Abstract
Background
Withdrawal from chronic ethanol enhances ventral tegmental area (VTA) GABA neuron excitability and reduces mesolimbic dopamine (DA) neurotransmission, which is suppressed by acupuncture at Shenmen (HT7) points (Zhao et al., 2006). The aim of this study was to evaluate the effects of HT7 acupuncture on VTA GABA neuron excitability, ethanol inhibition of VTA GABA neuron firing rate, and ethanol self-administration. A role for opioid receptors (ORs) in ethanol and acupuncture effects is also explored.
Methods
Using electrophysiological methods in mature rats, we evaluated the effects of HT7 stimulation and opioid antagonists on VTA GABA neuron firing rate. Using behavioral paradigms in rats, we evaluated the effects of HT7 stimulation and opioid antagonists on ethanol self-administration using a modification of the sucrose fading procedure.
Results
HT7 stimulation produced a biphasic modulation of VTA GABA neuron firing rate characterized by transient enhancement followed by inhibition and subsequent recovery in 5 min. HT7 inhibition of VTA GABA neuron firing rate was blocked by systemic administration of the non-selective μ-opioid receptor (MOR) antagonist naloxone. HT7 stimulation significantly reduced ethanol suppression of VTA GABA neuron firing rate, which was also blocked by naloxone. HT7 acupuncture reduced ethanol self-administration without affecting sucrose consumption. Systemic administration of the δ-opioid receptor (DOR) antagonist naltrindole blocked ethanol suppression of VTA GABA neuron firing rate and significantly reduced ethanol self-administration without affecting sucrose consumption.
Conclusions
These findings suggest that DOR-mediated opioid modulation of VTA GABA neurons may mediate acupuncture’s role in modulating mesolimbic DA release and suppressing the reinforcing effects of ethanol.
Keywords: GABA, opioid, VTA, ethanol, acupuncture, dopamine
INTRODUCTION
Acupuncture has proven to be an effective treatment for reducing nausea, pain (for review see (Jindal et al., 2008)), and drug abuse (Kim et al., 2005). Electroacupuncture activates enkephalinergic and β-endorphinergic neurons in the arcuate nucleus of the hypothalamus (Wang et al., 1990a; Wang et al., 1990b), and endorphinergic fibers projecting from the arcuate nucleus can in turn activate opioid receptors (ORs) on GABA neurons in the ventral tegmental area (VTA) and nucleus accumbens (NAcc) (Mansour et al., 1988), structures implicated in natural and drug reward. The involvement of VTA ORs in drug reward is supported by studies showing that animals will self-administer opioids (Bozarth and Wise, 1981) and μ-opioid receptor (MOR) agonists (Bals-Kubik et al., 1993; Olmstead and Franklin, 1997; Terashvili et al., 2004; Zangen et al., 2002) directly into the VTA.
Endogenous opioid systems have been linked to the positive reinforcing properties of ethanol (for review see (Herz, 1998)). Pharmacological studies have shown that selective (e.g., δ and μ) and non-selective (e.g., naloxone, naltrexone, etc…) opioid antagonists can decrease ethanol self-administration, indicating that endogenous opioid systems contribute to ethanol reward (for review see (June et al., 2004; June et al., 2003)). Thus, it is not unexpected that non-selective opioid antagonists attenuate alcohol drinking and relapse in alcoholics (Mason et al., 1999; O'Malley et al., 2002). Indeed, it has been proposed that endogenous opioid release in the VTA is sufficient to modulate ethanol reinforcement (Bechtholt and Cunningham, 2005).
We have previously demonstrated that acupuncture suppresses the enhancement in NAcc DA release induced by an ethanol challenge as well as the reduction of extracellular dopamine (DA) levels in the NAcc during ethanol withdrawal (Zhao et al., 2006). Although mesencephalic DA neurons are excited by ethanol, it has been suggested that their excitation may be attributed to disinhibition produced by a primary inhibitory effect on GABA-containing neurons of the midbrain (Mereu and Gessa, 1985). Accordingly, we have demonstrated that acute ethanol reduces VTA GABA neuron firing rate (Gallegos et al., 1999), with an IC50 of 1.0 g/kg (100 mg % blood alcohol level), a moderately intoxicating dose, at a substantial fraction of the EC50 for ethanol excitation of DA neurons in vitro (Brodie et al., 1999). Moreover, VTA GABA neurons become hyperexcitable during ethanol withdrawal and evince tolerance to ethanol inhibition of firing rate during chronic ethanol (Gallegos et al., 1999), suggesting that GABA neurons in the VTA constitute a critical substrate for the acute and chronic effects of ethanol on mesocorticolimbic DA release (Diana et al., 2003). Theoretically, inhibition of VTA GABA neurons by acute ethanol would result in enhanced DA neuron activity and DA release in the NAcc, while chronic ethanol would result in hyperexcitability of GABA neurons, reduced DA neuron activity and DA release in the NAcc. As VTA GABA neurons express MORs (Steffensen et al., 2006) and accumbal GABA input to VTA GABA neurons is modulated by DORs (Margolis et al., 2008), we hypothesized that acupuncture would alter the activity of VTA GABA neurons, their response to ethanol, and ethanol self-administration, presumably through activation of endorphinergic input to the VTA and NAcc from the arcuate nucleus.
METHODS
Animal Subjects
Male Wistar rats were used in both electrophysiological and behavioral experiments. For electrophysiological studies, rats (270 g) were obtained from Charles River Laboratory (Hollister, CA). For ethanol self-administration studies, rats (270 g) were obtained from Daehan Animal (Seoul, Korea). Rats were kept on ad libitum food and water and maintained on a 12 hr light-dark cycle except during the sucrose training period. Animal care, maintenance and experimental procedures were in accordance with the Brigham Young University and Daegu Haany University Animal Research Committees and met or exceeded National Institutes of Health guidelines for the care and use of laboratory animals.
Surgical Procedure and Single Cell Electrophysiology
For acute electrophysiological recordings of VTA GABA neurons, rats were anesthetized using Isoflurane and placed in a stereotaxic apparatus. Anesthesia level was maintained at 1% throughout the experiments. Body temperature was maintained at 37.4 ± 0.4° C by a feedback regulated heating pad. With the skull exposed, holes were drilled for placement of stimulating and recording electrodes. Extracellular potentials were recorded by 3.0 M KCl-filled micropipettes (2–4 MΩ; 1 µm inside diameter). Potentials were amplified with an Axon Instruments Multiclamp 700A amplifier (Union City, CA). Microelectrodes were oriented, via stereotaxic coordinates, into the VTA (from bregma: 5.6 – 6.5 posterior (P), 0.5 – 1.0 lateral (L), 6.5 – 7.8 ventral (V)) with a piezoelectric microdrive (EXFO Burleigh 8200 controller and Inchworm, Victor, NY). Single cell activity was filtered at 0.3–10 kHz (−3dB) with the Multiclamp 700A amplifier and displayed on Tektronix (Beaverton, OR) digital oscilloscopes. Potentials were sampled at 20 kHz (12 bit resolution) with National Instruments data acquisition boards in Macintosh computers (Apple Computer, Cupertino, CA). Extracellularly-recorded action potentials were discriminated with a World Precision Instruments WP-121 Spike Discriminator (Sarasota, Fl) and converted to computer-level pulses. Single-unit potentials, discriminated spikes and stimulation events were captured by National Instruments NB-MIO-16 digital I/O and counter/timer data acquisition boards (Austin, TX) in Macintosh computers.
Characterization of VTA GABA Neurons in vivo
VTA GABA neurons were identified by previously-established stereotaxic coordinates and by spontaneous and stimulus-evoked electrophysiological criteria (Steffensen et al., 1998). They included: Relatively fast firing rate (>10Hz); ON-OFF phasic non-bursting activity; spike duration less than 200 µsec; and multiple post-stimulus spike discharges (PSDs) produced by stimulation of the internal capsule (IC; coordinates: −1.0–1.3 P, 2.3–3.0 L, 5.0–6.0 V). Activation of the IC was accomplished by stimulating with insulated, bipolar stainless-steel electrodes with square-wave constant current stimulus pulses (500–1000 µA; 0.15 ms duration; average frequency, 0.1 Hz) that was generated by an AMPI IsoFlex isolation unit controlled by an AMPI MASTER-8 Pulse Generator (Jerusalem, Israel). We evaluated only those spikes that had greater than 5:1 signal-to-noise ratio and were driven by IC stimulation.
Ethanol Self-administration Procedures
Ethanol self-administration took place in operant chambers (MED Associates Inc., Georgia, VT), equipped with two response levers and with a house light that was illuminated during each self-administration session. Ethanol self-administration was performed in daily 30 min session for five days a week (Monday to Friday) during the dark cycle. Responses on the active lever produced a 0.1 mL drop of 10% (v/v) ethanol solution to one of two drinking cups which was placed in the center panel of the operant chamber, whereas responses on the inactive lever were recorded, but had no consequence. Rats were trained to orally self-administer ethanol using the modification of sucrose fading method, as previously described (Samson, 1986). Rats were initially trained to self-administer a sucrose solution (20% w/v) to facilitate lever pressing. Access to water was restricted, but food remained available in their home cage during the course of sucrose training period. When animals were pressing the lever reliably on a continuous schedule of reinforcement (FR1) for 20% sucrose solution, 10% sucrose solution was used as the reinforcer for lever pressing. At this time water was available. After stable baseline responding was established, the sucrose concentrations were gradually decreased to 0%, and ethanol concentrations were increased to 10%. The detailed procedure was as follows: 3 to 4 sessions with 10% sucrose, 3 sessions with 2% ethanol in 10 % sucrose, 3 sessions with 5% ethanol in 10% sucrose, 4 sessions with 10% ethanol in 5% sucrose. Finally, 10% ethanol alone was presented as the reinforcer. All ethanol solutions were volume/volume. After rats showed stable responding for 10% ethanol and met an established criterion for ethanol baseline responding, behavioral testing was initiated. Baseline responding was defined as the average value in three consecutive responses with less than 20% variation. Typically, this required approximately 6–8 weeks following initiation of sucrose training. Acupuncture was given to rats whose baseline responding had been determined prior to the test session. The effect of acupuncture on ethanol self-administration was evaluated in a between-session and within-subjects Latin square design. Additionally, the effects of naltrindole (15 mg/kg, i.p.) on ethanol self-administration were investigated in different groups of rats using the same sucrose fading procedures.
Sucrose self-administration
For sucrose reinforcement, rats were initially food restricted to maintain 85% of initial body weight and trained to lever-press for 45 mg sucrose pellets on a FR1 schedule for at least 3 consecutive days. After rats demonstrated stable responding, they self-administered a maximum of 50 sucrose pellets per session on a FR1 schedule of reinforcement with a 15 sec time-out period. Following acquisition of FR1, the reinforcement schedule was increased to FR5. During the time-out period, house light and cue light were turned off, and responses on the active lever were recorded, but not reinforced. Following the establishment of the stable responses (<10% latency to consume 50 sucrose pellets for three consecutive days), rats received acupuncture at bilateral HT7 points immediately before the testing. To evaluate the effect of naltrindole on sucrose reinforcement, rats were given naltrindole (15 mg/kg) intraperitoneally 30 min before the start of sucrose self-administration.
Acupuncture Treatments
The anatomical location of acupuncture points stimulated in rats corresponded to the acupoints in man as described previously (Stux et al., 2003) and in animal acupuncture atlas (Schoen, 2001). HT7 is located on the transverse crease of the wrist of the forepaw, radial to the tendon of the m. flexor carpi ulnaris. Neiguan (PC6) is located between the tendons of the m. palmaris longus and flexor carpi radialis, 4 mm proximal to the transverse crease of the wrist of the forepaw. For single-unit studies, acupuncture was accomplished with finely-controlled electrical stimulation. Insulated tungsten sharp microelectrodes (1–5 MΩ; ET-10-5, A–M Systems, Everette, WA) were inserted orthogonally into HT7 around 3 mm from skin surface. For tail stimulation, electrodes were inserted bilaterally at the base of the tail at a 30 degree angle to a depth of 2–3 mm. Activation of acupuncture points and non-acupuncture points was accomplished by stimulating with square-wave constant current stimulus pulses (1.0 msec duration; frequency, 2 Hz) that were generated by an AMPI IsoFlex isolation unit that were controlled by an AMPI MASTER-8 Pulse Generator. The stimulation current was adjusted until muscle twitch was visually detected (300–800 µA) either at the digits for HT7 stimulation or at the tail muscles for Tail stimulation. For acupuncture treatment in freely-behaving rats, acupuncture was accomplished with mechanical stimulation. We have used the same acupuncture paradigm as described by our previous study (Zhao et al., 2006). Stainless-steel needles (0.18 mm diameter and 20 mm length) were inserted vertically to a depth of 3 mm into acupuncture points of rats lightly restrained by hands for 1-min under unanesthetized condition. Since our previous work showed that brief manual acupuncture (1 min) was effective in reducing ethanol-induced dopamine overflow in the nucleus accumbens (Yoon et al., 2004) and electroacupuncture and manual acupuncture may recruit the identical peripheral afferent mechanisms involved in acupuncture analgesia (Zhao, 2008), rats were subjected to manual acupuncture at bilateral HT7 for 1 min. The acupuncture stimulation was manually delivered by twisting acupuncture needles at a frequency of twice per sec for a total of two sec of stimulation while needles were inserted and withdrawn from acupoints. Also, acupuncture was applied at non-acupoints one-fifth of tail length from the proximal region of the tail to avoid the two tail acupoints. In one group of rats the tail was used as a stimulation control site to determine the effect of mechanical stimulation at non-acupoints. In another group of rats, Neiguan (PC6) was used as nonspecific control points. In the control group, rats were lightly restrained by hands as identical method of each acupuncture treatment group but acupuncture was not applied. The rats were pre-handled for 2 min/day for 5 consecutive days before exposure to acupuncture for the reduction of stress.
Drug Preparation and Administration
For single-unit studies, systemic administration of 1.0 g/kg ethanol (16% w/v ethanol in saline) was accomplished by intraperitoneal injections. Naloxone hydrochloride and naltrindole hydrochloride were dissolved in saline at 1.0 mg/mL and 15 mg/mL, respectively, and administered intravenously at a volume corresponding to each rat’s weight in µL/gm. An equal volume of saline was administered intravenously to a paired rat for comparisons of ethanol effects on VTA GABA neuron firing rate.
Analysis of Responses and Statistics
For analysis of single-unit data, discriminated spikes and stimulation events were processed with National Instruments LabVIEW and IGOR Pro software (Wavemetrics, Lake Oswego, OR). Extracellularly recorded single-unit action potentials were discriminated by a peak detector digital processing LabVIEW algorithm. The effects of HT7 stimulation on VTA GABA neuron activity were determined by rectangular activation of ratemeter records over 5 min of stimulation with IGOR PRO software. To determine changes in VTA GABA neuron firing rate produced by ethanol administration, firing rate was determined by averaging 5 min epochs of activity before and 5 min epochs at 10 min after ethanol injection, by rectangular integration of ratemeter records with IGOR PRO software. The results for control and drug treatment groups were derived from calculations performed on ratemeter records and expressed as means ± S.E.M. A paired two sample for means t test was performed to determine statistical significance for within-subject drug vs saline comparisons with Microsoft Excel Statistical Analysis Toolpak and Igor Pro (Wavemetrics, Oswego, OR) Stat Pak with alpha=0.05 and level of confidence of 95%. A simple one-way ANOVA was used to compare the effects of drugs vs. saline for between-subjects firing rate. Figures were compiled by using IGOR Pro Software. For analysis of behavioral testing, statistical analysis of data was performed using the SPSS 11.0 software program. One-way ANOVA and post-hoc Tukey tests were carried out to compare the control group and each acupuncture treatment group. For comparison of sucrose self-administration, a paired t test was performed. Baseline responding was calculated as the mean absolute ethanol-paired lever responding from three consecutive responses exhibiting less than 20%.
RESULTS
Effects of HT7 stimulation on VTA GABA Neuron Firing Rate
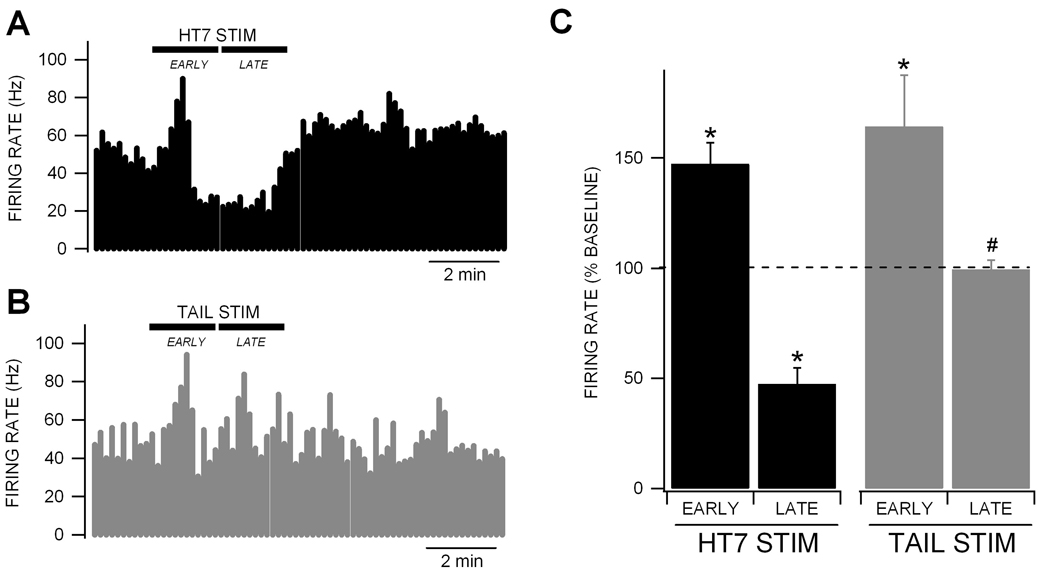
We compared the effects of HT7 and tail electrical stimulation on the firing rate of VTA GABA neurons, which were characterized by previously-established criteria including spontaneous activity, waveform properties, and response to IC stimulation (Steffensen et al., 1998). Both tail and HT7 electrical stimulation (2 Hz at threshold current for muscle twitch) produced transient increases in VTA GABA neuron firing rate, typically at the onset of stimulation (Fig. 1A,B). Of the 55 neurons tested for effects of HT7 stimulation on VTA GABA neuron activity, 24 (44%) showed an initial (EARLY) increase in firing rate (47.3 ± 9.6%) followed by a more sustained (LATE) decrease (52.5 ± 7.3%) in firing rate with recovery in 5 min (Fig. 1C; baseline firing rate for HT7 stimulation was 34.9 ± 2.5 Hz). Of the 15 neurons tested for effects of tail stimulation on VTA GABA neuron activity, 14 (93%) showed an EARLY increase (64.2 ± 23%) in firing rate, similar to HT7 stimulation. However, none showed a LATE inhibition (0.7 ± 4.2%) that was characteristic of HT7 stimulation (Fig. 1C; baseline firing rate for tail stimulation was 35.9 ± 4.2 Hz). While there was no significant difference in EARLY excitation between HT7 and tail stimulation (p > 0.05), there was a significant difference in LATE inhibition between HT7 and tail stimulation (p = 7.1E-06, F(1,30) = 29.8).
Figure 1. Modulation of VTA GABA neuron firing rate by HT7 vs. tail electrical stimulation.
(A) This ratemeter record shows a representative VTA GABA neuron with a baseline firing rate of approximately 50 Hz. HT7 electrical (2 Hz at threshold current for muscle twitch) stimulation produced an initial (EARLY) enhancement of VTA GABA neuron firing rate followed by a more prolonged (LATE) inhibition, with subsequent recovery in 5 min. (B) This ratemeter record shows a ratemeter record from the same neuron during tail stimulation. Tail electrical stimulation produces only an EARLY increase in firing rate. (C) Summary of HT7 and tail stimulation on VTA GABA neuron firing rate. HT7 stimulation produced a significant EARLY excitation (n = 24) followed by a significant LATE inhibition of VTA GABA neuron firing rate (n = 18). Tail stimulation produced only a significant EARLY excitation (n = 14). There was a significant difference between HT7 and tail LATE effects on VTA GABA neuron firing rate. Asterisks * represent p < 0.05 compared to baseline and # represents p = 7.2E-06 between HT7 vs. tail LATE responses.
Effects of Naloxone on HT7 Inhibition of VTA GABA neuron firing rate
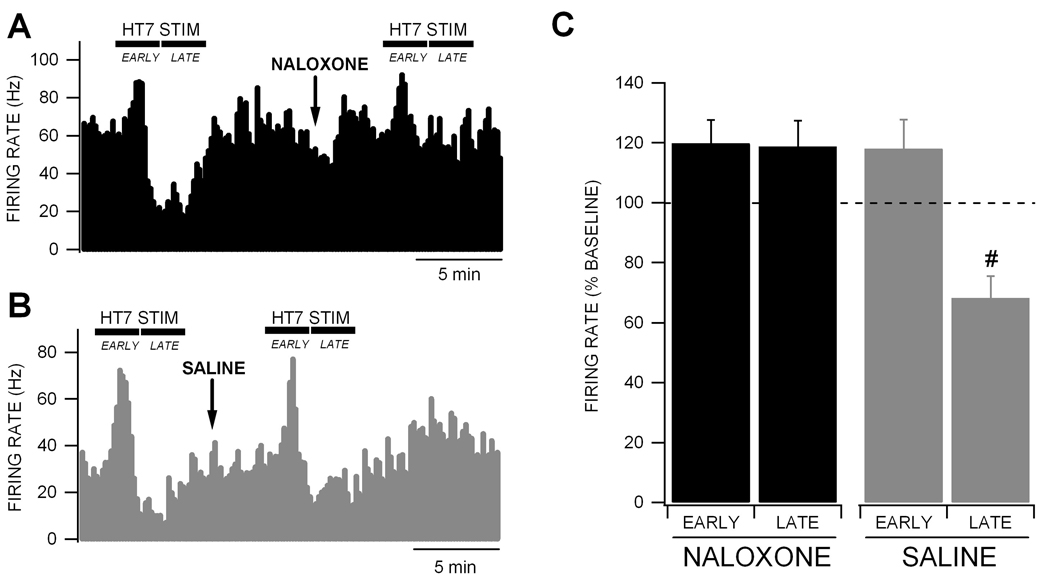
Since HT7 stimulation inhibited VTA GABA neuron firing rate similar to what we have demonstrated previously with opiates (Steffensen et al., 2006), we tested the effects of the non-selective OR antagonist naloxone on HT7 modulation of VTA GABA neuron firing rate. Compared to saline, intravenous administration of 1.0 mg/kg naloxone slightly, but not significantly, increased VTA GABA neuron firing rate (14.5 ± 10.2%; p = 0.5; n = 18). Naloxone blocked the LATE inhibition produced by HT7 stimulation, but did not affect the EARLY enhancement of VTA GABA neuron firing rate (Fig. 2A). Intravenous administration of saline (isovolumic with naloxone injection) had no effect on ability of HT7 modulation to reduce VTA GABA neuron firing rate (Fig. 2B). There was a significant difference between naloxone and saline on the LATE inhibition of VTA GABA neuron firing rate produced by HT7 stimulation (p = 0.006, F(1,11) = 12.1; n = 6,7, respectively).
Figure 2. Naloxone blocks the inhibition of VTA GABA neuron firing rate by HT7 electrical stimulation.
(A) This ratemeter record shows a representative VTA GABA neuron with a baseline firing rate of approximately 65 Hz. Intravenous administration of the µ-OR antagonist naloxone blocked the LATE inhibition, but not the EARLY activation produced by HT7 electrical stimulation. (B) This ratemeter record shows a VTA GABA neuron in a separate experiment with a baseline firing rate of approximately 30 Hz. Intravenous administration of saline (isovolumic to naloxone injection) did not affect either the EARLY or LATE phases of HT7 stimulation. (C) There was a significant difference between saline (n = 7) and naloxone (n = 6) on the LATE inhibition of VTA GABA neuron firing rate. Most importantly, there was a significant difference between naloxone and saline HT7 stimulation LATE effects on VTA GABA neuron firing rate. Asterisks * represent p < 0.05 compared to baseline and # represents p = 7.2E-06 between HT7 vs. tail LATE responses.
Effects of HT7 stimulation on Ethanol inhibition of VTA GABA Neuron Firing Rate
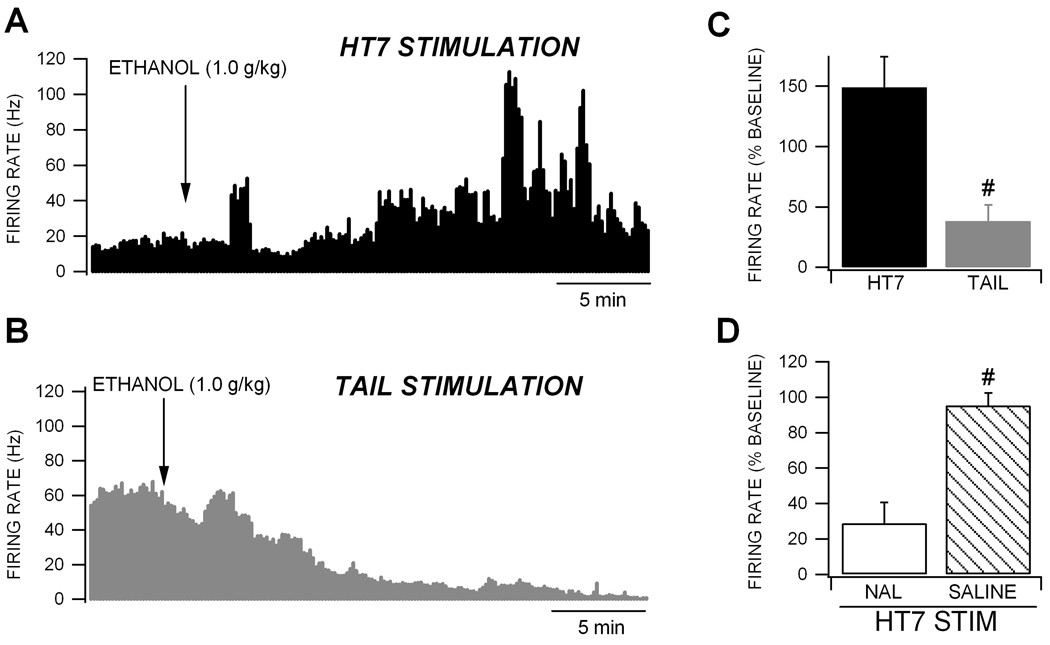
We then evaluated the effects of continuous HT7 and tail stimulation on ethanol inhibition of firing rate of VTA GABA neurons. We have previously reported that acute intoxicating doses of ethanol inhibit VTA GABA neuron firing rate with an IC50 of 1.0 g/kg (Gallegos et al., 1999; Ludlow et al., 2009; Steffensen et al., 2009; Stobbs et al., 2004). Thus, we evaluated the effects of HT7 stimulation at the IC50 dose for ethanol. Ten minutes after HT7 or tail stimulation a dose of 1.0 g/kg ethanol was administered intraperitoneally. Only neurons that were inhibited by HT7 stimulation were studied, only one neuron was studied in each rat and HT7 or tail stimulation was administered continuously throughout each experiment. Figure 3 shows the effects of continuous HT7 (Fig. 3A) or tail (Fig. 3B) stimulation on acute ethanol effects on VTA GABA neuron firing rate. While this dose of ethanol markedly decreased VTA GABA neuron firing rate in rats receiving tail stimulation (61.8 ± 13.6%), there was an increase in firing rate in those receiving HT7 stimulation (48.8 ± 25.6%; Fig. 3C). There was a significant difference in ethanol effects between HT7-stimulated and tail-stimulated rats (Fig. 3C; p = 0.002, F(1,14) = 15.7; n = 7,8, respectively). Since naloxone abolished the inhibition produced by HT7 stimulation, we evaluated its effects on HT7 block of ethanol inhibition of VTA GABA neuron firing rate. Compared to saline, intravenous administration of naloxone (1.0 mg/kg) restored ethanol’s ability to suppress the firing rate of VTA GABA neurons (Fig. 3D; saline vs naloxone: p = 0.001, F(1,17) = 17.1; n = 11,7, respectively).
Figure 3. Comparison between continuous HT7 and Tail electrical stimulation on acute ethanol inhibition of VTA GABA neuron firing rate.
(A) This ratemeter record shows a representative VTA GABA neuron with a baseline firing rate of approximately 19 Hz during continuous HT7 electrical stimulation. Intraperitoneal administration of 1.0 g/kg ethanol produced a prolonged enhancement of this neuron’s activity during HT7 stimulation. (B) This ratemeter shows the firing rate of a VTA GABA neuron in a separate experiment with a baseline firing rate of 60 Hz during continuous tail stimulation. Intraperitoneal administration of 1.0 g/kg ethanol produced its typical inhibition of firing rate (Gallegos et al., 1999; Ludlow et al., 2009; Steffensen et al., 2009; Stobbs et al., 2004). (C) This graph summarizes the effects of HT7 vs. tail stimulation on ethanol effects on VTA GABA neuron firing rate. There was an increase in firing rate produced by ethanol during continuous HT7 stimulation (n = 7). Ethanol produced its typical inhibition during continous tail stimulation (n = 8). There was a significant difference in ethanol effects between HT7 vs. tail electroacupuncture (p = 0.002). (D) There was a significant difference between naloxone vs. saline on HT7 block of ethanol inhibition of VTA GABA neuron firing rate (p = 0.001). # represents p = 0.0008 between naloxone vs. saline effects.
Role for Delta Opioid Receptors in Ethanol Inhibition of VTA GABA Neuron Activity
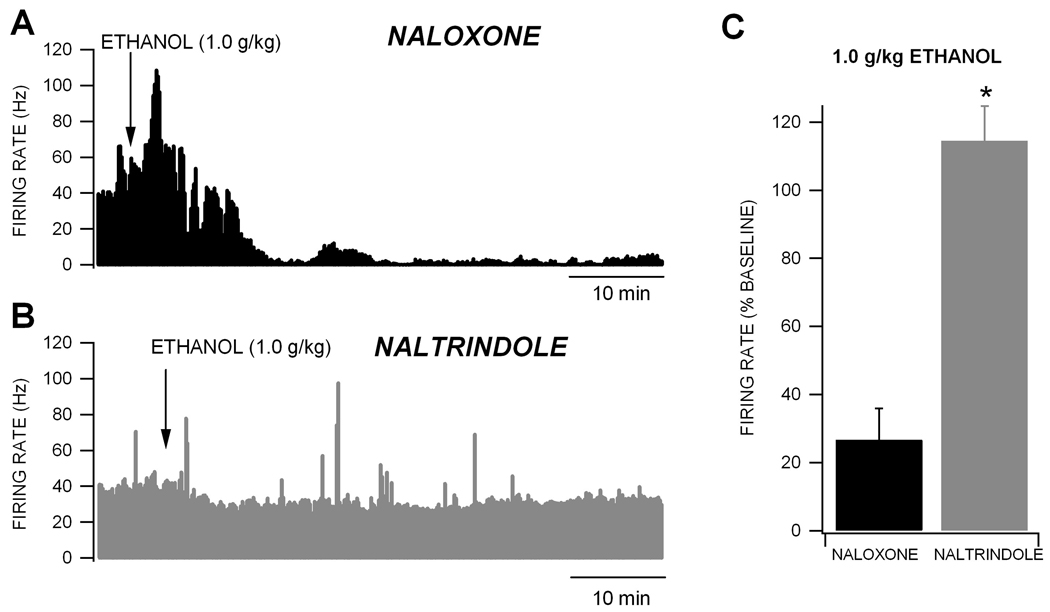
We compared the effects of the non-selective OR antagonist naloxone and the DOR antagonist naltrindole on VTA GABA neuron firing rate and ethanol inhibition of their firing rate. Compared to saline, intravenous administration of 15 mg/kg naltrindole slightly, but not significantly, decreased VTA GABA neuron firing rate (11.5 ± 13.1%; p = 0.5; n = 13). Also, there was no difference between naloxone and naltrindole on baseline firing rate (naloxone vs naltrindole: p = 0.12, F(1,30) = 2.5; n = 13,18, respectively). Naloxone administered 15 min prior to an intraperitoneal injection of 1.0 g/kg ethanol slightly, but not significantly, enhanced ethanol’s inhibition of VTA GABA neuron firing rate (Fig. 4A), Naltrindole (15 mg/kg) administered 15 min prior to an intraperitoneal injection of 1.0 g/kg ethanol abolished ethanol’s inhibition of VTA GABA neuron firing rate (Fig. 4B). There was a significant difference between naloxone and naltrindole for ethanol effects on VTA GABA neuron firing rate (Fig. 4C; naloxone vs naltrindole: p = 0.001, F(1,26) = 13.7; n = 12, 15, respectively).
Figure 4. Naltrindole, but not naloxone, blocks ethanol inhibition of VTA GABA neuron firing rate.
(A) This ratemeter record shows a representative VTA GABA neuron with a baseline firing rate of approximately 37 Hz in the presence of 1.0 g/kg naloxone. Intraperitoneal administration of 1.0 g/kg ethanol produced its typical inhibition of VTA GABA neuron firing rate when naloxone is present. (B) This ratemeter shows the firing rate of a VTA GABA neuron in a separate experiment with a baseline firing rate of 39 Hz in the presence of 15 mg/kg naltrindole, which blocked the inhibition of VTA GABA neuron firing rate by 1.0 g/kg ethanol. (C) This graph summarizes the effects of naloxone and naltrindole on ethanol inhibition of VTA GABA neuron firing rate. Naltrindole significantly reduced ethanol inhibition of VTA GABA neuron firing rate. * represents p = 0.001 between naloxone vs. naltrindole effects.
Effects of Acupuncture at HT7 on Ethanol-reinforced Responding
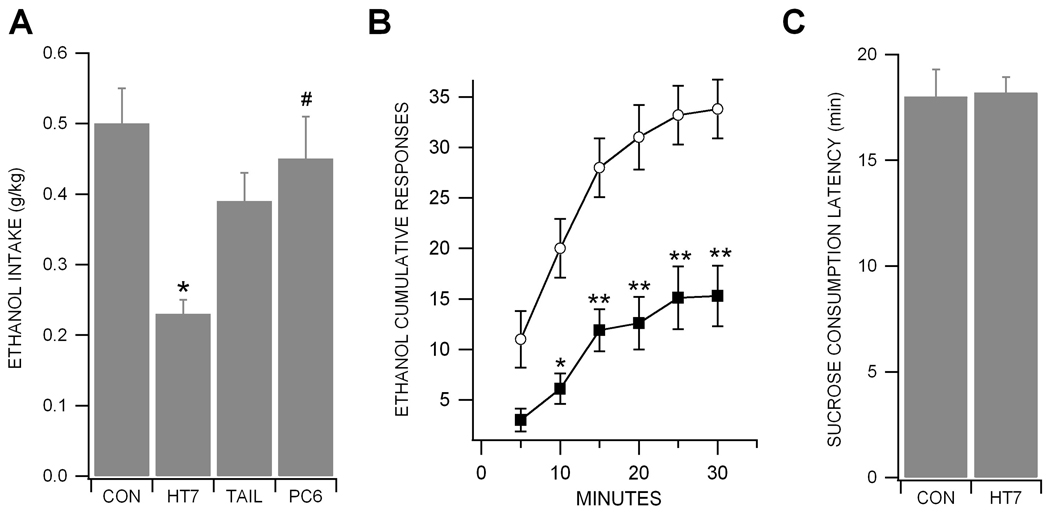
We evaluated the effects of HT7, Tail and PC6 acupuncture on ethanol-reinforced responding. The ethanol responding and the amount of ethanol consumption across the last 3 sessions (baseline) before acupuncture did not vary significantly by group (p > 0.05; control: 27.1 ± 3.0 responses, 0.44 ± 0.05 g/kg, n = 8; HT7: 29.1 ± 2.9 responses, 0.47 ± 0.05 g/kg, n = 8; Tail: 23.8 ± 1.9 responses, 0.50 ± 0.05 g/kg, n = 9; and PC6: 30.6 ± 3.1 responses, 0.42 ± 0.03 g/kg; n = 7). However, one-way ANOVA analysis revealed a significant main effect of acupuncture treatment (F(3,28) = 10.554, p < 0.001). Post hoc Tukey tests showed that there was a significant reduction in the amount of ethanol consumption in the HT7 group compared to the control group (p < 0.01; control = 0.50 ± 0.05 g/kg vs HT7 = 0.23 ± 0.02 g/kg; Fig. 5A). This effect of HT7 did not seem to be a long-term effect as the baseline responding for ethanol returned the following day. Additionally, Post hoc Tukey tests also showed that HT7 acupuncture produced the significant decreased in ethanol-reinforced responding compared to acupuncture at PC6 (p < 0.05; PC6 = 0.45 ± 0.06 g/kg; Fig. 5A). The HT7 group demonstrated initial suppression of ethanol responding and highly significant decreases in cumulative responses starting at 5 min and continuing to the end of the test session at 30 min compared with the control group (Fig. 5B; n = 8). To control for the possibility that acupuncture causes generalized suppression of operant responding, sucrose self-administration was performed using ethanol-naive rats. Before HT7 acupuncture treatment, the baseline values for each treatment group were 20.9 ± 1.13 min for control and 19.5 ± 0.71 min for HT7. Acupuncture at HT7 did not alter the response rate of sucrose self-administration compared to control group (Fig. 5C; t5=0.426, p = 0.208; n = 6). These results suggest that reduction in ethanol-reinforced responding by HT7 acupuncture can not be attributed to motor impairment.
Figure 5. Acupuncture at HT7 reduces ethanol, but not sucrose, reinforced behavior.
(A) Rats manually received the stimulation of bilateral HT7 (Shenmen, n = 8) points for 1 min immediately before testing session. Acupuncture points corresponding to bilateral tail (Tail, n = 9) and PC6 (Neiguan, n = 7) points were used as control points. Results are expressed as the mean ± S.E.M. for the amount of ethanol consumption (in g/kg) during the 30 min self-administration session. HT7, but not tail or PC6, stimulation significantly reduced ethanol intake (*p < 0.01, control group vs. HT7 group; #p < 0.05, HT7 group vs. PC6 group. (B) Cumulative responses for ethanol during the entire 30 min session time after HT7 stimulation (n = 8). HT7 acupuncture significantly reduces ethanol self-administration during the entire session time except 5 min after the starting of session compared to control (n = 8). Asterisks *, ** represent p < 0.01 and p < 0.001 between control group vs. HT7 group, respectively. (C) To control for the possibility that acupuncture affects generalized suppression of operant responding, sucrose pellet self-administration was performed using ethanol-naive rats (n = 6). HT7 acupuncture did not alter the time to self-administer 50 sucrose pellets.
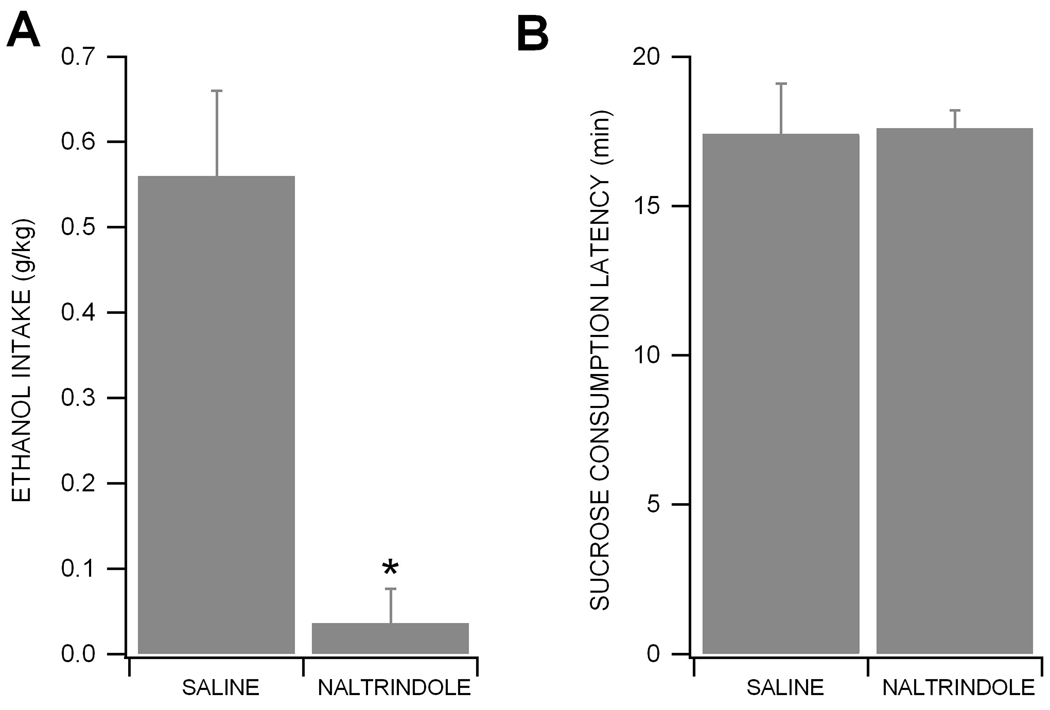
Effects of Naltrindole on Ethanol-reinforced Responding
To determine the effects of the DOR antagonist naltrindole on ethanol consumption, rats were given saline or naltrindole (15 mg/kg) 30 min before the start of ethanol self-administration. There was no significant difference in baseline ethanol consumption between groups (p = 0.213; saline group: 0.61 ± 0.07 g/kg, n = 6; naltrindole group; 0.54 ± 0.08 g/kg, n = 6). Administration of naltrindole markedly reduced ethanol self-administration (t5 = 4.163, p < 0.01; n = 6) without affecting the latency to self-administer 50 sucrose pellets compared to saline group (t4 = 0.1367, p = 0.8978; n = 5), suggesting that naltrindole does not produce generalized effects on response rates.
DISCUSSION
VTA GABA neuron activity was affected by HT7 electrical stimulation, which consistently produced a biphasic modulation of firing rate characterized by a transient enhancement followed by a more prolonged inhibition with recovery in 5 min. Tail stimulation produced only a transient enhancement of firing rate, which we have reported previously occurs in association with generalized sensory stimulation (Ludlow et al., 2009). Most importantly, HT7 inhibition of VTA GABA neuron firing rate was blocked by naloxone, suggesting that stimulation of this acupuncture point activates opioidergic inputs to VTA GABA neurons, perhaps from the acupuncture-sensitive arcuate nucleus (Wang et al., 1990a; Wang et al., 1990b).
The typical inhibition produced by an intoxicating dose of ethanol (Gallegos et al., 1999; Ludlow et al., 2009; Steffensen et al., 2009; Stobbs et al., 2004) was unaffected by continuous tail stimulation, while the same dose level of ethanol excited VTA GABA neurons during continuous HT7 stimulation, suggesting that HT7 acupuncture reverses ethanol’s inhibitory effects on VTA GABA neurons directly, or indirectly through interaction with synaptic inputs to these neurons. Since VTA GABA neurons express MORs, and are inhibited by opioids (Steffensen et al., 2006), and acupuncture enhances opioid-mediated transmission in the arcuate nucleus, which projects to the VTA, we speculated that OR modulation by HT7 electroacupuncture might be contributing to its ability to reduce ethanol inhibition of VTA GABA neurons. Accordingly, systemic administration of naloxone not only blocked HT7 inhibition, but also HT7 block of ethanol inhibition of VTA GABA neuron activity. At first glance this might seem counterintuitive, since HT7 and ethanol both inhibit VTA GABA neuron activity. However, while ethanol inhibition is prolonged, HT7 OR-sensitive inhibition is transient, suggesting desensitization of MORs or parallel involvement of DORs on accumbal GABA terminals to VTA GABA neurons. Acupuncture may play a functional role as an OR agonist as well as a partial DOR agonist. Although naloxone blocked the effects of HT7 acupuncture, as well as its effects on ethanol inhibition of VTA GABA neuron firing rate, its mechanism of action is uncertain given that it is a non-selective OR antagonist. Although MORs are sensitive to naloxone, it likely also blocks κ- and DORs at the high doses used to block HT7 inhibition of VTA GABA neuron firing rate. A role for ORs other than MORs cannot be ruled out. Mainly, its effects could result from a combination of actions on MORs and/or DORs in the VTA, NAcc, or accumbal GABA inputs to the VTA. Accumbal GABAergic inhibitory input to VTA GABA neurons is formidable, and a combined effect on MORs and DORs is likely occurring, as a recent report has implicated DORs on accumbal GABA terminals on VTA GABA neurons in ethanol consumption (Margolis et al., 2008). Interestingly, it is somewhat paradoxical that naloxone had no effect or slightly enhanced ethanol inhibition of VTA GABA neuron firing rate. Thus, in order to clarify the role of DORs in ethanol effects on VTA GABA neuron activity, we compared the effects of naloxone vs the DOR antagonist naltrindole. Unlike naloxone, naltrindole abolished the inhibitory effect of ethanol on VTA GABA neuron firing rate, suggesting the involvement of DORs for ethanol effects in the VTA. However, future studies are necessary to determine if the effect is on DORs on accumbal GABAergic terminals on VTA GABA neurons (Margolis et al., 2008) or on NAcc DORs (Lu et al., 1998; Mansour et al., 1995; Weiner et al., 1991).
Acupuncture at HT7, but not at PC6 or tail, significantly decreased ethanol-reinforced behavior, suggesting that this effect is specific to HT7 acupoints. Since insertion of needles into acupoints on the forepaw could give rise to motor impairment, we attempted to control for the possibility of generalized effects of acupuncture on response rates by monitoring sucrose-reinforced responding. Importantly, results showed that acupuncture did not alter the responding for sucrose pellets suggesting that acupuncture may exert an inhibitory effect on ethanol self-administration by reducing motivational, rather than performance-related influences. As suggested by many studies, ethanol enhancement of DA neurotransmission in the mesolimbic system has long been associated with positive reinforcement contributing to ethanol-seeking and-taking behaviors (Czachowski et al., 2001; Melendez et al., 2002; Rodd et al., 2004; Walker and Ettenberg, 2007; Weiss et al., 1993). In a previous study, we demonstrated that acupuncture at HT7 significantly inhibited ethanol-induced DA release in the NAcc through the GABAB receptor (Yoon et al., 2004). Based on these observations, our results indicate that the reduced ethanol-maintenance response produced by acupuncture at HT7 is most likely mediated via a suppression of DAergic activity in the NAcc.
Results obtained in genetic models of high preference for ethanol support the view that its intake depends on the activity of the endogenous opioid reward system, and that ethanol consumption may serve to compensate for inherent deficits in this system (Herz, 1997). This hypothetical model proposes that reward, including ethanol reward, results from activation of MORs and DORs in the VTA and/or DORs in the NAcc. How might ethanol and opioids interact in the VTA? First, they have similar effects on VTA GABA neurons. We and others have shown that ethanol and MOR agonists inhibit VTA GABA neuron firing rate (Gallegos et al., 1999; Johnson and North, 1992; Ludlow et al., 2009; Margolis et al., 2006; Stobbs et al., 2004; Xiao and Ye, 2008; Xiao et al., 2007), suppress GABA inhibition of VTA DA neurons (Xiao and Ye, 2008; Xiao et al., 2007), and excite VTA DA neurons (Gessa et al., 1985; Johnson and North, 1992; Margolis et al., 2003; Xiao et al., 2007), likely via disinhibition (Johnson and North, 1992; Xiao and Ye, 2008; Xiao et al., 2007). Second, ethanol potentiates GABA IPSCs in VTA DA neurons in the presence of saturating concentrations of MOR agonists (Xiao et al., 2007). Contrary to the typical potentiating effects of ethanol on GABA transmission in other brain areas (Siggins et al., 2005; Weiner and Valenzuela, 2006), ethanol inhibits GABA neurons in the VTA and consequently inhibits GABA IPSCs in VTA DA neurons (Xiao et al., 2007). Since VTA GABA neurons are the primary source of inhibitory input to VTA DA neurons, ethanol inhibition of GABA IPSCs appears to dominate over the potentiating effect of ethanol on other GABAergic or opioidergic inputs to VTA neurons. Third, ethanol enhances the release of β-endorphin (Herz, 1997; Marinelli et al., 2004; Stein, 1993), which might activate MORs on VTA GABA neurons. Thus, acupuncture effects on VTA GABA neurons might result from combined effects on opiodergic actions on VTA GABA neurons via MORs or on accumbal GABA input to VTA GABA neurons via DORs. In support of the latter, a recent study has shown that activation of DORs in the VTA decreases ethanol intake in rats (Margolis et al., 2008). This study implicates presynaptic modulation of GABA release in the VTA by DOR since the DOR agonist DPDPE inhibits evoked, spontaneous and mini GABA IPSCs. Accordingly, ethanol consumption up-regulates DORs, but down-regulates MORs in the VTA (Mendez et al., 2001). Thus, although many studies have reported a role for both MOR and DOR receptors in regulating ethanol intake, the evidence for the involvement of ORs in the VTA remains complex and controversial. Our findings provide some insight into the role of MOR and DOR involvement in ethanol effects and acupuncture modulation of ethanol effects. We show that both acupuncture and naltrindole reverse ethanol inhibition of VTA GABA neurons and reduce ethanol self-administration without affecting performance. We can only speculate that ethanol and acupuncture influence opioidergic mechanisms in both the VTA and NAcc, but that the effect of ethanol on VTA GABA neurons is predominantly mediated via DORs and not MORs.
We have recently proposed a hypothetical model that might explain how HT7 acupuncture might inhibit VTA GABA neuron activity (Yang et al., 2008). In brief, acupuncture or electroacupuncture may activate the ulnar nerve, which lies adjacent to HT7 points (Peuker and Cummings, 2003). Subsequently, sensory stimulation activates enkephalinergic and β-endorphinergic neurons in the arcuate nucleus of the hypothalamus (Wang et al., 1990a; Wang et al., 1990b), and endorphinergic fibers projecting from the arcuate nucleus can in turn activate MORs on VTA GABA neurons (Mansour et al., 1988). The inhibition of VTA GABA neuron firing rate by HT7 stimulation recovered in approximately 5 min, suggesting that opioidergic processes are being recruited to counteract the direct effects of opioid inhibition on VTA GABA neurons. Indeed, our previous studies have indicated that opioid modulation of VTA GABA neurons is complex, involving direct and indirect effects. For example, opioid inhibition of VTA GABA neurons involves not only direct effects on GABA neuron MORs (via in situ MOR agonist activation), but also latent indirect effects on accumbal GABAergic inhibition of VTA GABA neurons (Steffensen et al., 2006), and perhaps influenced by DOR modulation of accumbal GABAergic inhibition on VTA GABA neurons (Margolis et al., 2008). Notwithstanding the complexities of opioid effects on VTA GABA neurons, HT7 inhibition of their firing rate by MOR activation might lead to short-term enhancement of mesolimbic DA release via disinhibition of VTA DA neurons. Taken together, these findings support the hypothesis that opioid-mediated ethanol inhibition of GABAergic synaptic transmission to VTA DA neurons may have a critical role in ethanol reward. Moreover, they support the emerging view in the alcohol literature of an interaction between endogenous opioids and ethanol in the mesolimbic system (Herz, 1997), and the current clinical use of opioid antagonists to prevent relapse in alcoholics (Oswald and Wand, 2004). Finally, acupuncture may be an effective adjunct therapy for the treatment of alcoholism with OR antagonists.
Figure 6. Naltrindole reduces ethanol self-administration.
(A) This figure shows the effects of naltrindole on ethanol self-administration. Intraperitoneal injection of naltrindole (n = 6) but not saline (n = 6) significantly suppressed ethanol-reinforced responding. Data represent the mean ± S.E.M. * represents p = 0.01 between saline vs. naltrindole effects (paired t test). (B) This graph shows the latency to self-administer 50 sucrose pellets. Data represent the mean ± S.E.M. of the time to consume 50 sucrose pellets. Neither saline (n = 5) nor naltrindole (n = 5) did alter response rates of sucrose self-administration.
Acknowledgements
This work was supported by PHS grant AA13666 to SCS and the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund)(KRF-2007-313-E00595).
REFERENCES
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Diana M, Brodie M, Muntoni A, Puddu MC, Pillolla G, Steffensen S, Spiga Little HJ. Enduring Effects of Chronic Ethanol in the CNS: Basis for Alcoholism. Alcohol Clin Exp Res. 2003;27:354–361. doi: 10.1097/01.ALC.0000057121.36127.19. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Criado JR, Lee RS, Henriksen SJ, Steffensen SC. Adaptive responses of GABAergic neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons of the ventral tegmental area. Brain Res. 1985;348:201. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Herz A. Opioid reward mechanisms: a key role in drug abuse? 1998;76:252–258. doi: 10.1139/cjpp-76-3-252. [DOI] [PubMed] [Google Scholar]
- Jindal V, Ge A, Mansky PJ. Safety and efficacy of acupuncture in children: a review of the evidence. J Pediatr Hematol Oncol. 2008;30:431–442. doi: 10.1097/MPH.0b013e318165b2cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, 2nd, Foster KL, McKay PF, Seyoum R, Garcia M, McCane S, Grey C, Hawkins SE, Mason D. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Kim YH, Schiff E, Waalen J, Hovell M. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005;24:115–132. doi: 10.1300/j069v24n04_09. [DOI] [PubMed] [Google Scholar]
- Lu YF, Xu H, Liu-Chen LY, Chen C, Partilla JS, Brine GA, Carroll FI, Rice KC, Lai J, Porreca F, Sadee W, Rothman RB. Opioid peptide receptor studies. 7. The methylfentanyl congener RTI-4614-4 and its four enantiomers bind to different domains of the rat mu opioid receptor. Synapse. 1998;28:117–124. doi: 10.1002/(SICI)1098-2396(199802)28:2<117::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN, Steffensen SC. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin Exp Res. 2009;33:804–811. doi: 10.1111/j.1530-0277.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry. 1999;56:719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Mendez M, Leriche M, Calva JC. Acute ethanol administration differentially modulates mu opioid receptors in the rat meso-accumbens and mesocortical pathways. Brain Res Mol Brain Res. 2001;94:148–156. doi: 10.1016/s0169-328x(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Mereu G, Gessa GL. Low doses of ethanol inhibit the firing of neurons in the substantia nigra, pars reticulata: a GABAergic effect? Brain Research. 1985;348:201–203. doi: 10.1016/0006-8993(85)91249-1. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Peuker E, Cummings M. Anatomy for the acupuncturist--facts & fiction. 3: Upper & lower extremity. Acupunct Med. 2003;21:122–132. [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schoen AM. Veterinary acupuncture : ancient art to modern medicine. 2nd ed. St. Louis, Mo: Mosby; 2001. [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202:139–151. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA. Ventral tegmental self-stimulation selectively induces opioid peptide release in rat CNS. Synapse. 1993;13:63–73. doi: 10.1002/syn.890130109. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves NMDA receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Stux G, Berman B, Pomeranz B. Basics of acupuncture. 5th rev. ed. Berlin; New York: Springer; 2003. [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mao L, Han J. Analgesic electrical stimulation of the hypothalamic arcuate nucleus: tolerance and its cross-tolerance to 2 Hz or 100 Hz electroacupuncture. Brain Res. 1990a;518:40–46. doi: 10.1016/0006-8993(90)90951-7. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mao L, Han J. The arcuate nucleus of hypothalamus mediates low but not high frequency electroacupuncture analgesia in rats. Brain Res. 1990b;513:60–66. doi: 10.1016/0006-8993(90)91088-x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiner S, Shaikh MB, Shaikh AB, Siegel A. Enkephalinergic involvement in periaqueductal gray control of hypothalamically elicited predatory attack in the cat. Physiol Behav. 1991;49:1099–1105. doi: 10.1016/0031-9384(91)90337-n. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors. Neuroscience. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Yang CH, Lee BH, Sohn SH. A possible mechanism underlying the effectiveness of acupuncture in the treatment of drug addiction. Evid Based Complement Alternat Med. 2008;5:257–266. doi: 10.1093/ecam/nem081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SS, Kwon YK, Kim MR, Shim I, Kim KJ, Lee MH, Lee YS, Golden GT, Yang CH. Acupuncture-mediated inhibition of ethanol-induced dopamine release in the rat nucleus accumbens through the GABAB receptor. Neurosci Lett. 2004;369:234–238. doi: 10.1016/j.neulet.2004.07.095. [DOI] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RJ, Yoon SS, Lee BH, Kwon YK, Kim KJ, Shim I, Choi KH, Kim MR, Golden GT, Yang CH. Acupuncture normalizes the release of accumbal dopamine during the withdrawal period and after the ethanol challenge in chronic ethanol-treated rats. Neurosci Lett. 2006;395:28–32. doi: 10.1016/j.neulet.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]