Abstract
Background
Age-specific characteristics may contribute to the elevation in ethanol intake commonly reported among adolescents compared to adults. The present study was designed to examine age-related differences in sensitivity to ethanol’s aversive properties using a conditioned taste aversion (CTA) procedure with sucrose serving as the conditioned stimulus. Given that ontogenetic differences in responsiveness to stressors have been previously reported, the role of stressor exposure on the development of CTA was also assessed.
Methods
Experiment 1 examined the influence of 5 days of prior restraint stress exposure on the expression of CTA in a 2-bottle test following 1 pairing of a sucrose solution with ethanol. In Experiment 2, the effects of 7 days of social isolation on the development of CTA were observed using a 1-bottle test following multiple sucrose-ethanol pairings.
Results
The present study revealed age-related differences in the development of ethanol-induced CTA. In Experiment 1, adolescents required a higher dose of ethanol than adults to demonstrate an aversion. In Experiment 2, adolescents required not only a higher ethanol dose but also more pairings of ethanol with the sucrose conditioned stimulus. No effects of prior stressor exposure were observed in either experiment.
Conclusions
Together, these experiments demonstrate an adolescent-specific insensitivity to the aversive properties of ethanol that elicit CTA, a pattern not influenced by repeated restraint stress or housing in social isolation. This age-related insensitivity to the dysphoric effects of ethanol is consistent with other work from our laboratory, adding further to the evidence that adolescent rats are less susceptible to negative consequences of ethanol that may serve as cues to curb consumption.
Keywords: adolescent, rat, ethanol, conditioned taste aversion, stress exposure
Adolescence is a developmental period characterized by considerable transformations that are highly conserved across species. In addition to neural and hormonal changes, increases in social activity, novelty seeking, impulsivity and risk-taking are frequently reported during this transitional phase (see Spear, 2000; 2010). In line with these behavioral changes, alcohol use is commonly initiated during adolescence. Survey results from the 2008 Monitoring the Future study indicate that as many as 72% of high school seniors have tried alcohol at least once and that nearly 30% have been drunk in the past 30 days (Johnston et al., 2009).
Elevated ethanol intake is characteristic of rodent models of adolescence as well, using a conservative age range (postnatal days [P] 28 to 42) to typify the developmental period during which adolescent-characteristic neural and behavioral features are evident in both males and females (Spear, 2000). Adolescent rats often voluntarily consume 2–3 times more ethanol than do adults (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007). For this reason, animal models of adolescence are particularly useful in examining factors that may contribute to the age-related elevation of ethanol consumption, given ethical constraints limiting this line of research in human subjects.
There are several age-specific characteristics that may lead to elevated ethanol consumption during adolescence. For example, adolescents appear to be more sensitive to the social-facilitating effects of ethanol (Varlinskaya and Spear, 2002) and less sensitive than adults to many of the aversive consequences of ethanol that may serve as cues to curb intake, such as motor impairment (Hollstedt et al., 1980; Silveri and Spear, 2001; White et al., 2002), suppression of locomotor activity (Little et al., 1996), social impairment (Varlinskaya and Spear, 2002), and sedation (Draski et al., 2001; Moy et al., 1998; Silveri and Spear, 1998; 1999; 2002; 2004). Additionally, adolescent rats experience attenuated anxiogenesis compared to adults following acute ethanol withdrawal (Doremus et al., 2003; Varlinskaya and Spear, 2004). Together these age-related differences in sensitivity to various effects of ethanol may permit or even encourage higher ethanol consumption by adolescents relative to adults.
Conditioned taste aversion (CTA) is one method used to assess the dysphoric effects of alcohol and other drugs. This procedure involves the ingestion of a novel-flavored solution (conditioned stimulus; CS) paired with the effects of a particular drug (unconditioned stimulus; US). When given a subsequent opportunity to consume the drug-paired flavor, the degree to which an animal avoids the solution provides an index of the relative dysphoria experienced following the previous encounter with the drug. Supporting this interpretation, Green and Grahame (2008) report that CTA negatively correlates with ethanol intake across a wide variety of strains and selected lines of rats and mice, suggesting that low sensitivity to the aversive properties of ethanol may facilitate high ethanol intake. A recent study in our laboratory (Vetter-O'Hagen et al., 2009) demonstrated an age-related insensitivity to ethanol-induced taste aversion using saccharin as a CS, with both male and female adolescents requiring higher doses of ethanol than their adult counterparts to elicit a CTA. This initial study exploring the role of social context in sensitivity to the dysphonic effects of ethanol reported that adolescent males (but not females) who were exposed to an unfamiliar social partner for 24 hr following the pairing of ethanol with a saccharin solution CS demonstrated an attenuated sensitivity to the aversive properties of ethanol relative to males that remained isolated for this period. While the previously reported reduction in sensitivity to ethanol’s effect in the presence of a partner among adolescent males was interpreted as an effect of social experience during intoxication (Vetter-O’Hagen et al., 2009), we cannot preclude that exposure to an unfamiliar conspecific may have been stressful for a test subject. Therefore, the decreases in sensitivity to the aversive properties of ethanol seen in that CTA paradigm might have been stress-induced.
Human adolescents are confronted with a number of social and environmental challenges that are potentially stressful (Buchanan et al., 1992; Spear, 2000), and drinking to cope with problems is associated with extensive alcohol use in adolescence (Cooper et al., 2000). Research conducted in laboratory animals, although still limited, has revealed age-related differences in responsiveness to stressors, with adolescent rodents sometimes displaying increased sensitivity to stressors when indexed via hormonal responses as well as behavioral alterations (Brunell and Spear, 2005; Doremus-Fitzwater et al., 2009; Romeo et al., 2006; Stone and Quartermain, 1997). Given these ontogenetic differences in stress responses, it is possible that exposure to stressors might differentially impact sensitivity to the aversive effects of ethanol in adolescents compared to adults. Some experimental evidence suggests that exposure to stressors either immediately before or after ingestion of the CS may attenuate CTA (Misanin et al., 2006; Bourne et al., 1991; Revusky & Reilly, 1989), likely via an interruption in the learned association between the CS and US. However, no attempts have been made to assess age-related differences in stress-induced alterations using ethanol-induced CTA. Therefore, the effects of two different stressors on ethanol-induced CTA were examined in adolescent and adult animals in the present experiments: Repeated restraint (Experiment 1) and isolate-housing (Experiment 2).
An additional explanation for the elevated ethanol consumption commonly reported during adolescence involves possible age-related differences in hedonic sensitivity. In general, consummatory behaviors toward appetitive stimuli (such as food and other natural rewards) increase during adolescence. This change may be due to alterations in the hedonic value of the stimuli; that is, individuals at this age may consume more of a particular reinforcer either because they find it more rewarding or because they find it less rewarding and must consume more in order to achieve the positive consequences. If adolescents do indeed exhibit a partial anhedonia, they may seek out more natural and drug rewards to compensate for this age-related insensitivity to natural reinforcers. Sucrose consumption and preference are measures commonly used as a presumptive index of anhedonia (Katz, 1982; Konkle et al., 2003; Matthews et al., 1995; Willner et al., 1987), given evidence that animals subjected to chronic mild stress (an animal model of depression) typically reduce their intake of sweetened solutions (Gronli et al., 2004; Willner et al., 1987). There is some evidence that sucrose intake is generally more affected by prior stress exposure than that of saccharin (Harris et al., 1998; Grønli et al., 2005). In order to allow for assessment of hedonic sensitivity at both ages, sucrose, but not saccharin (Vetter-O’Hagen et al., 2009) was chosen as the CS in the present study, with the baseline sucrose consumption (prior to ethanol exposure) serving as an index of relative of stress-induced anhedonia.
Given that our previous study revealed no sex differences in ethanol-induced CTA in adult animals and decreased sensitivity to the aversive properties of ethanol when intoxication occurred in the presence of the peer in adolescent male but not female rats (Vetter-O’Hagen et al., 2009), only male subjects were used in the present experiments.
EXPERIMENT 1: The influence of repeated stressor exposure on ethanol-induced conditioned taste aversion in adolescent and adult rats
Adolescents are less sensitive than adults to many of the aversive consequences of ethanol that may serve as cues to curb intake. Because adolescence may be a particularly stressful developmental period (Arnett, 1999) and because stress may interact with ethanol’s effects (see Pohorecky, 1981; 1990), this experiment was designed to assess the influence of stressor exposure on ethanol’s negative effects when indexed via CTA in both stressed and non-stressed adolescent and adult rats. Restraint stress was chosen as a stressor since it is primarily psychological in nature and does not involve physical pain or harm to the animal (Herman and Cullinan, 1997; Weinberg et al., 2007). Furthermore, this stressor has been shown to produce sobering effects in adolescent and adult rats (Doremus-Fitzwater et al., 2007).
Subjects
A total of 172 male Sprague-Dawley rats bred in our colony at Binghamton University were used in Experiment 1. On postnatal day (P) 1, litters were culled to eight to 10 pups, with a ratio of six males to four females maintained whenever possible. Subjects were weaned on P21 and housed in pairs with a same-sex littermate unless otherwise specified. Animals were maintained in a temperature-controlled vivarium on a 14:10-hr light:dark cycle (lights on at 7 AM) and were at all times treated in accordance with guidelines for animal care established by the National Institutes of Health under protocols approved by the Binghamton University Institutional Animal Care and Use Committee. Subjects had ad libitum access to food (Purina lab chow, Lowell, MA) and water prior to experimentation. Eight to nine animals were assigned to each of the 20 experimental conditions defined by the 2 (age: adolescents, adults) × 2 (stress condition: no manipulation, restraint) × 5 (ethanol dose: 0, 0.75, 1.0, 1.5, 2.25 g/kg) factorial design. No more than one animal per litter was assigned to a given test group and all testing occurred between the hours of 10AM and 3PM.
Procedure
Subjects were re-housed with a same-age non-littermate on either P21 (adolescents) or P65–67 (adults). Seven days thereafter, subjects in the restraint stress condition were placed in restraint tubes for 90 min in a novel holding cage (a standard acrylic tub with no bedding) every day for experimental days 1–5. Restraint tubes (Tailveiner, Braintree Scientific, Braintree, MA) were Plexiglas cylinders measuring 18 × 4.7 cm for adolescents and 23 × 8.0 cm for adults (length × diameter). Each cylinder had a slot across the top and a sliding stopper that allowed for adjustment of the tube’s length in order to appropriately restrain animals of varying sizes.
While subjects in the stress condition were in the restraint tubes, non-stressed animals remained in their home cages and were not manipulated. Following stressor exposure on experimental day 5, all pairs of subjects were 50% water-deprived. For this calculation, water intake for each cage over the previous 24 hr was measured and divided in half. This amount was prepared in a 100-ml bottle in addition to an extra 5 ml of water to account for fluid in the bottle inaccessible to subjects. At no time following this water deprivation procedure did animal body weights drop below 85% of age-appropriate free-feeding weights.
On experimental day 6, animals in each housing pair were separated by a mesh divider for 30 min prior to conditioning. For conditioning, each subject was given access to a single bottle containing a 10% sucrose solution for 30 min. The mesh divider allowed for measurement of individual consumption while avoiding any potential effects of social isolation (Hall, 1998). Immediately following the 30-min sucrose access period, each subject was given an i.p. injection of 0 (0.9% saline), 0.75, 1.5 or 2.25 g/kg ethanol (18.9% v/v in a 0.9% saline solution). Injection volume (rather than ethanol concentration) was adjusted to deliver varying doses (Linakis and Cunningham, 1979). Saline control animals were injected with a volume equivalent to the volume of the highest ethanol dose, and all solutions were administered at room temperature. Animals in each housing pair were assigned to the same experimental condition (i.e., animals housed together always received the same dose of ethanol or saline and were assigned to the same stressor condition).
The mesh divider was removed approximately 15 min post-injection, and a freshly-filled water bottle returned to the cage for 24 hr. On experimental day 7, fluid intake of each pair over the preceding 24 hr period (including sucrose solution consumed during conditioning) was again measured, with 50% of this volume given to each pair over the 24 hr period prior to preference testing. On test day, animals in each housing pair were again separated by a mesh divider for 30 min prior to the 2-bottle choice test. During the 1-hr test, each subject had access to two bottles: one containing a 10% sucrose solution and the other containing water.
Data Analysis
Percent body weight gain across the stressor exposure period (calculated by the formula [(post-stress weight – pre-stress weight)/(pre-stress weight)]*100), g/kg sucrose intake during conditioning and percent sucrose preference on the test day served as the dependent variables of interest. Sucrose preference scores were determined using the formula [(sucrose consumption during test)/(total fluid consumption during test)]*100; thus, sucrose intake scores higher than 50% reflected greater intake of sucrose than water, while scores lower than 50% reflected relative avoidance of the sucrose solution. Body weight gain and sucrose intake during conditioning were analyzed using 2 (age) × 2 (stress) factorial analyses of variance (ANOVAs). Percent sucrose intake on the test day was analyzed using a 2 (age) × 2 (stress) × 5 (EtOH dose) factorial ANOVA, with Dunnett’s post-hoc tests used to explore main effects and interactions of interest. Subjects who did not consume any sucrose on the conditioning day were eliminated from the study.
Results
Percent Body Weight Gain
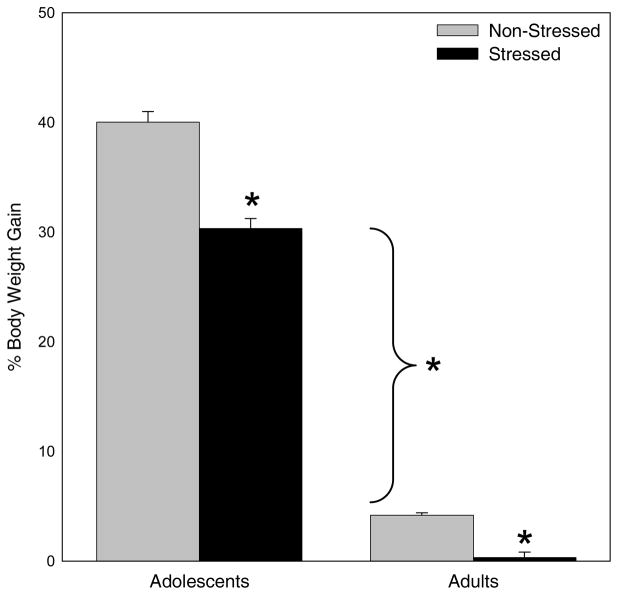
As expected, adolescents gained more weight than adults [main effect of age, F(1,118) = 2247, p < 0.01]. Additionally, there was a significant main effect of stress, F(1,118) = 95, p < 0.01, with both stressed adolescents and stressed adults gaining less weight than their non-stressed counterparts (see Figure 1).
Figure 1.
Percent Body Weight Gain Before and After 5 Days of Restraint Stress. Overall, adolescents showed greater increases in body weight across the 5 days of restraint stress than adults. Stressed animals of both ages gained significantly less weight than their non-stressed counterparts.
Sucrose Intake during Conditioning
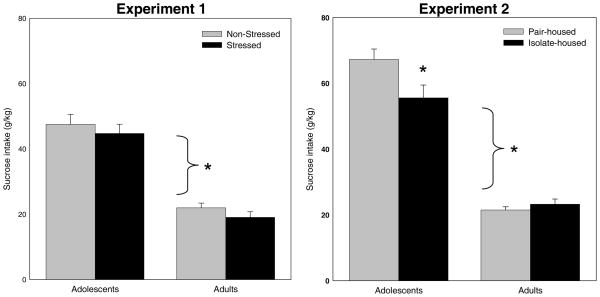
As shown in Figure 2 (left panel), adolescents consumed significantly more sucrose solution per kg body weight on the conditioning day than did adults [main effect of age, F(1,168) = 120, p < 0.01]. However, there was no impact of stress condition on intake during conditioning at either age.
Figure 2.
Baseline Sucrose Intake. For Experiment 1, adolescents consumed more sucrose (g/kg) than their adult counterparts. No differences in sucrose intake among stressed and non-stressed animals were observed in animals of either age. For Experiment 2, adolescents again consumed more sucrose than adults. While isolate- and pair-housed adults showed similar sucrose consumption, isolate-housed adolescents consumed significantly less sucrose than pair-housed adolescents.
Percent Sucrose Intake on Test Day
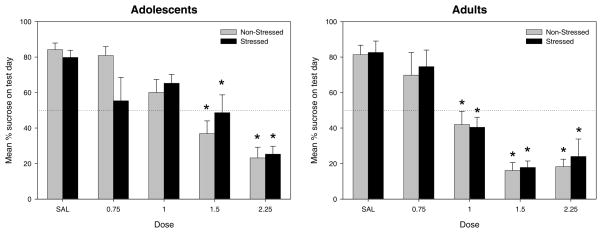
Analysis of sucrose consumption relative to total intake on the test day revealed a significant age × dose interaction [F(4,152) = 3, p < 0.02]. Saline-treated animals at both ages demonstrated relatively high sucrose preferences. Dunnett’s post-hoc tests revealed significant reductions in sucrose consumption relative to the saline-injected controls following ethanol doses of 1.0 g/kg and higher in adults, but only at ethanol doses of 1.5 g/kg and higher among adolescents (see Figure 3). Prior stressor exposure did not influence sucrose preference in animals of either age.
Figure 3.
Mean Percent Sucrose on Test Day. Adolescent subjects injected with ethanol doses of 1.5 g/kg or higher demonstrated a significant reduction compared to saline controls in percent sucrose consumed on the test day. Adult subjects injected with ethanol doses of 1.0 g/kg or higher exhibited an attenuation of percent sucrose intake on the test day.
EXPERIMENT 2: The influence of isolation stress on the development of ethanol-induced conditioned taste aversion in adolescent and adult rats
Previous results from our laboratory have indicated that isolate-housing can influence home cage ethanol consumption in adults, with isolated adults consuming less ethanol than their pair-housed counterparts—an effect that did not reach significance in adolescents (Doremus et al., 2005). The influence of post-weaning housing conditions on ethanol-induced conditioned taste aversion has been examined previously in adult female Hooded Lister rats, with no differences reported between subjects housed in a socially enriched environment or in isolation (Smith et al., 1997); however, the effects of isolate-housing on conditioned taste aversion in adolescent animals are unknown.
While conditioned taste aversion is often assessed via a two-bottle consumption test (see Exp. 1), this procedure has been modified by the Cunningham group to allow tracking of the development of the aversion across repeated pairings of ethanol using a one-bottle conditioning/test procedure (e.g., Risinger and Cunningham, 1995). This method was used in Experiment 2 to explore the influence of social deprivation on ethanol’s effects by examining the development of ethanol-induced conditioned taste aversion across five conditioning days in isolate-housed and pair-housed adolescent and adult rats.
Subjects
A total of 128 adolescent and adult animals were tested across the 16 experimental conditions defined by the 2 (age: adolescents, adults) × 2 (housing condition: pair-housed, isolate-housed) × 4 (ethanol dose: 0, 0.5, 1.0, 1.5 g/kg) factorial design, with 7–8 animals placed into each experimental group.
Procedure
As in Experiment 1, animals in the pair-housed condition were placed with a same-age non-littermate assigned to the same experimental group on P21 (adolescent-tested) or P65 (adult-tested). At the same time, animals in the isolate-housed condition at each age were placed in a cage alone. Subjects were then not manipulated for an acclimation period of eight days before the onset of the experimental procedure.
On experimental day 1 (24 hr before the first conditioning day), all subjects were 50% water-deprived as described for Experiment 1. The following day, pair-housed animals were separated by a mesh divider 30 min prior to each being given access to a single bottle containing 10% sucrose for 1 hr. Following this 1-hr access period, animals were injected i.p. with one of the four doses of ethanol (0, 0.5, 1.0 or 1.5 g/kg). Each pair of animals housed together received the same ethanol dose. Approximately 15 min thereafter, mesh dividers were removed and regular water bottles were returned to each cage for 24 hr of ad libitum access, with intake during this period used to calculate the 50% fluid amount to be given over the next 24 hr. This procedure was repeated on experimental days 3–10, with every other day serving as a sucrose intake conditioning/test day. On day 10, the final intake test day, no post-test injections were given.
Data Analysis
Baseline sucrose intake was analyzed in a 2 (age) × 2 (housing) factorial ANOVA. Sucrose intake (g/kg) on each of the four test days (i.e., experimental days 3, 5, 7, and 9) was analyzed via a 2 (age) × 2 (housing) × 4 (ethanol dose) × 5 (day) repeated measures ANOVA, as was percent of baseline sucrose intake across days. Significant effects were then subjected to Tukey’s post hoc tests.
The baseline sucrose intake of four subjects was more than two standard deviations less than the respective group mean. Because significant exposure to the CS is a prerequisite for the development of conditioned taste aversion, these subjects were identified as outliers and eliminated from all analyses. Three of these subjects were isolate-housed adolescents (one each from the 0.5, 1.0 and 1.5 g/kg ethanol groups) and the remaining subject was an isolate-housed adult (from the saline control group). Additionally, 11 sucrose intake data points (i.e., <2% of the total) from various conditioning days were replaced with group means because they were either two standard deviations above or below the group mean—variations likely attributable to bottle leakage or measurement error.
Results
Baseline sucrose intake
As shown in Figure 2 (right panel), housing conditions had no effect on baseline sucrose intake among adults, whereas isolate-housed adolescents consumed significantly less sucrose than their pair-housed counterparts [age × housing interaction: F(1,120) = 6.54, p < 0.02]. There was also a main effect of age [F(1,120) = 220.8, p < 0.01], with adolescents again consuming notably more sucrose than adults.
Sucrose consumption on test days
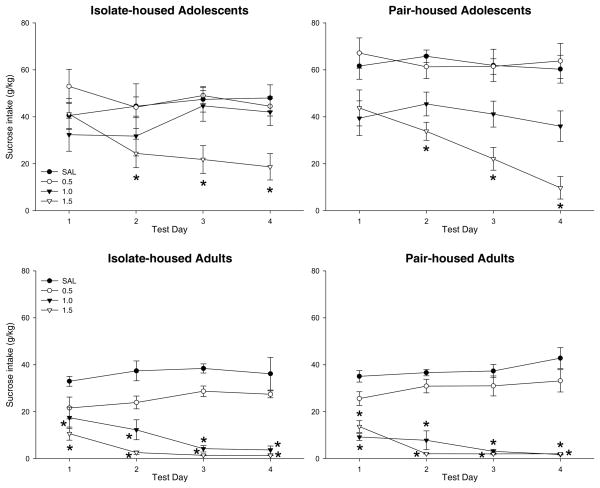
A significant age × dose × day interaction, F(12,432) = 3.78, p < 0.01, emerged during analysis of the sucrose intake on the test days (see Figure 4). For adults, Tukey’s post-hoc tests revealed significant decreases in sucrose consumption on all four test days in the 1.0 and 1.5 g/kg ethanol groups (p < 0.05) when compared to saline-injected controls. For adolescents, Tukey’s post-hoc tests revealed significant decreases in sucrose consumption from saline control animals only on test days 2–4 and only in the 1.5 g/kg ethanol group (p < 0.01). No effects of housing condition were seen.
Figure 4.
Sucrose Intake Across Test Days. During the test days following the first conditioning session, adolescent animals in both housing conditions significantly reduced their sucrose intake compared to saline-injected controls following a 1.5 g/kg dose of ethanol on test days 2–4. Adult animals demonstrated a reduction in sucrose consumption on test days 1–4 following ethanol doses of 1.0 g/kg or higher.
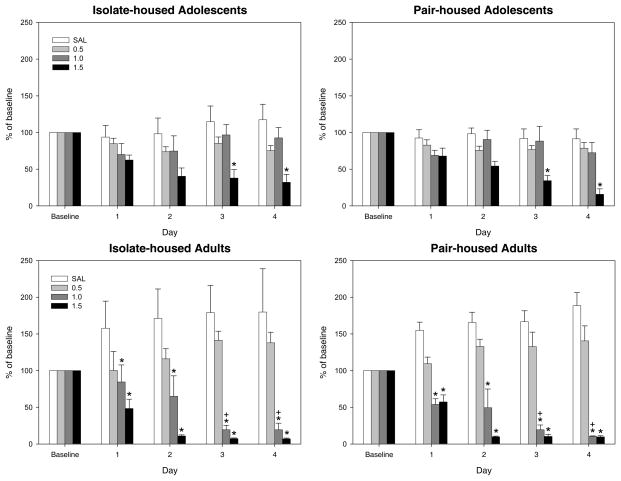
Percent of baseline across 4 test days
Given the difference in baseline sucrose consumption on day 1 across housing condition and across age, these data were also analyzed following transformation into percent of baseline [(Test day sucrose intake/Baseline day sucrose intake)*100] prior to further analysis. As presented in Figure 5, a significant age × dose × day interaction, F(9,324) = 4.87, p < 0.01, also emerged in the analysis of these data, with Tukey’s post-hoc tests revealing a generally similar pattern of findings as for the raw consumption findings, with significantly reduced consumption relative to saline controls on all four test days for adult subjects in the 1.0 and 1.5 g/kg ethanol groups, but with significant reductions occurring only on test days 3–4 in the 1.5 g/kg ethanol group for adolescents. Tukey’s post-hoc tests also confirmed the disparity between adolescents and adults at the 1.0 g/kg ethanol dose: adults showed a significant reduction from baseline sucrose intake compared to adolescents on test days 3–4. Adolescents and adults did not differ at any other dose. Again, no effects of housing condition were evident in animals of either age.
Figure 5.
Percent of Baseline Sucrose Intake Across Test Days. Across the four test days following the first conditioning session, adolescent animals in both housing conditions consumed a significantly smaller percent of their baseline sucrose intake compared to saline-injected controls on test days 3–4 following an ethanol dose of 1.5 g/kg. Adult animals, however, demonstrated a significant decrease from baseline sucrose consumption on all four test days following ethanol doses of 1.0 g/kg and higher. Adults showed a significant reduction from baseline sucrose intake compared to adolescents on test days 3–4 following conditioning with the 1.0 g/kg ethanol dose (* indicates significant difference from saline controls; + indicates significant decrease relative to adolescents).
DISCUSSION
Results from the present study revealed age-related differences in the development of ethanol-induced CTA, with adolescent males requiring higher doses of ethanol than adult males to demonstrate an aversion. These results extend findings reported in an earlier study from our lab (Vetter-O’Hagen et al., 2009) across multiple CTA paradigms using different solutions as a CS. In the current study, a 10% sucrose solution served as a highly appetitive, caloric CS in two different CTA procedures. Data from the initial sucrose consumption tests revealed greater baseline g/kg sucrose intake among adolescent than adult animals in both experiments, and hence do not support the hypothesis that adolescents are less sensitive to hedonic stimuli than adults. These results are in agreement with previous taste reactivity data from our lab demonstrating greater positive oral responses to sucrose among adolescent animals relative to their adult counterparts (Wilmouth and Spear, 2009). Interestingly, using baseline sucrose consumption as an index of relative anhedonia revealed age differences in response to different types of stressors. For both adolescents and adults, prior restraint effectively reduced body weight but the stressor did not influence sucrose consumption in animals of either age. Isolation housing, however, resulted in decreased sucrose consumption among adolescent but not adult animals. No effects of prior stressor exposure on ethanol-induced CTA were observed in animals of either age.
The CTA procedure can be varied in several ways that may influence experimental outcomes. In the earlier study from our lab, the conditioning paradigm involved one pairing of ethanol with the saccharin CS followed by a one-bottle test 48 hr later. There has been some debate regarding the optimal testing conditions in CTA models: while some researchers have favored a one-bottle test (Batsell and Best, 1993), others have argued that a two-bottle test (i.e., providing the animal with a choice between the drug-paired solution and water) is a more sensitive measure (Klein et al., 1975). According to Batsell and Best (1993), the two-bottle testing method, while appropriate for revealing an aversion, is less effective for detecting differences between groups that demonstrate varying degrees of aversion. In the present study, the two-bottle test in Experiment 1 and the one-bottle test in Experiment 2 yielded similar results. Adults developed an aversion to sucrose following a 1.0 g/kg dose of ethanol under both one-bottle and two-bottle testing conditions. Likewise, adolescents demonstrated aversion at the same ethanol dose (1.5 g/kg) regardless of test procedure. The two experiments also differed in whether animals were given one (Experiment 1) or multiple (Experiment 2) pairings of the flavored solution with ethanol. The procedure used in Experiment 2, during which subjects received a total of five pairings of sucrose with ethanol, with the latter four pairings serving as both test and conditioning sessions, was found to provide a particularly sensitive assessment of the development of CTA, amplifying age-related differences. Adolescents receiving the 1.5 g/kg ethanol dose did not demonstrate the aversion to sucrose that was evident following one pairing in adults, but instead required multiple pairings of sucrose with ethanol to display CTA. Taken together with the previous report (Vetter-O’Hagen et al., 2009), the results of the present study suggest that adolescent-associated insensitivity to the aversive properties of ethanol in the CTA paradigm is a robust phenomenon that generalizes across a number of procedural differences, including housing conditions, the nature of the CS, number of CS-US pairings, and test procedures.
Neither stressor in our experiments influenced the aversive properties of ethanol in the CTA paradigm. The results of Experiment 2 are reminiscent of the findings of a previous study (Smith et al., 1997) where no effects of extensive (12-week) post-weaning isolate-housing were found on CTA to a 1.5 g/kg ethanol dose in adult female Hooded Lister rats. A number of other studies, however, have found various types of stressors to modify the discriminative and rewarding properties of ethanol. For example, in an experiment examining the influence of stressor exposure on rewarding and aversive effects of ethanol in Wistar rats using a place conditioning paradigm, Funk and colleagues (2005) found that conditioned place aversion produced by a 1.0 g/kg ethanol dose was blocked by exposure to either footshock stress or social defeat prior to ethanol injection. In a recent study, prolonged exposure to a stressor (corticosterone in the drinking water for 7 days) was reported to induce an adaptive decrease in plasma corticosterone levels in male Long-Evans rats as well as to diminish the interoceptive effects of ethanol as demonstrated in a drug discrimination task (Besheer et al., 2009). In the Vetter-O’Hagen et al. (2009) study, however, the aversive properties of ethanol were eliminated in adolescent males by a presence of a peer during an intoxication period – an exposure to an unfamiliar conspecific that might be considered stressful. It seems unlikely, however, that experimental animals perceived any amount of stress in that study, given that the presence of an unfamiliar conspecific has pronounced stress-ameliorating (i.e., social buffering) effects in rats (Cirulli et al., 1996; Kiyokawa et al., 2004).
Previous research suggests that stressor exposure can influence expression of CTA under some circumstances. Footshock, tail-pinch stress, and swim stress have all been shown to attenuate CTA to unconditioned stimuli such as lithium chloride and morphine when administered after conditioning, between the US and CS pairing, or prior to CS presentation (Misanin et al., 2006; Bourne et al., 1991; Revusky & Reilly, 1989). Such findings suggest that examination of the effects of other types of stressors on ethanol-induced CTA may have yielded different results. It is also possible that the lack of stress effects on CTA in the present experiments could be associated with the use of a sucrose CS or water deprivation. Sucrose has been reported to attenuate physiological responses to stress (Ulrich-Lai et al., 2007), perhaps dampening the perception of stressor exposure (Foster et al., 2009). The modest water deprivation schedule employed to encourage fluid consumption during testing also might have been sufficiently stressful enough to mask any additional stressor effects. It is also feasible that the stressors used may not have been perceived as stressful at one or both ages. Indeed, when stress-induced anhedonia was indexed via baseline levels of sucrose consumption, only isolate-housed adolescents consumed significantly less sucrose than pair-housed adolescents—an age effect not seen in adult animals in Experiment 2 nor at either age following restraint stress in Experiment 1. This finding should not be surprising, given that interactions with peers are more rewarding for adolescents than for adults (Douglas et al., 2004), and deprivation of these social interactions appears especially stressful for adolescent animals relative to their more mature counterparts (see Hall, 1998). Although restraint stress was ineffective in inducing an anhedonia response to sucrose at either age in Experiment 1, this stressor was effective in reducing body weight at both ages, suggesting that the restraint stress effects on CTA are not simply due to a lack of perception of the restraint manipulation as stressful.
Attenuated expression of CTAs among adolescents is not unique to ethanol: relative to adults, adolescent animals have demonstrated a reduced susceptibility for development of CTAs to a variety of unconditioned drug stimuli, including amphetamine (Infurna and Spear, 1979), THC (Schramm-Sapyta et al., 2007), nicotine (Shram et al., 2006; Wilmouth and Spear, 2004), and cocaine (Schramm-Sapyta et al., 2006). In the latter study, adolescents showed attenuated CTA to both cocaine and lithium chloride. These data suggest that when compared to adult animals, adolescents show a general reduction in sensitivity to CTA whether the unconditioned stimulus is addictive or not, supporting the idea that adolescents may process aversive stimuli differently than more mature animals. An alternative explanation for reduced expression of CTA in adolescent subjects is that animals of this age may not be able to learn associations between the conditioned stimulus and the effects of the drug with which it was paired. This explanation seems unlikely, given that studies within the same experimental series found that, relative to adult animals, adolescents exhibited weaker aversive responses to nicotine when indexed by means of nicotine-induced CTA, but greater sensitivity to nicotine-induced conditioned place preference (Shram et al., 2006). Indeed, there is an emerging literature showing that, whereas adolescents are less sensitive than adults to the aversive properties of drug (and even non-drug) stimuli, their sensitivity to the rewarding properties of drugs such as nicotine and cocaine appears to be greater (Badanich et al., 2006; Brenhouse and Andersen, 2008; Brenhouse et al., 2008; Shram et al., 2006; Torres et al., 2008; Vastola et al., 2002; Zakharova et al., 2009a; Zakharova et al., 2009b). Furthermore, adolescents are more sensitive to the rewarding properties of social stimuli (Douglas et al., 2004) and novelty (Douglas et al., 2003).
In summary, the results of these experiments demonstrate that adolescent rats are less sensitive than their adult counterparts to the aversive properties of ethanol when indexed via CTA. This age-related pattern of insensitivity was still evident following variation in housing conditions, the nature of the CS, number of CS-US pairings, and test procedure. Consistent with other recent work from our laboratory (Vetter-O'Hagen et al., 2009), the present study provides further evidence that adolescent rats, while demonstrating elevated levels of ethanol intake relative to adults in a number of different paradigms (Brunell and Spear, 2005; Doremus et al., 2005; Smith et al., 1997; Vetter et al., 2007), are less susceptible to negative consequences of ethanol. These findings add a developmental dimension to conclusions based on genetic analyses that lower sensitivity to ethanol-induced CTA is associated with higher levels of ethanol intake across a wide variety of selectively bred lines (Broadbent et al., 2002; Chester et al., 2003; Froehlich et al., 1988; Green and Grahame, 2008; Phillips et al., 2005). Together, these approaches suggest that aversive properties of ethanol serve as important cues for modulating consumption, and support the conclusion that age-typical insensitivities to the aversive properties of ethanol likely contribute to the elevated consumption commonly reported during adolescence.
Acknowledgments
The research presented in this paper was supported by NIAAA grants R37 AA12525, R01 AA018026, and R01 AA16887 to Linda P. Spear.
References
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Batsell WR, Best MR. One bottle too many? Method of testing determines the detection of overshadowing and retention of taste aversions. Anim Learn Behav. 1993;21:154–158. [Google Scholar]
- Besheer J, Grondin JJM, Hodge CW. Prolonged activation of the HPA axis by corticosterone blunts the interoceptive properties of alcohol. Alcohol Clin Exp Res. 2009;33:152A. [Google Scholar]
- Bourne MJ, Calton JL, Gustavson KK, Schachtman TR. Effects of acute swim stress on LiCl-induced conditioned taste aversions. Physiol Behav. 1992;51:1227–1234. doi: 10.1016/0031-9384(92)90313-q. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Terranova ML, Laviola G. Affiliation in periadolescent rats: behavioral and corticosterone response to social reunion with familiar or unfamiliar partners. Pharmacol Biochem Behav. 1996;54:99–105. doi: 10.1016/0091-3057(95)02169-8. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: the role of personality and affect regulatory processes. J Pers. 2000;68:1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Impact of repeated stress on responsivity to ethanol-induced changes in social behavior in adolescent and adult rats. Poster presented at the annual meeting of the Society of Neuroscience; 2007. [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70:387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J, Murison R, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–147. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- Grønli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo- pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008 (NIH Publication No. 09-7401) Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16:965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner's stress status influences social buffering effects in rats. Behav Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Klein SB, Domato GC, Hallstead C, Stephens I, Mikulka PJ. Acquisition of a conditioned aversion as a function of age and measurement technique. Physiological Psychology. 1975;3:379–384. [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Matthews K, Forbes N, Reid IC. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Kaufhold SE, Paul RL, Hinderliter CF, Anderson MJ. A time contraction effect of acute tail-pinch stress on the associative learning of rats. Behav Processes. 2006;71:16–20. doi: 10.1016/j.beproc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, McKinnon CS, Cunningham CL. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci. 2005;119:892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. The interaction of alcohol and stress. A review. Neurosci Biobehav Rev. 1981;5:209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals--an update. Alcohol Alcohol. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Revusky S, Reilly S. Attenuation of conditioned taste aversions by external stressors. Pharmacol Biochem Behav. 1989;33:219–226. doi: 10.1016/0091-3057(89)90453-x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcohol Clin Exp Res. 2004;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehavl Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. New York: W. W. Norton; 2010. [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) Rockville, MD: 2008. [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150:478–486. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009b;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]