Abstract
Background
Adolescent rats are less sensitive to the motor-impairing effects of ethanol than adults. However, the cellular and molecular mechanisms underlying this age dependent effect of ethanol have yet to be fully elucidated.
Method
Male rats of various ages were used to investigate ethanol-induced ataxia and its underlying cellular correlates. In addition, Purkinje neurons from adolescent and adult rats were recorded both in vivo and in vitro. Finally, PKCγ expression was determine in three brain regions in both adolescent and adult rats.
Results
The present multi-methodological investigation confirms that adolescents are less sensitive to the motor impairing effects of ethanol, and this differential effect is not due to differential blood ethanol levels. In addition, we identify a particular cellular correlate that may underlie the reduced motor impairment. Specifically, the in vivo firing rate of cerebellar Purkinje neurons recorded from adolescent rats are insensitive to an acute ethanol challenge, while the firing rate of adult cerebellar Purkinje neurons are significantly depressed. Finally, it is demonstrated that PKCγ expression in the cortex and cerebellum mirrors the age-dependent effect of ethanol: adolescents have significantly less PKCγ expression compared to adults.
Conclusions
Adolescents are less sensitive than adults to the motor-impairing effects of ethanol, and a similar effect is seen with in vivo electrophysiological recordings of cerebellar Purkinje neurons. While still under investigation, PKCγ expression mirrors the age effect of ethanol and may contribute to the age-dependent differences in the ataxic effects of ethanol.
Keywords: ethanol, adolescence, ataxia, PKC gamma, cerebellum, Purkinje
INTRODUCTION
Reduced initial sensitivity to ethanol and acute tolerance to ethanol exposure are risk factors for the development of alcoholism (Schuckit, 1994). Compared to adults, adolescents are less sensitive to the motor-impairing effects of alcohol, which serve as cues for moderating alcohol intake (Hefner and Holmes, 2007; Lisenbardt, 2009; Little et al., 1996; Pian et al., 2008; Ristuccia and Spear, 2008; Silveri and Spear, 2001; Spear and Varlinskaya, 2005; Varlinskaya and Spear, 2002; White et al., 2002 for review). Such reduced sensitivity is troublesome considering that binge and heavy alcohol consumption increases throughout human adolescence and peaks at 21-25 years of age (National Survey on Drug Use and Health 2007). Therefore understanding the mechanisms that underlie the reduced sensitivity to alcohol during adolescence is critical.
Adolescence is a unique period of altered sensitivity to many of ethanol's effects. Not only are adolescents less sensitive to ethanol-induced ataxia and sedation compared to adults, they are also less sensitive to the anxiolytic effects (Varlinskaya and Spear, 2002) and the hypothermic effects of low-dose ethanol (Ristuccia and Spear, 2004; Swartzwelder et al., 1998). Conversely, adolescents are more sensitive than adults to hypothermia at higher doses (Brasser and Spear, 2002; Ristuccia and Spear, 2005; 2008). Adolescents will also self-administer ethanol in greater quantities than adults (Doremus et al., 2005; Walker et al., 2008), consuming 2-3 times more ethanol relative to body weight (Lancaster et al., 1996). Hippocampal memory impairments have been shown to be more pronounced in adolescents (Markwiese et al., 1998); however, more recent studies have found no differences in age-related sensitivity to these memory impairments (Chin et al., 2010; Rajendran and Spear, 2004).
Although some of the age-dependent effects of ethanol are controversial, there is universal agreement on the relative insensitivity of adolescents to the motor-impairing effects of ethanol; however, the mechanisms responsible for this difference have yet to be fully elucidated. The cerebellum plays a critical role in the coordination of movement, although its regulatory effect on behavioral affect and many different aspects of cognition should not be overlooked (Baillieux et al., 2008). Since ethanol produces a wide array of effects, including alterations of coordination, speech, and cognitive function, the cerebellum is an important target of investigation. Purkinje neurons, which are GABAergic, constitute the sole output of the cerebellar cortex, are involved in motor function (Dow and Moruzzi, 1958) and genetic deletions of Purkinje neurons (for example theLurcher mouse) can produce motor impairments (Vogel et al., 2007 for review). Ethanol has been shown to increase spontaneous GABA release from the presynaptic terminal onto Purkinje neurons (Hirono et al., 2009; Kelm et al., 2010; Mameli et al., 2008) to increase the frequency of miniature inhibitory synaptic currents (mIPSCs), as well as spontaneous inhibitory synaptic currents (sIPSCs) in these neurons (Mameli et al., 2008). Specific modulation of fast GABAergic transmission onto Purkinje neurons, using engineered GABAA receptors, translates directly to impairment on a complex motor task (Wulff et al., 2007). Therefore ethanol-mediated inhibition of Purkinje neurons through GABAergic mechanisms is likely to contribute to some of the motor impairments produced by ethanol consumption.
PKC phosphorylates the β; and γ2 subunits of GABAA receptors to modulate receptor sensitivity to ethanol (Kellenberger et al., 1992; Krishek et al., 1994; Qi et al., 2007; Wafford et al., 1991; for review see Song and Messing, 2005), with PKCγ enhancing the effect of ethanol on GABAA receptors (Kumar et al., 2005; Song and Messing, 2005). Mice lacking the γ isoform of protein kinase C (PKCγ) exhibit reduced hypnotic and anxiolytic sensitivity in response to ethanol compared to wild-type littermate controls (Bowers et al., 1999; Bowers et al., 2001; Harris et al., 1995). Interestingly, PKCγ knock-out mice are affected by ethanol in ways that are strikingly similar to adolescent rats: PKCγ knock-outs show an enhanced preference for ethanol compared to wild-type littermates (Bowers and Wehner, 2001) and show reduced hypnotic and anxiolytic sensitivity to ethanol (Bowers et al., 1999; Bowers et al., 2001; Harris et al., 1995). Given the similarity between ethanol responsiveness in adolescent rats and PKCγ knockout mice, the current work investigates if expression of PKCγ varies in accordance with the age-dependent effects of ethanol on motor behavior and cerebellar neurophysiology. It is hypothesized that when compared to adults, adolescents will have an attenuated behavioral response to ethanol, altered cerebellar Purkinje activity in response to ethanol and will also have lower PKCγ peptide expression in the cerebellum.
METHODS
Subjects
All studies used male Sprague-Dawley rats (16 periadolescent (Postnatal Day (PD) 28), 55 adolescent (PD 30-43), 54 adult (PD 58-120), and 16 aged (~19 months); see individual studies for more exact ages) obtained from Harlan (Indianapolis, IN). All animals were group housed in an IACUC-approved animal colony at the University of Memphis, Baylor University, or University of New Mexico. All animals had ad libidum access to food and water.
Open Field Testing
Adolescent (Postnatal day (PD) 30-38, n=9) and adult (PD 60-70, n=9) rats were run individually in squads of 6 (3 adolescent and 3 adult; one per box) in Med Associates ENV-520 Activity Monitor boxes. Animals were weighed and moved from the housing room to the testing room 10 minutes prior to the procedure. Animals were then given a six-minute baseline to explore the open field and total distance travelled was recorded via computer. Immediately following the baseline period, animals were removed from the chamber, administered 1.5 g/kg of 10% w/v ethanol i.p., and placed back into the recording chamber for an additional 30 minutes. Following completion of the test, animals were returned to their home cage, the apparatus was cleaned, and the next squad of animals was tested.
Data Analysis
A two-way analysis of variance (Age [adolescent, adult] × Time [baseline, test]) was performed.
Aerial Righting Reflex Test
Periadolescent (PD 28, n = 8), adolescent (PD 43, n = 8), adult (PD ~120, n = 8), and aged (PD ~19 months, n = 8) rats were used to determine the effect of age on ethanol-induced ataxia using an aerial righting reflex (ARR) task as previously described (VanDoren et al., 2000). ARR was determined immediately prior to a 2.0 g/kg i.p. 10% w/v ethanol administration, as well as 10, 20, 30, and 40 minutes post-administration. An animal's righting reflex was considered intact if, twice from the same height, it successfully rotated from a supine position onto a 10 inch foam pad. Animals were initially released 5 inches (12.7 cm) above the pad; if righting was not intact, the height was raised in 5 inch increments up to a maximum release height of 25 inches.
Data Analysis
Data were analyzed with a two-way analysis of variance (Age [periadolescent, adolescent, adult, aged] × Time [baseline, 10 min, 20 min, 30 min, 40 min post ethanol injection), with Bonferroni post hoc t-tests where appropriate to discern the nature of the interaction.
Electrophysiological Studies
In vivo electrophysiology
Neurons were recorded as previously described (Matthews et al., 2000; Tokanaga et al., 2003; 2006; VanDoren et al., 2000). Briefly, adolescent (PD 30-38, n = 10) and adult (PD > 60, n = 9) rats were anesthetized with urethane (approximately 1.5 g/kg, i.p.) and placed in a stereotaxic frame with flat skull orientation. Urethane was used as an anesthetic because previous research has indicated urethane does not interact with ethanol to alter neural firing rates (Givens and Breese, 1990), and also to be consistent with previous research investigating the effects of ethanol on cerebellar Purkinje neurons (Yang et al., 2000; 1999). An incision was made in the skin, the skull surface was cleaned, and a burr hole was drilled through the skull over the cerebellum. The center of the hole was roughly 1.5 mm posterior Lambda. Single-barrel glass micropipettes (A-M Systems, Carlsborg, WA) were pulled (using Model PE-2; Narishige, Tokyo, Japan), and the tip was broken back to ~1.0 mm and filled with a 0.9 M NaCl solution saturated with Chicago sky-blue dye (Sigma Chemical). The electrode was lowered into the cerebellum via a hydraulic microdrive (Trent Wells, South Gate, CA). Extracellular action potentials were amplified, filtered (400HZ and 8kHZ; Fintronics, Orange, CT), and monitored with a Tektronix oscilloscope and audiomonitor. Neurons were classified as cerebellar Purkinje neurons based on previously published studies (Yang et al., 1998). Individual action potentials were isolated from background activity with at least 3:1 signal-to-noise ratio.
Following the isolation of a cerebellar Purkinje neuron, a 6-minute baseline of spontaneous neural activity was collected prior to an i.p. injection of 1.5 g/kg ethanol. An additional 30 minutes of spontaneous neural activity was then recorded. Neural firing rates were summed over 10 second bins, averaged over 6 minute intervals and recorded on computer for later analysis. The location of the electrode was micromanipulated during the recording to prevent waveform alterations. No more than one neuron was recorded per animal.
Data Analysis
A preliminary t-test was conducted to determine if age affected the baseline firing rates of the Purkinje neurons. Given that no statistical difference was found (see result section), data was analyzed by a two-way analysis of variance (Age [adolescent or adult] × Time [6, 12, 18, 24, 30 min post ethanol injection]) to discern the effect of age and ethanol on Purkinje neuron firing rates.
In vitro electrophysiology
Brain slice preparation
Experiments were performed in parasagittal vermis cerebellar slices that were prepared from adolescent (PD 30, n = 8) and adult (PD 58-60, n = 8) rats. Animals were euthanized by rapid decapitation under deep anesthesia with ketamine (250 mg/kg i.p.) and 200 μm thick slices were prepared with a vibratome (Technical Products International, St. Louis, MO). Slices were cut in cold solution containing (in mM) 220 sucrose, 26 NaHCO3, 10 glucose, 6 MgSO4, 2 KCl, 1.25 NaH2PO4, 0.2 CaCl2 and 0.43 ketamine; this solution was pre-equilibrated with 95% O2 plus 5% CO2. Immediately after this procedure, slices were transferred to a chamber containing artificial cerebrospinal fluid (ACSF) and allowed to recover at 35-36°C for 35 min, followed by storage at room temperature. ACSF contained (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose equilibrated with 95% O2 plus 5% CO2. After storage for 1.5-9 hrs, slices were transferred to a recording chamber perfused with ACSF at a rate of 2-3 ml/min and maintained at 32-33°C.
Loose-seal Cell-attached Patch-Clamp Recordings
Neurons were visualized using infrared-differential interference contrast microscopy and recordings performed with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Purkinje neurons were primarily identified on the basis of their location in the Purkinje cell layer, large size, and the presence of spontaneous action potential firing. Patch pipettes had resistances of 2-5 MΩ. Each slice was exposed once to a single ethanol concentration and the duration of ethanol exposure was limited to 5 min to avoid the development of rapid tolerance.
The loose-patch cell-attached configuration (seal resistance = 8–30 MΩ) was used to record action currents. The patch pipettes were filled with regular ACSF and the holding potential was 0 mV. Action currents were detected by the presence of a downward and upward deflection in the current trace.
Data Analyses
Data were filtered at 2 kHz and digitized at 5-50 kHz with 1322A pClamp-9 (Molecular Devices, Sunnyvale, CA) and analyzed with Clampfit-9 (Molecular Devices) and MiniAnalysis-6.0.3. (Synaptosoft, Decatur, GA). Effects of ethanol were calculated with respect to the average of control and washout responses. Data were statistically analyzed with Prizm 4 (GraphPad, San Diego, CA) and are presented as mean ± SEM.
Blood ethanol determination
Preadolescent (PD 28, n = 8), adolescent (PD 43, n = 8), adult (PD ~120, n = 8), and aged (PD ~19 months, n = 8) rats were given 2.0 g/kg i.p. ethanol. The tail was nicked 20 and 40 minutes post-administration for blood collection. The collected blood was immediately centrifuged to separate plasma. Blood ethanol levels were determined via Analox AM1 protocol.
Data Analysis
A two-way ANOVA (Age [periadolescent, adolescent, adult, aged] × Time [20, 40 min post ethanol injection]) was performed, followed by Bonferoni post hoc t-tests tests where appropriate.
Protein Detection: Western Blot
Tissue collection
Adolescent (PD 40, n = 12) and adult (PD 120, n = 12) rats received a single i.p. administration of 2.0 g/kg 10% (w/v) ethanol (n = 6 per age) or equivalent volume of saline (n = 6 per age). Animals were sacrificed 40 minutes post-injection, a time point when BECs are equivalent but differential motor impairment between adolescent and adult animals exists. In addition, this time point following ethanol administration provides unique information concerning ethanol's effect of PKCγ expression compared to previously published work (Kumar et al., 2006). Whole cortex, cerebellum, and hippocampus were rapidly dissected over ice and stored at −80°C until assayed.
Tissue Preparation
P2 fractions of individual brain regions were prepared by homogenizing the tissue sample in 0.32M sucrose in phosphate-buffered saline (PBS), followed by centrifugation at 1000g for 10 minutes. The resulting supernatant was centrifuged again at 12,000g for 20 minutes. This pellet (P2 fraction) was resuspended in PBS and stored at −80°C.
Western Blot Analysis
Each P2 fraction was assessed with a Bradford assay to determine protein concentration. Equivalent amounts of protein (20 μg) were loaded into Tris-Glycine gels (8-16%), counterbalanced across conditions. Proteins were separated by SDS-PAGE, electroblotted to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA), and targeted with PKCγ (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibody, diluted with blocking buffer (50mL PBS, 25μL Tween-20, 0.5g milk powder) to a concentration of 1:2000. A horseradish peroxidase (HRP)conjugated secondary antibody targeted against a rabbit host (Santa Cruz Biotechnology) was applied, with antibody concentration diluted to 1:2000. Peptide labeling was detected by chemiluminescent substrates (Thermo/Scientific Pierce Rockford, IL), and exposed to x-ray film under non-saturating conditions. Densitometric measurements of the resulting bands were made with NIH Image J software. All blots were re-probed with β-actin (1:2000) and an HRP conjugated secondary antibody targeted against a goat host (1:7500) to verify equivalent protein loading and transfer by normalizing all data to actin expression. Blots were not stripped before application of β-actin; however, the molecular weights of PKCγ and β-actin are significantly different (78 kDa and 42 kDa, respectively). This difference makes the two proteins easily distinguishable on the x-ray film, and the bands corresponding to the appropriate molecular weight were analyzed (as also described in Kumar et al., 2010 and Matthews et al., 1998; 2000). Furthermore, the host animals for the two secondary antibodies are of different species to ensure appropriate binding, although there was some residual PKCγ expression present when β-actin was developed since the blots were not stripped.
Data Analysis
Densitometric measurements normalized to actin expression were analyzed with a two-way repeated ANOVA (Age [adolescent, adult] × Drug [saline, ethanol]).
RESULTS
Ethanol reduces movement more significantly in adult rats than adolescent rats
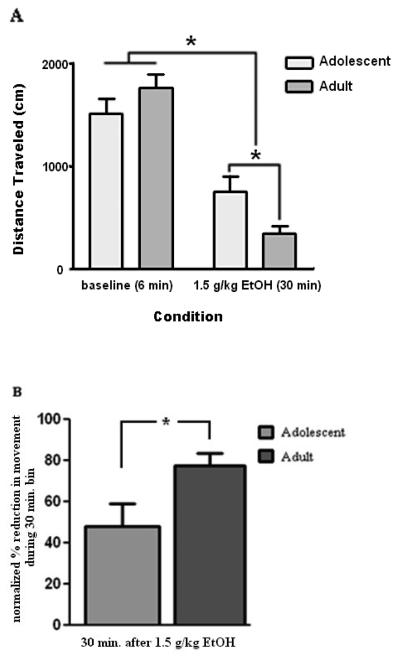
The age of the animals did not affect the total distance travelled in the baseline open field session, but was a significant determining factor on the total amount of movement inhibition produced by 1.5 g/kg ethanol (Age × Time interaction, df (1,16), p < 0.05). Post-hoc t-tests revealed that while no significant difference between adolescent and adult animals was found at baseline (t (16) = 1.31, p > 0.20), ethanol induced inhibition of movement between adolescent animals and adult animals was significantly different when total distance travelled during the 30 minute test session was analyzed (t (16) = 2.44, p < 0.03). To further investigate the effect of ethanol on the differential reduction in distance travelled between adolescent and adult animals, total distance in the 30 minute test session (post-ethanol) was normalized to distance in the 6 minute baseline session (pre-ethanol). As expected, a significant difference in ethanol-induced reduction in total movement was found, with adolescent animals showing a reduction of ~47% while adult animals demonstrated a reduction of ~78% (t (16) = 2.37, p < 0.03). See Figure 1A and 1B.
Figure 1. Acute ethanol administration results in a significant reduction in the total amount of distance traveled in the open field test.
(A) Mean distance travelled during a 6 minute open field test before and 30 minutes after a 1.5 g/kg ethanol administration and (B) mean reduction in distance traveled during a 30 minute bin compared to baseline. Error bars denote S.E.M while * < 0.05.
Ethanol impairs the aerial righting reflex in an age-dependent manner
The age of the animal did not affect baseline aerial righting reflex (ARR), but the effect of acute ethanol exposure on ARR was significantly affected by animals' age (Age × Time interaction, F (12, 112) = 3.25, p < 0.001, Table 1). Bonferroni post-hoc tests (all p's < 0.05) revealed that pre-adolescent and adolescent animals never significantly differed from one another on righting distance, while adult and aged animals also did not significantly differ from one another. However, pre-adolescent animals had significantly lower ARR scores than adults at the 10, 30, and 40 minute time points and significantly lower ARR scores than the aged animals at all time points tested after ethanol administration. Adolescent animals showed a similar trend, having significantly lower ARR 10 and 20 minutes post-ethanol administration compared to the adult animals and significantly lower ARR than the aged animals at the 10, 20, 30 and 40 minute time points.
Table 1.
Mean height required for successful righting and significant differences assessed by Bonferoni post hoc tests at p < 0.05. SEM shown in parenthesis.
| Aerial Righting Reflex | |||||
|---|---|---|---|---|---|
| Periadolescent (1) |
Adolescent (2) |
Adult (3) |
Aged (4) |
Significant Findings |
|
| Baseline | 5.63 (0.63) |
5.00 (0.00) |
5.00 (0.00) |
6.25 (0.82) |
n.s. |
| 10 min. | 16.25 (2.63) |
16.88 (1.62) |
28.13 (0.92) |
25.00 (2.50) |
1 vs 3 & 4; 2 vs 3 & 4 |
| 20 min. | 15.63 (3.05) |
12.50 (2.11) |
22.50 (3.27) |
26.25 (2.06) |
1 vs 4; 2 vs 3 & 4 |
| 30 min. | 11.88 (3.13) |
16.88 (2.30) |
24.38 (2.75) |
25.00 (2.11) |
1 vs 3 & 4; 2 vs 4 |
| 40 min. | 11.25 (1.83) |
15.00 (2.32) |
20.00 (2.50) |
24.38 (2.40) |
1 vs 3 & 4; 2 vs 4 |
Ethanol decreases the in vivo spontaneous firing rate of cerebellar Purkinje neurons in adult rats, but does not alter firing rates of adolescent rats
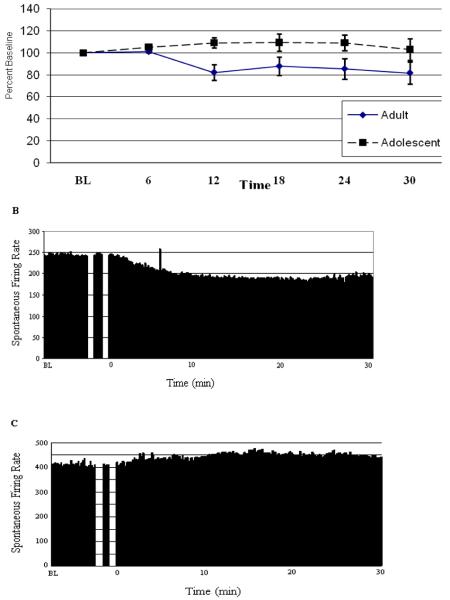
The age of the animal did not affect the baseline firing rate of cerebellar Purkinje neurons recorded using single barrel glass micropipettes (Independent t-test, t = 0.98, p > 0.30, data not shown). However, the effect of acute ethanol exposure on cerebellar Purkinje neural activity was significantly affected by age of the animal (Age, F (1,68) = 5.50, p < 0.05, Fig. 2). Specifically, Purkinje neurons recorded from adult animals showed approximately 20% inhibition while the Purkinje neurons recorded from adolescent animals demonstrated slight, approximately 5%, excitation.
Figure 2. Acute ethanol administration reduces the in vivo recorded spontaneous firing rate of Purkinje neurons from adult rats without altering the spontaneous firing rate of Purkinje neurons from adolescent rats.
(A) Mean spontaneous firing rates of cerebellar Purkinje neurons from adolescent and adult animals during the course of an acute ethanol administration, expressed as a percentage of baseline spontaneous activity. Overall a significant difference in the firing rate was found that was dependent on the age of the subject. The spontaneous firing rate of a single representative Purkinje neuron from (B) an adult animal and (C) an adolescent animal prior to, during, and 30 minutes after a 1.5 g/kg ethanol administration. Error bars denote S.E.M.
Ethanol slightly increases spontaneous firing frequency (f) of cerebellar Purkinje neurons independently of the age in vitro
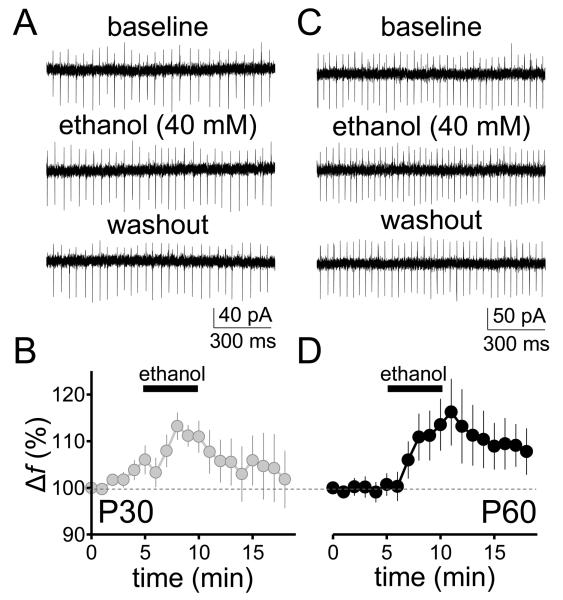
We investigated the effect of 40 mM ethanol on the pacemaker activity of Purkinje neurons in two experimental groups in vitro: PD 30 (adolescent) and PD 58-60 (adult) day old rats. In neurons from adolescent rats, ethanol reversibly increased the firing f by approximately 10-15% from a baseline level of 33.7 ± 3.4 Hz (p < 0.01 by one-sample t-test vs. 100; Fig. 3 A,B; firing f change, expressed in Hz: 2.1 ± 0.3 Hz, p < 0.001 by one-sample t-test vs. zero). In neurons from adult rats, we found that 40 mM ethanol also increased the firing f by a similar extent from a baseline level of 41.7 ± 5.3 Hz (p < 0.05 by one-sample t-test vs. 100; Fig. 3 C, D; firing f change, expressed in Hz: 2.1 ± 0.3 Hz, p < 0.05 by one-sample t-test vs. zero). However, unlike the in vivo data, no significant difference was found between the effects of ethanol on firing f between the two age groups (unpaired t-test, p > 0.05). It should be noted that the acute effect of ethanol on spontaneous Purkinje neuron firing frequency that we report here is of greater magnitude than the small but statistically significant effect that we previously observed in slices from younger rats (PD 19-30) under similar experimental conditions (Mameli et al., 2008). Taken together, these findings suggest that the sensitivity of spontaneous Purkinje neuron firing to acute ethanol exposure may increase between the juvenile and adolescent developmental periods.
Figure 3. Acute ethanol administration increases the spontaneous firing rate of Purkinje neurons in cerebellar slices from adolescent and adult rats.
Effect of ethanol (40 mM) on the pacemaker activity of Purkinje neurons in parasagittal cerebellar vermis slices. (A), representative traces showing action currents recorded in loose-seal cell-attached configuration in Purkinje neurons from 30 day-old Sprague-Dawley male rats before (baseline), during (ethanol) and after (washout) application of 40 mM ethanol. (B), time course of the normalized firing frequency recorded from these Purkinje neurons (n = 8). (C), Same as in A but for 58-60 day-old rats. (D), Same as in B but for 58-60 day-old rats (n = 8). All values are expressed as mean ± SEM.
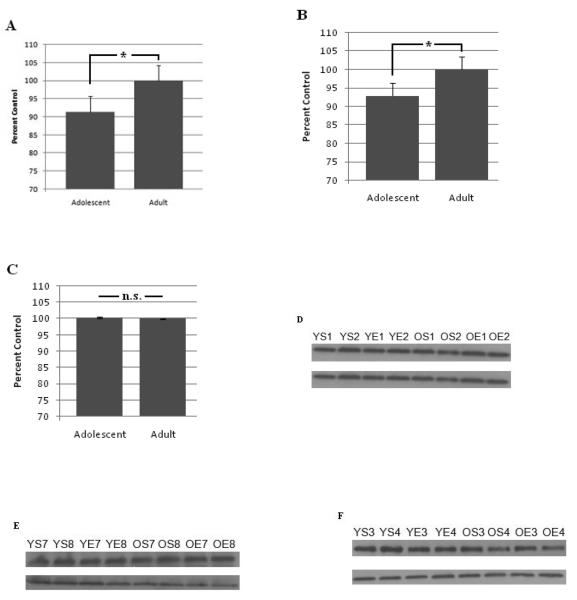
Adolescent rats have less PKCγ peptide expression in the cortex and cerebellum than adult rats
PKCγ expression in adolescent rats is significantly less in cortex (Age, F (7,14) = 16.91, p < 0.01, Fig. 4A) and cerebellum (Age, F (7,14) = 5.08, p < 0.05, Fig. 4B) compared to adults. PKCγ expression in the hippocampus was not significantly affected by age (F (7,14) = 0.34, p = n.s., Fig. 4C). Interestingly, acute ethanol administration did not have a significant effect on PKCγ expression in any brain region and did not significantly interact with age to alter expression.
Figure 4. Adolescent rats have less PKCγ expression in the cerebellum and cortex, but not hippocampus, compared to adult rats.
Optical density of PKCγ expression normalized to β-actin (adult set as control condition) in (A) the cortex, (B) cerebellum, and (C) hippocampus. Representative PKCγ expression (top) and β-Actin expression (bottom) in the (D) cortex, (E) cerebellum, and (F) hippocampus. YS = adolescent saline, YE = adolescent ethanol, OS = adult saline, OE = adult ethanol, and the number indicates animal number. Error bars indicate S.E.M. and * p < 0.05.
Age significantly affected blood ethanol concentrations following an acute ethanol administration
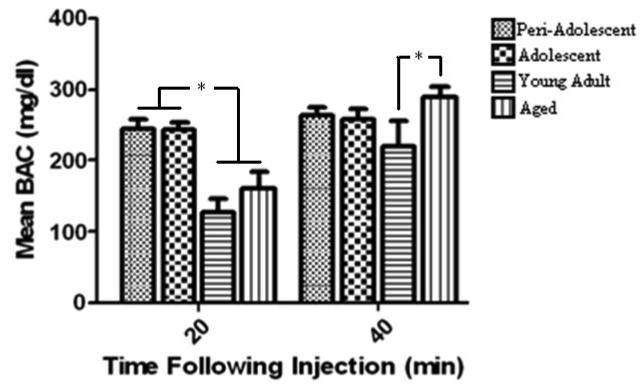
Blood alcohol levels were differentially affected by the interaction of age of the animal and time following ethanol administration (Age × Time interaction F (3,22) = 4.88, p < 0.02). Bonferroni post-hoc tests revealed that preadolescent and adolescent rats displayed similar BAC levels at both time points while preadolescent and adolescent animals had higher blood ethanol levels than either adult and aged animals at the 20 minute time point but not the 40 minute time point (p's < 0.05). Interestingly, adult animals had significantly lower BAC levels compared to aged animals at the 40 minute time point (p < 0.05). See Figure 5.
Fig. 5. Blood ethanol concentrations are significantly affected by both time and subjects age.
Mean blood ethanol concentration at 20 and 40 minutes after a 2.0 g/kg ethanol administration. Error bars denote S.E.M. and * p < 0.05
DISCUSSION
Age-dependent motor impairments produced by ethanol have been well documented (Hefner and Holmes, 2007; Lisenbardt, 2009; Little et al., 1996; Pian et al., 2008; Ristuccia and Spear, 2008; Silveri and Spear, 2001; Varlinskaya and Spear, 2002; White et al., 2002). It stands to reason that reduced sensitivity to alcohol-induced motor impairments, which serve as cues to moderate alcohol intake (Spear and Varlinskaya, 2005), may contribute to amplified alcohol use during adolescence. Recent national survey data reveal that adolescents are consuming alcohol at alarming rates (Underage Alcohol Use: Findings from the 2002-2006 National Survey on Drug Use and Health http://oas.samhsa.gov/underage2k8/toc.htm), yet the neural and molecular mechanisms underlying the differential alcohol sensitivity, which potentially underlies the high drinking rates, has yet to be determined.
The present study provides a macroscopic view of age-dependent motor impairments during the life span through the inclusion of periadolescent and aged animals. Results from the open field test and the aerial righting reflex task both indicate that periadolescent and adolescent rats show less motor impairments in response to ethanol than adults, which is consistent with previous research in the field (Hefner and Holmes, 2007; Lisenbardt, 2009; Little et al., 1996; Pian et al., 2008; Ristuccia and Spear, 2008; Silveri and Spear, 2001; Varlinskaya and Spear, 2002; White et al., 2002). However, a saline control condition was not included in the experiments and therefore our results should be interpreted with caution. In our open field test, adolescent rats showed less of an ethanol-induced reduction in total distance travelled compared to adults. Interestingly, in the aerial righting reflex task, periadolescent and adolescent rats did not show any significant differences from each other in terms of sensitivity. Similarly, adult and aged animals did not significantly differ from each other at the time points we tested. However, there appears to be some slight differences between adult and aged rats, since adolescent rats were significantly different from aged animals at all time points tested, while the adolescent rats were only significantly different in the 10 and 20 minute post-ethanol test compared to adult rats. Future studies should extend the post-ethanol ARR times and include more sensitive measures of ataxia to investigate if additional age-dependent differences exist that were not detected in the current work, especially considering the increasing life expectancy of our population.
It is important to note that baseline motor movement and baseline aerial righting reflex scores did not differ between any of the age groups. The lack of significant baseline differences rules out the possibility that a general motor difference exists due to age that could be responsible for the age-dependent ethanol-induced movement differences. However, the differences found in the open field test are more difficult to interpret, since it is expected that the distance travelled will be inversely proportional to the amount of time spent in the field as the novelty of the environment is reduced (Walsh and Cummins, 1976). Consequently, it is expected that a decrease in movement compared to baseline would be found during the 30-minute testing period. As such, decreased exploration is a confounding variable in the ethanol-induced reduction in movement reported in the current set of studies. In addition, saline controls were not used in either of the behavioral experiments. However, when coupled with the ARR data as well as previous studies indicating young animals are less sensitive to ethanol's motor impairing effects (White et al., 2002), it is reasonable to conclude that periadolescent and adolescent animals are less sensitive to ethanol-induced motor impairments regardless of the task used.
The motor differences elicited by ethanol administration have been well established; however, there is not yet a solid neuronal effect to account for it. Since Purkinje neurons constitute the exclusive motor output of the cerebellum (Cesa and Strata, 2009 for review), alterations in the neuronal firing rates may be a critical index underlying changes in motor functioning. The current study is, to our knowledge, the first to directly compare the in vivo spontaneous firing of adolescent and adult cerebellar Purkinje neurons in response to ethanol. Results from the present study partially mirror the behavioral effect of ethanol: the spontaneous firing rate from adult Purkinje neurons decreased approximately 20% from baseline, suggestive of a potential motor impairment. However while adolescents did show a modest motor impairment, the firing rates from adolescent Purkinje neurons actually were not dramatically changed in response to ethanol, and in fact showed a 5% increase in firing rate. One potential weakness of the current work is the use of a general anesthetic during the recording preparation. Although urethane is a commonly used anesthetic in this type of study (Matthews et al., 2001; Tokunaga et al., 2003; 2006), it is possible that Purkinje neural activity is altered by the combination of urethane and ethanol.
Ethanol increases spontaneous GABA release onto Purkinje neurons from the presynaptic terminal (Hirono et al., 2009; Kelm et al., 2010; Mameli et al., 2008). In fact, specific modulation of fast GABAergic transmission onto Purkinje neurons, using engineered GABAA receptors to confer unique zolpidem sensitivity of Purkinje cells, translates directly to impairment on a complex motor task (Wulff et al., 2007). Zolpidem is a positive allosteric modulator of the GABAA receptor (Campo-Soria et al., 2006). Therefore ethanol-mediated inhibition of Purkinje neurons through GABAergic mechanisms is likely to contribute to some of the motor impairments produced by ethanol consumption.
This ethanol-induced reduction of spontaneous Purkinje firing rates in neurons from adult rats could partially explain the greater sensitivity to ethanol's motor impairing effects in adult rats compared to adolescents. Although ethanol-induced inhibition of Purkinje neurons is plausibly one component of the differential behavioral effects, there are likely to be contributions from other systems involved in the final behavioral manifestation. The altered firing rate of the adult rat correlates well with the behavior; however, the interpretation for the adolescent rat is not as straightforward. The Purkinje neurons of adolescent rats are relatively ethanol insensitive, leading to the prediction that adolescents should not show any motor impairment. However, adolescent rats did show ethanol-induced impairments in the open field test and aerial righting reflex task, although the impairments were not as profound in comparison to adult rats. Therefore, it is obvious that ethanol-induced ataxia involves other neural systems in addition to alterations in Purkinje neural firing rates.
Previous research has established similar in vivo neurophysiological alterations of the limbic system in adult rats. For example, ethanol inhibits the firing rate of medial septal neurons (VanDoren et al., 2000) and chronic ethanol alters the recovery of the spontaneous firing rate of medial septal neurons following GABA microiontophoresis (Matthews et al., 2000). In addition, ethanol enhances GABA-mediated inhibition in the medial septal area, which correlates with behavioral sedation in anesthetized and freely behaving rats (Givens and Breese, 1990) and inhibits NMDA-evoked activity in the medial septum (Simson et al., 1991) and hippocampus (Simson et al., 1993). Similarly, ethanol decreases the specificity of the place fields of hippocampal pyramidal cells (Matthews et al., 1996; White and Best, 2000) recorded from awake and freely behaving rats. However, these results were demonstrated in adult animals, and it should be obvious from the present work that additional studies are needed to investigate if neurons from adolescent animals demonstrate similar alterations of neuronal firing rates in response to ethanol.
The in vivo effect we report has strong face validity when correlated with the age-dependent motor impairments we, and other researchers, have described (Hefner and Holmes, 2007; Lisenbardt, 2009; Little et al., 1996; Pian et al., 2008; Ristuccia and Spear, 2008; Varlinskaya and Spear, 2002; White et al., 2002). However, the in vivo data is in direct contrast with data from our in vitro recordings. Curiously, in vitro electrophysiological recordings revealed a small, but significant excitatory effect on the firing rates of cerebellar Purkinje neurons independently of age. The lack of an age-dependent neuronal effect is surprising, and seems contradictory in light of the abundance of age-dependent behavioral responses to ethanol. Furthermore, results from the in vivo and in vitro electrophysiological recordings from the adult rat are in direct opposition: the Purkinje firing rate was decreased with in vivo recording, while in vitro recordings indicated an excitation. However, results from the adolescent rat are more consistent, with both methods revealing an ethanol-induced excitation of Purkinje neuronal firing. One potential limitation of the in vitro data is that the age difference between adolescent and adult animals is not as diverse compared to the other analyses. In addition, future studies should examine whether the effects of ethanol on neuronal firing and synaptic transmission in other cerebellar neuronal populations (molecular layer interneurons, Golgi cells and granule cells) are age-dependent (Carta et al., 2004; Hirono et al., 2009; Mameli et al., 2008).
The contradictory conclusions may arise from the inherent differences between the two methodologies. In vitro electrophysiology allows recording of individual neurons from brain slices in a simplified system; however, the recording environment is artificially simulated. Furthermore, isolating specimens may alter neuronal behavior from that of the intact animal. In vivo electrophysiology records the net effect of a systemically administered drug in a live animal under general anesthesia. This provides a more physiologically relevant environment for studying neuronal effects; however, the effects cannot be localized as easily when compared to in vitro recordings. For these reasons, multi-methodological collaborations should be employed when possible.
The differing results could have also arisen from alterations located outside the slice from which the in vitro recordings were obtained. Some evidence suggests that motor impairments resulting from cerebellar damage may be confined to damage within the anterior lobe (lobules I-V and VIII) of the cerebellum (Schmahmann et al., 2009). In fact, the concept of the cerebellum as having a pure motor function may be outdated and incomplete, since the cerebellum likely plays a role in higher cognitive functioning (Schmahmann, 1991), specifically lobules VI and VII of the posterior lobe (Schmahmann et al., 2009). In vitro recordings in the present study were obtained from Purkinje neurons located in parasagittal slices of the cerebellar vermis. However, since the cerebellum is thought to have topographic organization (Schmahmann, 1991; Schmahmann et al., 2009), with lobules I-V and VII thought to be predominantly involved in sensorimotor function (Schmahmann et al., 2009), additional research should look specifically at individual lobules of the cerebellum to provide more insight into the exact location of motor modulation by ethanol. One plausible explanation of ethanol's age-dependent motor effects is that adolescents have an enhanced ethanol clearance rate for low (0.75 g/kg) and moderate (1.5 g/kg) ethanol doses (Walker and Ehlers, 2009). Results from the BAC portion of our study do reveal age-dependent effects, specifically that periadolescent and adolescent animals had significantly higher BACs than adult and aged animals 20 minutes after ethanol administration. However, at 40 minutes post-administration, the BACs of the two youngest age groups were no longer different from the adults. Fascinatingly, the aerial righting reflex data indicates that there is still a differential behavioral effect 40 minutes post-injection, despite comparable BACs between the adolescent and adult age groups at this time point. Although these studies are correlational in nature, it provides strong evidence that the enhanced ethanol clearance rate of adolescents is not the only factor contributing to behavioral differences. In fact, adolescent rats display shorter ethanol-induced sleep times, and regain their righting reflex with higher BACs than adult rats (Pian et al., 2008; Silveri and Spear, 1998; Silvers et al., 2003; Swartzwelder et al., 1998), further indicating that BAC levels are not necessarily a good predictor of motor impairment between the two age groups.
We propose that PKCγ peptide expression is part of the neurobiological mechanism that accounts for the inherent age-dependent differences in motor sensitivity to ethanol. PKC phosphorylates the β and γ2 subunits of the GABAA receptor (Brandon et al., 2000) to modulate the GABAergic mechanisms underlying the behavioral aspects of ethanol exposure (Kumar et al., 2009 for review). Interestingly, PKCγ knock-out mice show an enhanced preference for ethanol compared to wild-type littermates (Bowers and Wehner, 2001), have reduced hypnotic sensitivity to ethanol (Harris et al., 1995), and wake with higher blood ethanol concentrations (Proctor et al., 2003) independently of ethanol metabolism (Harris et al., 1995). Therefore, the PKCγ knock-out mouse shows many similarities to the adolescent rat in response to ethanol. Indeed, the present study reveals that adolescent rats have reduced PKCγ expression levels in the cortex and cerebellum compared to adults. The differences in expression levels were modest, with approximately 10% reduction in adolescent cortex and 7% reduction in adolescent cerebellum from adult expression levels. The small effect presented here could be due to several different factors. It is plausible that changes in PKCγ expression are localized to specific areas of the cortex, whereas we harvested and analyzed whole cortex, which could have effectively washed out the potentially large changes in individual cortical regions. Another possible explanation is that cortical expression of PKCγ in response to ethanol is time dependent: the expression level is stable 10 minutes following ethanol administration, but is decreased at 60 minutes post-administration in adult rats (Kumar et al., 2006). Our tissue was harvested 40 minutes post-ethanol, at a time when we demonstrated that the BACs of adolescent and adult rats are comparable, but age-related behavioral differences are still manifested and provides a unique data point when compared to previously published work (Kumar et al., 2006). PKCγ expression partially underlies the differences in cellular responsiveness to ethanol between adolescents and adults, which are effects that agree with the behavioral data indicating adolescent rats are less susceptible to ethanol-induced motor impairments. However, our investigation did not use protease or peptidase inhibitors in the preparation of the P2 fraction for the Western blot experiments. As such, it is possible that differential PKCγ expression could be due to differential protein degradation. Future studies should address this issue. Furthermore, additional studies should analyze the isolated motor cortex to ascertain if changes in PKCγ expression are enhanced in the motor cortex. Additional time points should also be considered in future studies since PKCγ expression levels are affected by time after ethanol administration (Kumar et al., 2006).
Interestingly, hippocampal PKCγ expression levels were similar in adolescent and adult rats. Contrary to what is frequently reported (Markwiese et al., 1998), data from our lab indicates that adolescent rats are not more sensitive to ethanol-induced impairments in spatial cognition; instead, both ages show similar decrements (Chin et al., 2010). The lack of age-dependent hippocampal impairments in response to ethanol is not completely unique: both adolescents and adults showed the same ethanol-induced impairment using an appetitive paradigm (Rajendran and Spear, 2004). When taken together, this indicates that the adolescent hippocampus may not be as fragile as previously thought.
The results from the current work focused on PKCγ expression of membrane-bound proteins and therefore only the enriched synaptosomal fraction was analyzed. We chose to focus initially on only the P2 fraction due to previous data demonstrating that ethanol can modulate PKCγ expression in this fraction (Kumar et al., 2006). Given the current findings, additional work should be performed to confirm our results by using immunohistochemical techniques to reveal relative PKCγ expression patterns, with a specific focus on Purkinje neurons. Additionally, future experiments could explore the age-dependent differences in PKCγ expression as it relates to the age-dependent differences in ethanol-induced alterations of Purkinje neuron currents, using single unit recording followed by single cell RTPCR to determine the PKC levels in RNA.
The results from the previous studies are limited for a variety of reasons. The exact age of the rats, although still within the appropriate limits, varies slightly across the studies presented. Adolescence is a period of ongoing development and the generally accepted estimate of adolescence in the rat is PD 28-42, which are the days surrounding sexual maturation during which physiological and behavioral markers of adolescence are present (Spear, 2007; Spear and Brake, 1983); although the exact dates delineating adolescence in the rat have been disputed (Odell, 1990). Another factor for consideration is that we have related several different studies that look at slightly different times and slightly different levels of ethanol administration. When reflecting on this data as a whole, the independence of the BAC, ARR, and PKCγ expression studies makes it difficult to draw any causational conclusions; however, it is perhaps less of an issue considering that the behavioral responses of rats show little variation in response to the same dose of ethanol. Nevertheless, the conclusions could be stronger if the same rats were used to collect data across all portions of these experiments. Other elements of these studies do not line up as well as the 3 aforementioned portions; but since ethanol's effects are so widespread and are comprised of specific physiological, behavioral, electrophysiological, and molecular changes, each portion of the study should be tailored to provide the most informative look at ethanol's effect on the specific target of the study. However future studies should collect data at more consistent time points to provide an in-depth, multiple-level portrait of the effects of ethanol.
This multi-methodological investigation uses several different methodologies at the behavioral, physiological, cellular, and molecular levels to discern the nature and origin of the age-dependent motor responses to ethanol. The combined results demonstrate that adolescents are less sensitive than adults to the motor-impairing effects of ethanol. In addition a similar effect is seen using in vivo electrophysiology. While still under investigation, PKCγ expression mirrors the age effect of ethanol, leading to the hypothesis that differential PKCγ expression underlies the age dependent differences in ethanol's ataxic effects.
ACKNOWLEDGEMENTS
This work was partially funded by AA13509 (DBM) and AA14973 (FV)
REFERENCES
- Baillieux H, De Smet HJ, Paquier PF, Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol. Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in (γ-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J Neurosci. 2001;21:RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Elliott KJ, Wehner JM. Differential sensitivity to the anxiolytic effects of ethanol and flunitrazepam in PKCgamma null mutant mice. Pharmachol Biochem Behav. 2001;69:99–110. doi: 10.1016/s0091-3057(01)00510-x. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghard W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase c-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa R, Strata P. Axonal competition in the synaptic wiring of the cerebellar cortex during development and in the mature cerebellum. Neuroscience. 2009;162:624–632. doi: 10.1016/j.neuroscience.2009.02.061. [DOI] [PubMed] [Google Scholar]
- Chin VS, Van Skike CE, Berry RB, Diaz-Granados JL, Matthews DB. Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol. 2010;44:3–14. doi: 10.1016/j.alcohol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Dow RS, Moruzzi G. The physiology and pathology of the cerebellum. University of Minnesota Press; Minneapolis: 1958. [Google Scholar]
- Givens BS, Breese GR. Electrophysiological evidence that ethanol alters function of medial septal area without affecting lateral septal function. J Pharmacol Exp Ther. 1990;253:95–103. [PMC free article] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abelovich A, Tonegawa S, Wehner JM. Mutant mice lacking the γ isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of (γ-aminobutyrate type A receptors. Proc Natl Acad Sci U S A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K. Ethanol enhances both action potential-dependent and action potential-indepent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology. 2009;57:109–120. doi: 10.1016/j.neuropharm.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Malherbe P, Sigel E. Function of the α1β2γ2S γ-aminobutyric acid type A receptor is modulated by protein kinase C via multiple phosphorylation sites. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Kumar S, Khisti RT, Morrow AL. Regulation of native GABAA receptors by PKC and protein phosphatase activity. Psychopharmacology. 2005;183:241–247. doi: 10.1007/s00213-005-0161-x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lane BM, Morrow AL. Differential effects of systemic ethanol administration on protein kinase cepsilon, gamma, and beta isoform expression, membrane translocation, and target phosphorylation: reversal by chronic ethanol exposure. J Pharmacol Exp Ther. 2006;319:1366–1375. doi: 10.1124/jpet.106.110890. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Golfarb KJ, Blackman LC, Boehm SL. Sensitivity and tolerance to the Hypnotic and Ataxic Effects of Ethanol in Adolescent and Adult C57BL/6J and DBA/2J Mice. Alcoholism: Clinical and Experimental Research. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABAa receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Criswell HE, Grobin AC, Morrow AL. Chronic ethanol consumption alters recovery of spontaneously active medial septal/diagonal band of broca neurons from GABA-microiontophoresis. Alcohol Clin Exp Res. 2000;24:1427–1432. [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Ethanol alters spatial processing of hippocampal place cells: a mechanism for impaired navigation while intoxicated. Alcohol Clin Exp Res. 1996;20:404–407. doi: 10.1111/j.1530-0277.1996.tb01660.x. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health Underage Alcohol Use: Findings from the 2002-2006 National Survey on Drug Use and Health. 2007 http://oas.samhsa.gov/underage2k8/toc.htm.
- Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Williams and Wilkins; Baltimore, MD: 1990. pp. 183–210. 1990. [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor WR, Poelchen W, Bowers BJ, Wehner JM, Messing RO, Dunwiddie TV. Ethanol differentially enhances hippocampal GABA A receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J Pharmacol Exp Ther. 2003;305:264–270. doi: 10.1124/jpet.102.045450. [DOI] [PubMed] [Google Scholar]
- Qi Z, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou W-H, Zhang C, Shokat KM, Messing RO. Protein kinase Cε regulates γ-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of γ2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent ethanol sensitivity: hypothermia and acute tolerance. Ann N Y Acad Sci. 2004;1021:445–447. doi: 10.1196/annals.1308.061. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Autonomic responses to ethanol in adolescent and adult rats: a dose-response analysis. Alcohol. 2008;42:623–629. doi: 10.1016/j.alcohol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept: the cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, MacMore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–861. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produces metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Breese GR. Inhibition of NMDA-evoked electorphysiological activity by ethanol in selected brain regions: evidence for ethanol-sensitive and ethanol-insensitive NMDA-evoked responses. Brain Res. 1993;607:9–16. doi: 10.1016/0006-8993(93)91483-9. [DOI] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Johnson KB, Hicks RE, Breese GR. Ethanol inhibits NMDA-evoked electrophysiological activity in vivo. Journal of Pharmacology and Experimental Theraputics. 1991;257:225–231. [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cellular and Molecular Life Sciences. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicology and Teratology. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, McDaniel JR, Morrow AL, Matthews DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Res. 2003;967:273–280. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JL, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol Clin Exp Res. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Vogel MW, Caston J, Yuzaki M, Mariani J. The Lurcher mouse: Fresh insights from an old mutant. Brain Res. 2007;1140:4–18. doi: 10.1016/j.brainres.2005.11.086. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, Dunwiddie TV, Harris RA, Sikela JM. Ethanol sensitivity of the GABAA receptor expressed in xenopus oocytes requires 8 amino acids contained in the gamma 2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of wistar rats. Pharmacology, Biochemistry, and Behavior. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open field test: a critical review. Psychological Bulletin. 1976;83:482–504. [PubMed] [Google Scholar]
- White AM, Best PJ. Effects of ethanol on hippocampal place-cell and interneuron activity. Brain Res. 2000;876:154–165. doi: 10.1016/s0006-8993(00)02629-9. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppa E, Linden A-M, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Action of ethanol on responses from cerebellar Purkinge neurons: relationship to methyllycaconitine (MLA) inhibition of nicotine responses. Neurochem Inter. 1999;35:185–194. doi: 10.1016/s0197-0186(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Ethanol modulation of gamma-aminobutyric acid (GABA-)mediated inhibition of cerebellar Purkinje neurons: relationship to GABAb receptor input. Alcohol Clin Exp Res. 2000;24:682–690. [PubMed] [Google Scholar]