Abstract
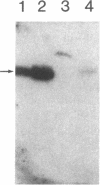
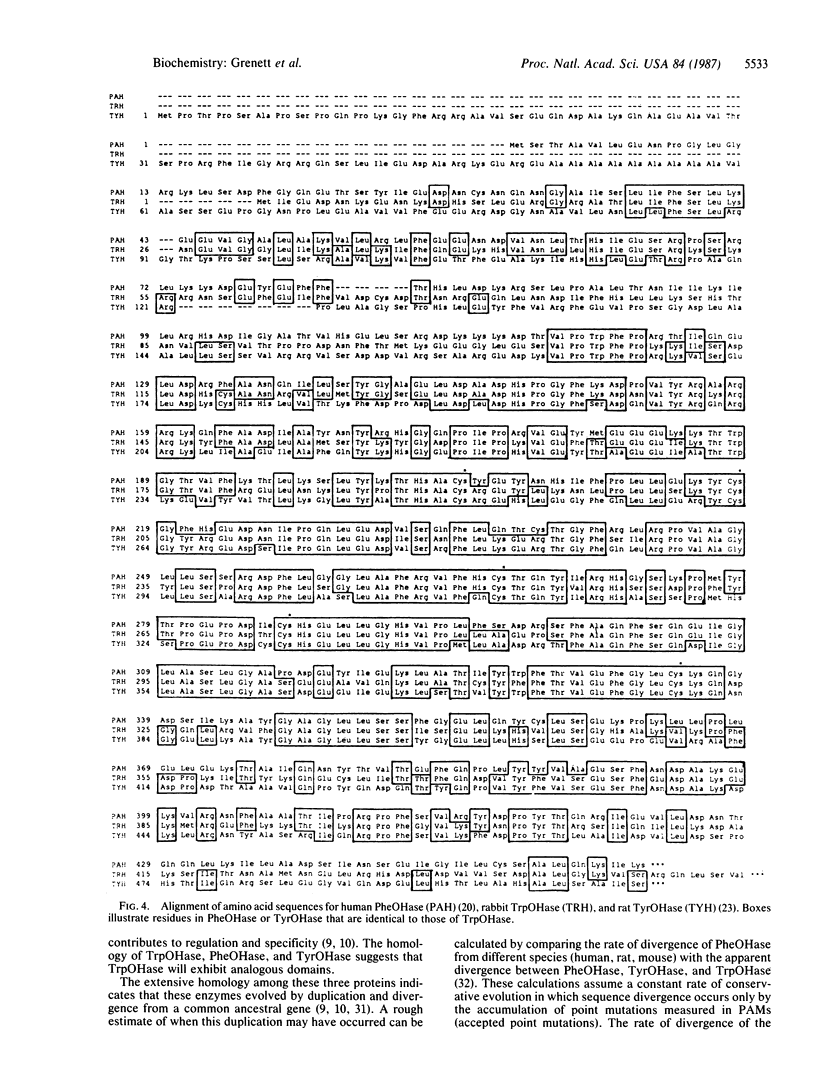
A full-length cDNA for tryptophan hydroxylase was cloned from rabbit pineal body by screening an expression library with antibody against rat phenylalanine hydroxylase, which crossreacts with rabbit tryptophan hydroxylase. Clones producing immunoreactive material contain sequences homologous to, yet distinct from, phenylalanine hydroxylase. The rabbit cDNA hybridizes to mRNA in pineal body and brainstem but not in liver. Comparison of the rabbit tryptophan hydroxylase sequence with the sequences of phenylalanine hydroxylase and tyrosine hydroxylase demonstrates that these three biopterin-dependent aromatic amino acid hydroxylases are highly homologous, reflecting a common evolutionary origin from a single primordial genetic locus. The pattern of sequence homology supports the hypothesis that the carboxyl-terminal two-thirds of the molecules constitute the enzymatic activity cores, and the amino-terminal thirds of the molecules constitute domains for substrate specificity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Parniak M., Kaufman S. The activation of rat liver phenylalanine hydroxylase by limited proteolysis, lysolecithin, and tocopherol phosphate. Changes in conformation and catalytic properties. J Biol Chem. 1984 Dec 10;259(23):14560–14566. [PubMed] [Google Scholar]
- BROWNLEE G., JOHNSON E. S. THE SITE OF THE 5-HYDROXYTRYPTAMINE RECEPTOR ON THE INTRAMURAL NERVOUS PLEXUS OF THE GUINEA-PIG ISOLATED ILEUM. Br J Pharmacol Chemother. 1963 Oct;21:306–322. doi: 10.1111/j.1476-5381.1963.tb01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetge E. E., Suh Y. H., Joh T. H. Complete nucleotide and deduced amino acid sequence of bovine phenylethanolamine N-methyltransferase: partial amino acid homology with rat tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5454–5458. doi: 10.1073/pnas.83.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Gershon M. D. 5-hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J Physiol. 1967 Oct;192(3):823–846. doi: 10.1113/jphysiol.1967.sp008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Culman J., Kiss A., Kvetnanský R. Serotonin and tryptophan hydroxylase in isolated hypothalamic and brain stem nuclei of rats exposed to acute and repeated immobilization stress. Exp Clin Endocrinol. 1984 Mar;83(1):28–36. doi: 10.1055/s-0029-1210309. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Darmon M. C., Grima B., Cash C. D., Maitre M., Mallet J. Isolation of a rat pineal gland cDNA clone homologous to tyrosine and phenylalanine hydroxylases. FEBS Lett. 1986 Sep 29;206(1):43–46. doi: 10.1016/0014-5793(86)81337-0. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O. The origin and evolution of protein superfamilies. Fed Proc. 1976 Aug;35(10):2132–2138. [PubMed] [Google Scholar]
- Friedman P. A., Kappelman A. H., Kaufman S. Partial purification and characterization of tryptophan hydroxylase from rabbit hindbrain. J Biol Chem. 1972 Jul 10;247(13):4165–4173. [PubMed] [Google Scholar]
- Friedman P. A., Lloyd T., Kaufman S. Production of antibodies to rat liver phenylalanine hydroxylase. Cross-reactivity with other pterin-dependent hydroxylases. Mol Pharmacol. 1972 Sep;8(5):501–510. [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ichiyama A., Hasegawa H., Tohyama C., Dohmoto C., Kataoka T. Some properties of bovine pineal tryptophan hydroxylase. Adv Exp Med Biol. 1976;74:103–117. doi: 10.1007/978-1-4684-3270-1_9. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Phillips R. S., Kaufman S. Proteolytic modification of the amino-terminal and carboxyl-terminal regions of rat hepatic phenylalanine hydroxylase. J Biol Chem. 1986 Feb 15;261(5):2051–2056. [PubMed] [Google Scholar]
- Joh T. H., Baetge E. E., Ross M. E., Reis D. J. Evidence for the existence of homologous gene coding regions for the catecholamine biosynthetic enzymes. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):327–335. doi: 10.1101/sqb.1983.048.01.036. [DOI] [PubMed] [Google Scholar]
- Jéquier E., Lovenberg W., Sjoerdsma A. Tryptophan hydroxylase inhibition: the mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Mol Pharmacol. 1967 May;3(3):274–278. [PubMed] [Google Scholar]
- Kaufman S. Regulatory properties of phenylalanine, tyrosine and tryptophan hydroxylases. Biochem Soc Trans. 1985 Apr;13(2):433–436. doi: 10.1042/bst0130433. [DOI] [PubMed] [Google Scholar]
- Kuhn D. M., Ruskin B., Lovenberg W. Tryptophan hydroxylase. The role of oxygen, iron, and sulfhydryl groups as determinants of stability and catalytic activity. J Biol Chem. 1980 May 10;255(9):4137–4143. [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., DiLella A. G., Kwok S. C., Woo S. L. Homology between phenylalanine and tyrosine hydroxylases reveals common structural and functional domains. Biochemistry. 1985 Jul 2;24(14):3389–3394. doi: 10.1021/bi00335a001. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., Woo S. L. Biochemical characterization of recombinant human phenylalanine hydroxylase produced in Escherichia coli. J Biol Chem. 1987 Feb 15;262(5):2228–2233. [PubMed] [Google Scholar]
- Lovenberg W., Jequier E., Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967 Jan 13;155(3759):217–219. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- Lysz T. W., Sze P. Y. Activation of brain tryptophan hydroxylase by a phosphorylating system. J Neurosci Res. 1978;3(5-6):411–418. doi: 10.1002/jnr.490030512. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H., Fujisawa H. Tryptophan 5-monooxygenase from mouse mastocytoma P815. A simple purification and general properties. Eur J Biochem. 1982 Jun;124(3):595–601. doi: 10.1111/j.1432-1033.1982.tb06636.x. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Chandra T., MacGillivray R. T., Woo S. L. Polysome immunoprecipitation of phenylalanine hydroxylase mRNA from rat liver and cloning of its cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4701–4705. doi: 10.1073/pnas.79.15.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J. H., Kaufman S. Tryptophan hydroxylase. Purification and some properties of the enzyme from rabbit hindbrain. J Biol Chem. 1975 Jun 10;250(11):4152–4158. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]