Abstract
Myotonic dystrophy is a multisystemic disorder characterized by repeat expansion mutations of the dystrophia myotonica protein kinase (DMPK) gene resulting in a defective muscular insulin receptor and insulin resistance. We describe a patient with myotonic dystrophy who developed biopsy-proven non-alcoholic steatohepatitis. We suggest that patients with myotonic dystrophy are at risk of developing steatohepatitis. The relationship between defective insulin receptor and development of steatohepatitis should be further investigated.
Key Words: Non-alcoholic steatohepatitis, Myotonic dystrophy, DMPK gene mutation, Insulin resistance
Introduction
Myotonic dystrophy in a complex multisystemic autosomal dominant disorder characterized by two different mutations resulting in an unstable CTG repeat expansion in the 3(untranslated region of the dystrophia myotonica protein kinase (DMPK) gene on chromosome 19 (myotonic dystrophy type 1) and a CCTG repeat expansion in intron 1 on zinc finger protein 9 on chromosome 3 (myotonic dystrophy type 2) [1,2]. It is the most common form of muscular dystrophy with an incidence of 1:8,000 worldwide [3]. Symptoms are multisystemic and include muscle hyperexcitability, progressive muscle weakness, cataract development, testicular atrophy and cardiac arrhythmias [4]. Insulin resistance in myotonic dystrophy correlates with a failure to express the insulin receptor splice variant that normally predominates in adult skeletal muscle, leading to the expression of an insulin receptor variant with a lower signaling capability. Muscles of myotonic dystrophy 1 patients contain lower than normal levels of insulin receptor RNA and protein. This results in reduced responsiveness of muscle to insulin compared to normal individuals [5,6], confirming previous studies which suggested that metabolic abnormalities which may lead to glucose intolerance were related to insulin response defects [7]. Hepatic dysfunction in patients with myotonic dystrophy is common and includes chronic liver disease and gallbladder dismotility [8]. One study showed that an approximate 66% of patients with myotonic dystrophy 1 had at least one abnormality of liver enzymes [9].
In this article we describe the case of a patient with myotonic dystrophy and non-alcoholic steatohepatitis (NASH) and discuss the possible links between the myotonic dystrophy genetic defect and development of steatohepatitis.
Case Report
The patient was a 39-year-old male with a history of myotonic dystrophy who was being evaluated for abnormal liver function tests. He denied alcohol or tobacco use. Family history was negative for liver disease. He had a medical history of hypertension that was well controlled on Diovan. His BMI was 38.
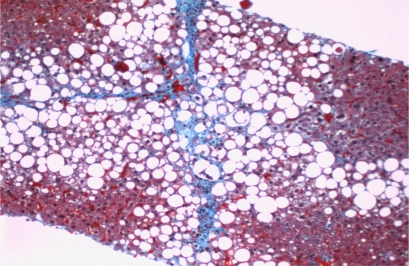
At physical examination the liver margin was felt at deep inspiration and was smooth. The spleen was not enlarged and there was no ascites. He had no stigmata of chronic liver disease. Abnormal laboratory values included total bilirubin 2.5 mg/dl (normal 0.2-1.2), indirect bilirubin 2.1 mg/dl, AST 71 U/l (normal 10-40), ALT 96 U/l (normal 9-60), gamma-GT 126 U/l (normal 3-90). Viral hepatitis serologies and autoimmune panel were negative. Glycemic control was normal. MRI of the abdomen showed moderate fatty infiltration of the liver. The gallbladder and biliary tree were unremarkable. A liver biopsy was performed which showed a moderate degree of macrovescicular steatosis with foci of hepatocellular inflammation suggestive of steatohepatitis. There was pericentral and perisinusoidal fibrosis with occasional septal extension (fig. 1).
Fig. 1.
Core needle biopsy of the liver. Findings include a moderate degree of macrovesicular steatosis with foci of hepatocellular inflammation. Trichrome stain demonstrates increased sinusoidal and pericentral fibrosis with occasional septal fibrosis.
Discussion
We suggest that patients with myotonic dystrophy are at increased risk of developing hepatocytic inflammation/NASH through the same pathways that are seen in patients with metabolic syndrome, i.e. abnormalities in insulin receptor and glucose intolerance leading to elevated free fatty acid oxidation and reactive oxygen species. These abnormal metabolic pathways promote hepatocyte inflammation which in turn can increase the risk of development of hepatic cirrhosis and carcinoma. NASH, a common cause of cryptogenic cirrhosis and hepatocellular carcinoma, is characterized by fatty infiltration of the liver, inflammation, hepatocellular damage and fibrosis. It is seen in a subset of patients with non-alcoholic fatty liver disease (NAFLD) which is a well-known hepatic manifestation seen in patients with the metabolic syndrome (which includes insulin resistance, hypertriglyceridemia, hypertension and obesity) [10].
The etiology of elevated liver tests in patients with myotonic dystrophy has been suggested to arise from two possible mechanisms: (1) elevated AST from skeletal muscle breakdown as correlated with creatine kinase elevations and (2) biliary contraction/bile excretion abnormalities. These laboratory abnormalities in patients with myotonic dystrophy type 1 include changes in serum creatine kinase, AST/ALT and fasting blood glucose [11]. Increase in ALT, alkaline phosphatase and gamma-GT activity has been reported to be specific of liver involvement in patients with myotonic dystrophy [12]. However the link between myotonic dystrophy and liver disease remains controversial. Biochemical abnormalities seen in myotonic dystrophy patients have been described to be mainly secondary to muscular damage and falsely attributed to the liver, leading to costly and unnecessary liver investigations [13,14]. Some have suggested alterations in the contractility of bile canaliculi and bile ductules that may lead to incomplete biliary obstruction and impaired bile excretion as an etiology [9].
A recent study showed decreased insulin receptor RNA and protein not only in skeletal muscle but also in the liver (up to an 80% decrease) [15]. We suggest that the resulting insulin resistance in hepatocytes and other tissues leads to hepatocyte damage similar to that seen in NASH. The mechanism of damage in NASH/NAFLD has been related to insulin resistance even in the absence of obesity or diabetes. Insulin resistance leads to an increase in circulating free fatty acids because of the decreased antilipolytic effects of insulin in adipose tissue. Also, mitochondrial dysfunction secondary to insulin resistance leads to increased formation of reactive oxygen species in hepatocytes leading to inflammation. In vitro studies have identified a CUG-binding protein that binds to the anomalous triplet expansions in myotonic dystrophy 1 [16,17]. CUG-binding protein activity is increased as a result of this interaction in myotonic dystrophy 1 which leads to aberrant splicing defects in chloride channel 1 and insulin receptor RNA resulting in myotonia and insulin receptor defects [5, 18, 19].
References
- 1.Brook JD, McCurrach ME, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3? end of a transcript encoding a protein kinase family member. Cell. 1992;69:385. doi: 10.1016/0092-8674(92)90418-c. [DOI] [PubMed] [Google Scholar]
- 2.Liquori CL, Ricker K, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 3.Harper PS. Myotonic Dystrophy. ed 2. WB Saunders; 1989. [Google Scholar]
- 4.Harper PS, Reardon W. Heart disease in myotonic dystrophy. Lancet. 1992;339:939. doi: 10.1016/0140-6736(92)90989-g. [DOI] [PubMed] [Google Scholar]
- 5.Savkur RS, Philips AV, et al. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29:40–47. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 6.Morrone A, Pegoraro E, et al. RNA metabolism in myotonic dystrophy: patient muscle shows decreased insulin receptor RNA and protein consistent with abnormal insulin resistance. J Clin Invest. 1997;99:1691–1698. doi: 10.1172/JCI119332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moxley RT, Corbett AJ, et al. Whole body insulin resistance in myotonic dystrophy. Ann Neurol. 1984;15:157–162. doi: 10.1002/ana.410150208. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Bedossa P, et al. Perisinusoidal cells (Ito-cells) enlargement in a family with myotonic dystrophy. Liver. 1989;9:276–278. doi: 10.1111/j.1600-0676.1989.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 9.Achiron A, Barak Y, et al. Abnormal liver test results in myotonic dystrophy. J Clin Gastroenterol. 1998;26:292–295. doi: 10.1097/00004836-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Bugianesi E, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 11.Heatwole CR, Miller JR, et al. Laboratory abnormalities in ambulatory patients with myotonic dystrophy type 1. Arch Neurol. 2006;63:1149–1153. doi: 10.1001/archneur.63.8.1149. [DOI] [PubMed] [Google Scholar]
- 12.Rönnemaa T, Alaranta H, et al. Increased activity of serum gamma-glutamyltransferase in myotonic dystrophy. Acta Med Scand. 1987;222:267–273. doi: 10.1111/j.0954-6820.1987.tb10669.x. [DOI] [PubMed] [Google Scholar]
- 13.Zamora S, Adams C, et al. Elevated aminotransferase activity as an indication of muscular dystrophy: case reports and review of the literature. Can J Gastroenterol. 1996;10:389–393. doi: 10.1155/1996/213209. [DOI] [PubMed] [Google Scholar]
- 14.Kamath BM, Dhawan A, Mieli-Vergani G. Raised serum transaminases: not always liver disease. Arch Dis Child. 2000;82:270–271. doi: 10.1136/adc.82.3.266l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guiraud-Dogan C, Huguet A, et al. DM1 CTG expansions affect insulin receptor isoforms expression in various tissues of transgenic mice. Biochim Biophys Acta. 2007;1772:1183–1191. doi: 10.1016/j.bbadis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Timchenko LT, Timchenko NA, et al. Novel proteins with binding specificity for DNA CTG repeats and RNA CUG repeats: implications for myotonic dystrophy. Hum Mol Genet. 1996;5:115–121. doi: 10.1093/hmg/5.1.115. [DOI] [PubMed] [Google Scholar]
- 17.Ladd AN, Charlet N, et al. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol. 2001;21:1285–1296. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timchenko NA, Cai ZJ, et al. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem. 2001;276:7820–7826. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 19.Charlet BN, Savkur RS, et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]