, Cassatella MA, Rossi F. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid[25-35] J Neuroimmunol. 1999;93:45–52. doi: 10.1016/s0165-5728(98)00188-x. [DOI] [PubMed] [Google Scholar]
- 4.Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation. 2005;2:23–28. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Liu L, Barger SW, Griffin WST. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M, Lorton D, Kuo YM, Roher AE. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood--brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- 8.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nature Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 9.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to β-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37–50. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Bebo BF, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139-151. J Neurosci Res. 1996;45:680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Walunas T, Bruce DS, Dustin L, Lob DY, Bluestone JA. Ly-6C is a marker of memory CD8+ T cells. J Immunol. 1995;155:1873–1883. [PubMed] [Google Scholar]
- 14.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn P. Splenic atrophy in experimental stroke is accompanied by increased Treg cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 16.Ajmo CT, Jr, Vernon DOL, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging. 2001;22:837–884. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Fonseca MI, Kayed R, Hernandez I, Webster SD, Yazan O, Cribbs DH, Glabe CG, Tenner AJ. Novel Aβ peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease. J Neuroinflammation. 2005;2:28–46. doi: 10.1186/1742-2094-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vale C, Alonso E, Rubiolo JA, Vieytes MR, LaFerla FM, Giménez-Llort L, Botana LM. Profile for amyloid-β and tau expression in primary cortical cultures from 3xTg-AD mice. Cell Mol Neurobiol. 2009 doi: 10.1007/s10571-009-9482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Fang H, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 21.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3α. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- 22.Lundy SK, Lira SA, Smit JJ, Cook DN, Berlin AA, Lukacs NW. Attenuation of allergen-induced responses in CCR6-/- mice is dependent upon altered pulmonary T lymphocyte activation. J Immunol. 2005;174:2054–2060. doi: 10.4049/jimmunol.174.4.2054. [DOI] [PubMed] [Google Scholar]
- 23.Ruth JH, Shahrara S, Park CC, Morel JCM, Kumar P, Qin S, Koch AE. Role of macrophage inflammatory protein-3α and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 24.Varona R, Cadenas V, Gomez L, Martinez C, Marquez G. CCR6 regulates CD4 T-cell-mediated acute graft-versus host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- 25.Jee SW, Cho JS, Kim CK, Hwang DY, Shim SB, Lee SH, Sin JS, Park JH, Kim YS, Choi SY, Kim YK. Oligonucleotide-based analysis of differentially expressed genes in hippocampus of transgenic mice expressing NSE-controlled APPsw. Neurochem Res. 2006;31:1035–1044. doi: 10.1007/s11064-006-9117-8. [DOI] [PubMed] [Google Scholar]
- 26.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol. 2010;40:1042–1052. doi: 10.1002/eji.200939778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGeer EG, McGeer PL. Abeta immunotherapy and other means to remove amyloid. Curr Drug Targets CNS Neurol Disord. 2005;4:569–573. doi: 10.2174/156800705774322067. [DOI] [PubMed] [Google Scholar]
- 28.Barger SW. Vascular consequences of passive Abeta immunization for Alzheimer's disease. Is avoidance of “malactivation” of microglia enough? J Neuroinflammation. 2005;2:2–5. doi: 10.1186/1742-2094-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab C, Klegeris A, McGeer PL. Inflammation in transgenic mouse models of neurodegenerative disorders. Biochem et Biophys Acta. 2009 Oct 31; doi: 10.1016/j.bbadis.2009.10.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Ethell DW, Shippy D, Cao C, Cracchiolo JR, Runfeldt M, Blake B, Arendash GW. Abeta-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer's mice. Neurobiol Dis. 2006;23:351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW. Abeta-specific Th2 cells provide cognitive and pathological benefits to Alzheimer's mice without infiltrating the CNS. Neurobiol Dis. 2009;34:63–70. doi: 10.1016/j.nbd.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark RK, Lee EV, White RF, Jonak ZL, Feuerstein GZ, Barone FC. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Res Bulletin. 1994;35:387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 33.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 34.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 35.Schindowski K, Peters J, Gorriz C, Schramm U, Weinandi T, Leutner S, Maurer K, Frölich L, Müller WE, Eckert A. Apoptosis of CD4+ T and natural killer cells in Alzheimer's disease. Pharmacopsychiatry. 2006;39:220–228. doi: 10.1055/s-2006-954591. [DOI] [PubMed] [Google Scholar]
- 36.Schindowski K, Eckert A, Peters J, Gorriz C, Schramm U, Weinandi T, Maurer K, Frölich L, Müller WE. Increased T-cell reactivity and elevated levels of CD8+ memory T-cells in Alzheimer's disease-patients and T-cell hyporeactivity in an Alzheimer's disease-mouse model: implications for immunotherapy. Neuromolecular Med. 2007;9:340–354. doi: 10.1007/s12017-007-8015-9. [DOI] [PubMed] [Google Scholar]
- 37.Vesosky B, Flaherty DK, Rottinghaus EK, Beamer GL, Turner J. Age dependent increase in early resistance of mice to Mycobacterium tuberculosis is associated with an increase in CD8 T cells that are capable of antigen independent IFN-gamma production. Exp Gerontol. 2006;41:1185–1194. doi: 10.1016/j.exger.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci USA. 2008;105:12997–3002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 40.Rocha B, Tanchot C. The Tower of Babel of CD8+ T-cell memory: known facts, deserted roads, muddy waters, and possible dead ends. Immunol Rev. 2006;211:182–196. doi: 10.1111/j.0105-2896.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 41.Jaakkola I, Merinen M, Jalkanen S, Hanninen A. Ly6C induces clustering of LFA-1 (CD11a/CD18) and is involved in subtype-specific adhesion of CD8 T cells. J Immunol. 2003;170:1283–1290. doi: 10.4049/jimmunol.170.3.1283. [DOI] [PubMed] [Google Scholar]
- 42.Hanninen A, Jaakkola I, Salmi M, Simell O, Jalkannen S. Ly-6C regulates endothelial adhesion and homing of CD8+ T cells by activating integrin-dependent adhesion pathways. Proc Natl Acad Sci USA. 1997;94:6898–6903. doi: 10.1073/pnas.94.13.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, Ehrenstein MR, Strauss HJ. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donia M, Mangano K, Amoroso A, Mazzarino MC, Imbesi R, Castrogiovanni P, Coco M, Meroni PL, Nicoletti F. Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmunity. 2009;33:135–140. doi: 10.1016/j.jaut.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LME. Increased plasma levels of interleukin-1, interleukin-6 and α-1 antichymotrypsin in patients with Alzheimer's disease: Peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 46.Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-α expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg PB, Carroll KAL, Cheng J, Allie R, Lyketsos CG, Calabresi P, Kaplin A. IL6 release by LPS-stimulated peripheral blood mononuclear cells as a potential biomarker in Alzheimer's disease. Int Psychogeriatr. 2009;21:413–414. doi: 10.1017/S1041610208008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 49.Ciramella A, Bizzioni F, Salani F, Vanni D, Spalletta G, Sanarico N, Vendetti S, Caltagirone C, Bossu P. Increased pro-inflammatory response by dendritic cells from patients with Alzheimer's disease. J Alz Dis. 2010;19:559–572. doi: 10.3233/JAD-2010-1257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Distribution of cell subsets among PBMCs in 5-6 month old female and male 3xTg-AD vs. WT mice. Blood mononuclear cells isolated from individual WT and 3xTg-AD mice were stained for T cells, B cells, macrophages, dendritic cells and granulocytes as well as for expression of CCR6 and Ly6C markers. Data are presented as mean ± SD with n=8-9 mice/group.
Supplementary Figure 2. Changes in cytokine levels in activated PBMC from 5-6 month old WT and 3xTg-AD female and male mice. PBMC from WT and 3xTg-AD mice were cultured in the presence of plate-bound anti-CD3 (5μg) and anti-CD28 (1μg) mAb for 24h and supernatants were assayed for cytokine levels by Luminex assay as described in Materials and Methods. Note increases in IL-6 and IFN-γ levels and a decrease in IL-2 levels in young 3xTg-AD females but not males compared to WT controls. Data are presented as mean ± SD with n=7-8 mice/group. ND = not detectable.