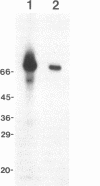
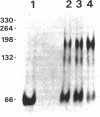
Abstract
An in vitro system was developed that results in the self-assembly of subunit precursors into complexes that resemble those found naturally in the endoplasmic reticulum. Subunits of glycinin, the predominant seed protein of soybeans, were synthesized from modified cDNAs using a combination of the SP6 transcription and the rabbit reticulocyte translation systems. Subunits produced from plasmid constructions that encoded either Gy4 or Gy5 gene products, but modified such that their signal sequences were absent, self-assembled into trimers equivalent in size to those precursors found in the endoplasmic reticulum. In contrast, proteins synthesized in vitro from Gy4 constructs failed to self-assemble when the signal sequence was left intact (e.g., preproglycinin) or when the coding sequence was modified to remove 27 amino acids from an internal hydrophobic region, which is highly conserved among the glycinin subunits. Various hybrid subunits were also produced by trading portions of Gy4 and Gy5 cDNAs and all self-assembled in our system. The in vitro assembly system provides an opportunity to study the self-assembly of precursors and to probe for regions important for assembly. It will also be helpful in attempts to engineer beneficial nutritional changes into this important food protein.
Keywords: soybean, seed storage protein, SP6 polymerase
Full text
PDF
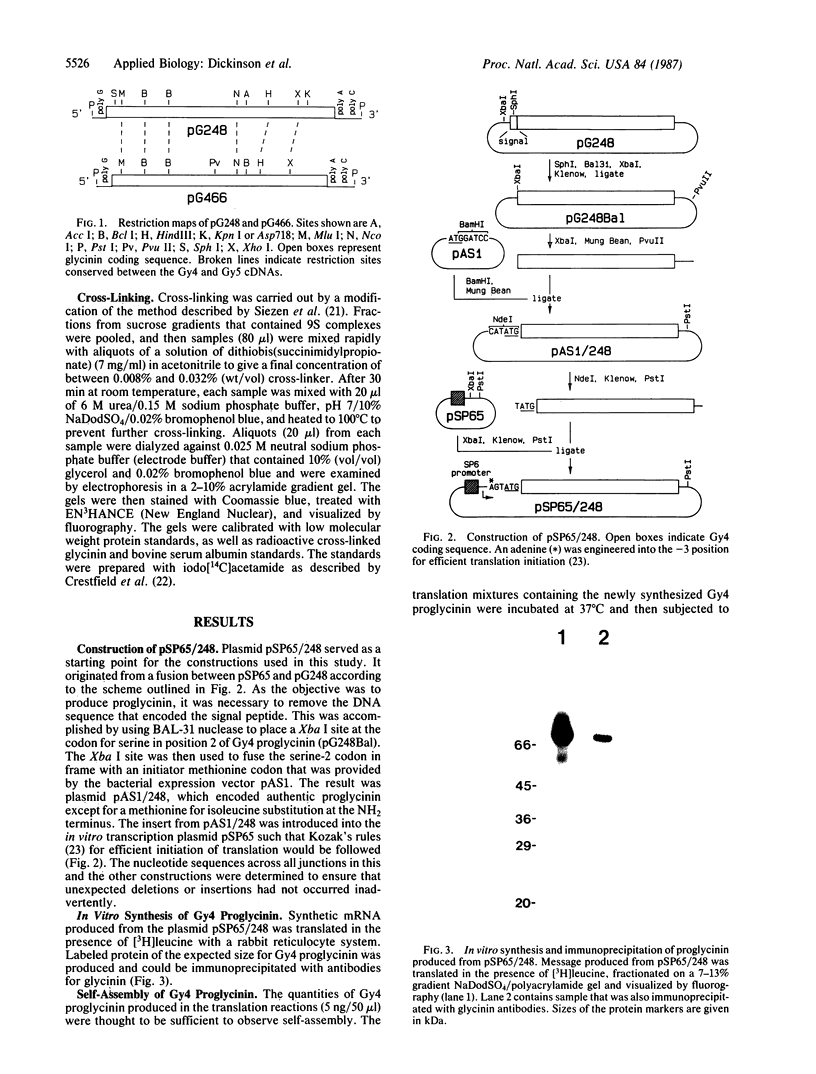
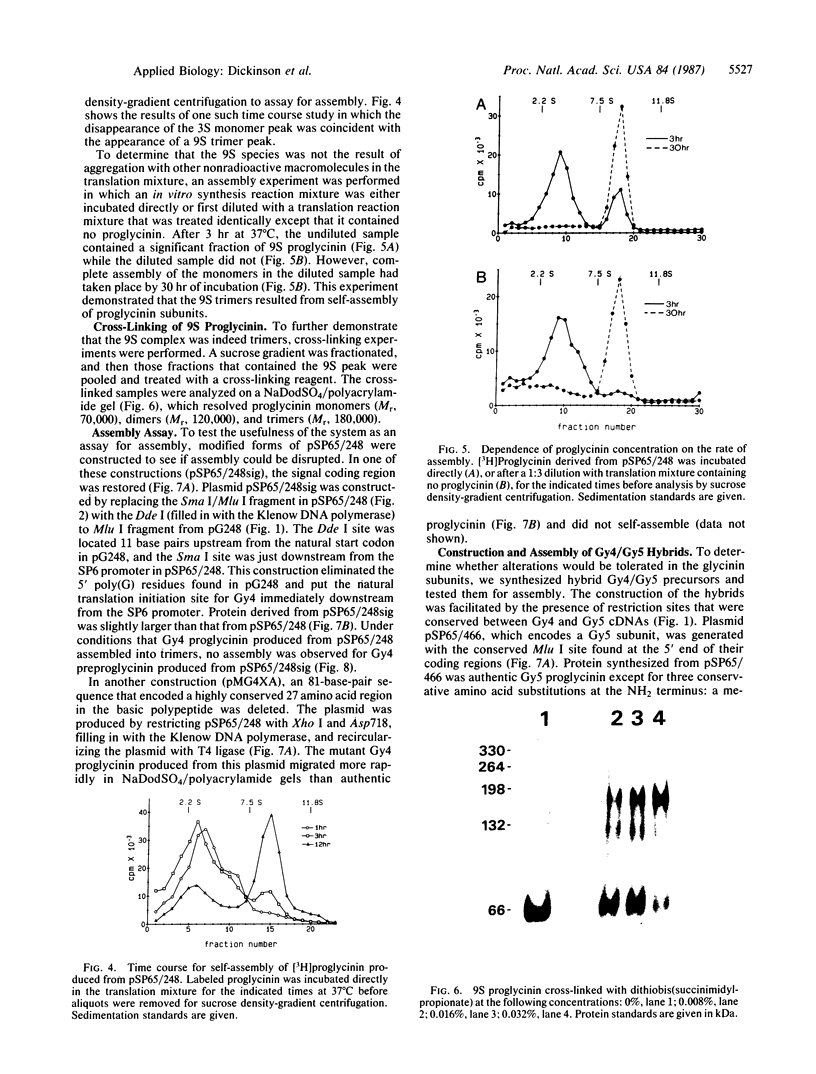
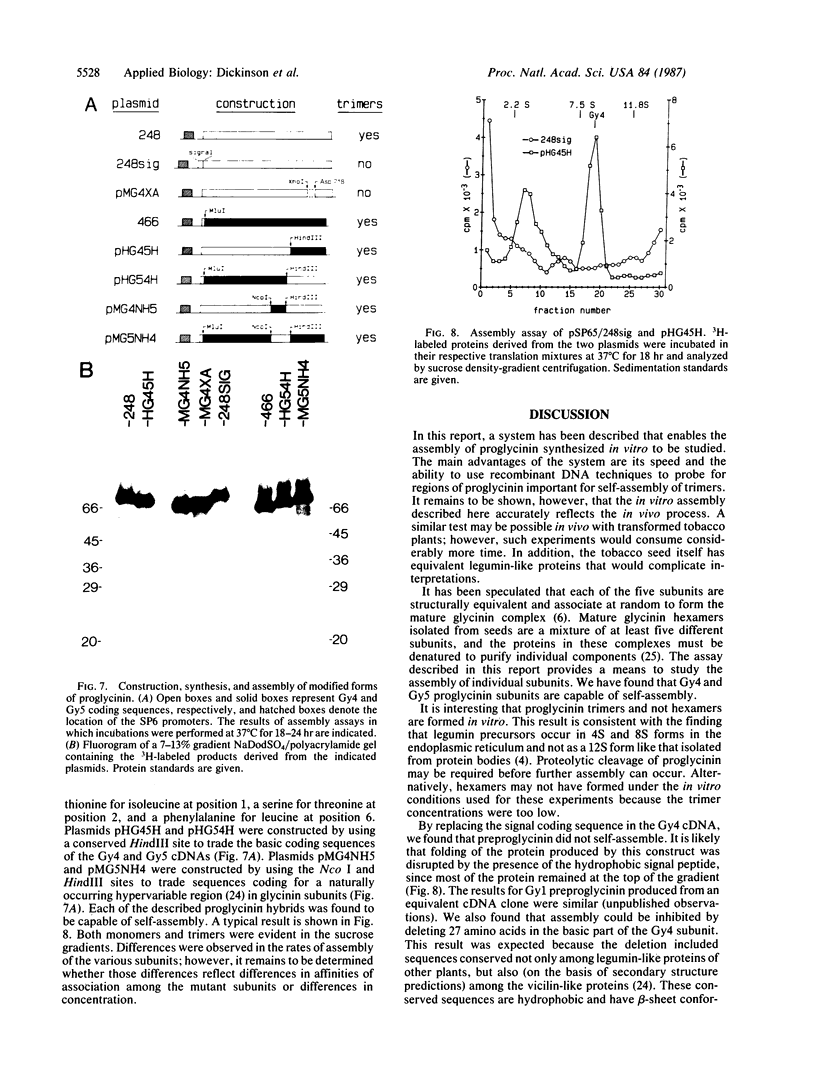

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Narayana S. V., Nielsen N. C. Structural similarity between legumin and vicilin storage proteins from legumes. EMBO J. 1985 May;4(5):1111–1117. doi: 10.1002/j.1460-2075.1985.tb03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereken-Tumer N., Richter J. D., Nielsen N. C. Structural characterization of the glycinin precursors. J Biol Chem. 1982 Apr 25;257(8):4016–4018. [PubMed] [Google Scholar]
- Fukazawa C., Momma T., Hirano H., Harada K., Udaka K. Glycinin A3B4 mRNA. Cloning and sequencing of double-stranded cDNA complementary to a soybean storage protein. J Biol Chem. 1985 May 25;260(10):6234–6239. [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of soybean seeds: I. Isolation and characterization of the major components. Plant Physiol. 1974 May;53(5):742–746. doi: 10.1104/pp.53.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw R. A., 3rd Dideoxy DNA sequencing with end-labeled oligonucleotide primers. Anal Biochem. 1984 Dec;143(2):298–303. doi: 10.1016/0003-2697(84)90666-3. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momma T., Negoro T., Hirano H., Matsumoto A., Udaka K., Fukazawa C. Glycinin A5A4B3 mRNA: cDNA cloning and nucleotide sequencing of a splitting storage protein subunit of soybean. Eur J Biochem. 1985 Jun 18;149(3):491–496. doi: 10.1111/j.1432-1033.1985.tb08951.x. [DOI] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Comparison of the primary structure of the acidic polypeptides of glycinin. Arch Biochem Biophys. 1981 Sep;210(2):633–642. doi: 10.1016/0003-9861(81)90230-7. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Active transepithelial potassium transport in frog skin via specific potassium channels in the apical membrane. Acta Physiol Scand. 1984 Feb;120(2):287–296. doi: 10.1111/j.1748-1716.1984.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Bindels J. G., Hoenders H. J. The quaternary structure of bovine alpha-crystallin. Chemical crosslinking with bifunctional imido esters. Eur J Biochem. 1980;107(1):243–249. doi: 10.1111/j.1432-1033.1980.tb04644.x. [DOI] [PubMed] [Google Scholar]
- Staswick P. E., Hermodson M. A., Nielsen N. C. Identification of the cystines which link the acidic and basic components of the glycinin subunits. J Biol Chem. 1984 Nov 10;259(21):13431–13435. [PubMed] [Google Scholar]
- Tumer N. E., Thanh V. H., Nielsen N. C. Purification and characterization of mRNA from soybean seeds. Identification of glycinin and beta-conglycinin precursors. J Biol Chem. 1981 Aug 25;256(16):8756–8760. [PubMed] [Google Scholar]
- WOLF W. J., BRIGGS D. R. Purification and characterization of the 11S component of soybean proteins. Arch Biochem Biophys. 1959 Nov;85:186–199. doi: 10.1016/0003-9861(59)90462-x. [DOI] [PubMed] [Google Scholar]