Abstract
Altered dynorphin opioid peptide systems contribute to increased ethanol self-administration during withdrawal following chronic alcohol exposure. We previously identified that the κ-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), selectively reduced ethanol self-administration in dependent animals. The purpose of this study was two-fold: 1) determine whether peripherally-administered nor-BNI could reduce dependence-induced ethanol self-administration and 2) confirm the selective κ-opioid effects of nor-BNI by administering it 24-hrs prior to ethanol self-administration sessions occurring during acute withdrawal. Nor-BNI decreased ethanol self-administration in ethanol-dependent animals, with no effect in nondependent animals. Thus, the κ-opioid/dynorphin system is a viable pharmacotherapeutic target for the treatment of alcoholism.
Keywords: Alcohol, Dependence, Dynorphin, nor-binaltorphimine (nor-BNI), Self-administration, Withdrawal
The comorbidity of affective disorders in individuals diagnosed with an alcohol use disorder is high and nearly 33% of those classified with alcoholism have experienced major depression (Roy et al. 1991). The opioid peptide dynorphin (DYN) is the endogenous ligand for the κ-opioid receptor (KOR) and this system has been implicated in affective responses and escalated ethanol self-administration resulting from ethanol dependence. Administration of KOR agonists induces depressive-like states (e.g., Todtenkopf et al. 2004), increased DYN transmission has been linked to dysphoria (Land et al. 2008) and DYN expression is upregulated in motivational and affective neurocircuitry by chronic ethanol exposure (e.g., Lindholm et al. 2000). Furthermore, acute intracerebroventricular administration of the KOR antagonist, nor-binaltorphimine (nor-BNI), has been shown to selectively reduce escalated ethanol self-administration in dependent animals compared to non-dependent animals (Walker and Koob, 2008).
The purpose of the present study was: 1) to confirm that systemic administration of nor-BNI shows efficacy for reducing ethanol self-administration in dependent animals and 2) to address any concerns regarding the specificity of nor-BNI for the κ-opioid receptor that might be raised with acute administration (Shippenberg et al. 2007).
Adult male Wistar rats (Charles River Laboratory, Kingston, NY) were housed and trained to self-administer 10% ethanol (w/v) using a sweetener fading technique as described in Walker and Koob (2008). Once operant ethanol self-administration stabilized, the animals were split into two groups (n=9/grp) that were matched for ethanol self-administration; one designated as the ethanol exposure group and the other the air-exposed control group. Dependence was induced using an intermittent vapor exposure schedule (14 h on/10 h off) for one-month (see Walker & Koob 2008 for a more detailed description). Target BALs were 175-225 mg% and plasma alcohol levels were determined using the Analox micro-stat GM7 (Analox Instruments, Lunenberg, MA) twice weekly.
Following dependence induction, the animals were tested in 30 min self-administration sessions twice weekly 6 h into withdrawal from ethanol vapor exposure (i.e., 6 h after the ethanol vapor was terminated for that day). Once ethanol self-administration stabilized for 3 test sessions, a vehicle (saline) injection (1 ml/kg, SC) was administered 24 h prior to the next self-administration session to confirm the lack of a vehicle effect (by this session, the length of vapor exposure corresponded to ~7 weeks). Nor-BNI (generously provided by the National Institute on Drug Abuse) was administered SC in 5 mg/kg doses 24 h prior to ethanol self-administration sessions over four test sessions. Following the four nor-BNI-treated ethanol self-administration sessions, the animals continued to have access to semiweekly self-administration sessions that were vehicle-pretreated (as described above).
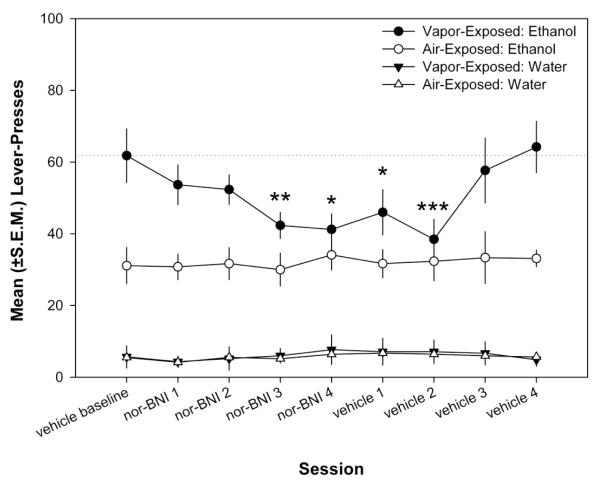
Following one month of intermittent vapor-exposure, dependence was induced as evidenced by the significantly escalated alcohol self-administration in the vapor-exposed group and prior to pharmacological challenges both the non-dependent and dependent animals showed stable responding (<10% deviation) for 3 sessions. The effects of nor-BNI on nondependent and dependent ethanol self-administration following four sessions of drug treatment and five sessions of vehicle treatments (one prior to and four following the nor-BNI challenges) are presented in Fig. 1. Using a mixed-factor two-way ANOVA, a main effect of session (F8,128 = 3.471, p ≤ 0.001) and a Session × Exposure interaction (F8,128 = 3.384, p ≤ 0.001) were found, indicating that ethanol responding was selectively altered by nor-BNI in dependent animals. Water responding was unaffected by nor-BNI for both the nondependent or ethanol-dependent animals.
Figure 1.
Mean (±S.E.M.) responses for ethanol and water following either vehicle or nor-BNI (5 mg/kg) pretreatments during acute withdrawal. Vapor-exposed animals selectively decreased responding (*p < 0.05, **p < 0.01, ***p < 0.001 compared with vehicle baseline).
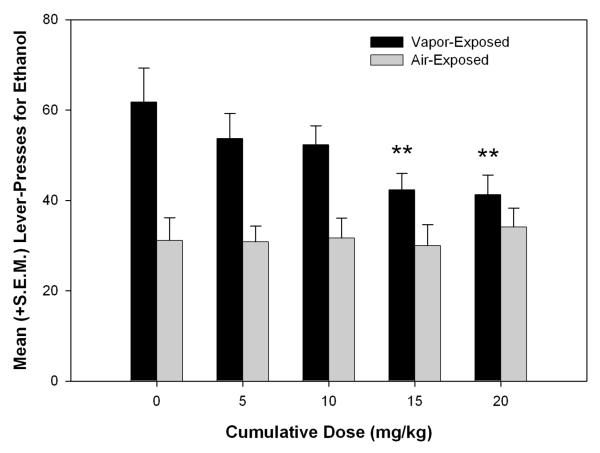
To evaluate changes in ethanol self-administration induced by different cumulative doses of nor-BNI compared with vehicle pretreatment (see Fig. 2), a two-way ANOVA was conducted. The ANOVA showed a main effect of dose (F4,64 = 3.359, p < 0.05) and exposure (F1,16 = 11.456, p < 0.01) and a Dose × Exposure interaction (F4,64 = 4.283, p < 0.01). Individual one-way ANOVAs showed a main effect of nor-BNI dose only in dependent animals (F4,32 = 6.046, p ≤ 0.001). Fisher’s LSD tests revealed that both the 15 and 20 mg/kg cumulative doses significantly suppressed responding for ethanol (p < 0.01).
Figure 2.
Mean (+S.E.M.) cumulative dose-response curve for ethanol self-administration following either vehicle or nor-BNI (5-20 mg/kg) pretreatments during acute withdrawal (**p < .01 compared with vehicle dose).
Two weeks of nor-BNI administration progressively attenuated the excessive alcohol self-administration observed in ethanol dependent animals compared to non-dependent controls. At cumulative doses of 15 and 20 mg/kg, nor-BNI selectively attenuated responding for ethanol in dependent animals while leaving control responding intact. The selective suppression of ethanol responding in dependent animals is consistent with previous behavioral data following acute ICV administration of nor-BNI (Walker & Koob 2008).
To understand the nature of the experimental design and results of the present study, the pharmacological (i.e., pharmacodynamic and/or pharmacokinetic) properties of nor-BNI must be considered. Critically important is nor-BNI’s long duration of action that can last for weeks (Broadbear et al. 1994). In the present study, nor-BNI was administered over a 2 week period under the assumption that additive effects would occur and a cumulative dose could be determined that was efficacious at reducing operant ethanol self-administration during acute withdrawal in dependent rats.
Another aspect of nor-BNI that was considered when designing the present study is evidence suggesting that nor-BNI has affinity for not only the KOR, but also mildly for the μ-opioid receptor (MOR) immediately after administration that appears to last at least 2 h (Broadbear et al. 1994) and more selective antagonism at the KOR 24 h after administration than 1 h after administration (Broadbear et al. 1994). Thus, in the present study, nor-BNI was administered 24 h prior to the acute withdrawal test sessions to address any concerns regarding specificity that might be raised with acute administration (Shippenberg et al. 2007). Notably, however, the transient MOR affinity of nor-BNI that has been observed in previous studies using mice (e.g., Broadbear et al. 1994) has not been replicated in rats (Picker et al. 1996). In addition, when administered immediately prior to ethanol self-administration sessions, nor-BNI did not impact rates of responding for ethanol in non-dependent animals as would be expected of an antagonist with a MOR mechanism of action (Gonzales & Weiss 1998; Walker & Koob 2008). Thus, it is questionable whether extended pretreatments to avoid an initial MOR affinity are truly necessary as both acute (immediately prior; Walker & Koob 2008) and extended (24 hrs prior; present experiment) pretreatments selectively attenuate increased ethanol self-administration in dependent animals.
In summary, dependence-induced increases in ethanol self-administration are selectively ameliorated by KOR antagonism. DYN systems may be recruited during the transition to dependence and thus produce a negative emotional state in the absence of alcohol during withdrawal. By blocking the DYN system, one reduces the negative emotional state that motivates an organism to continue consuming alcohol. The need for further study of alcohol-induced negative emotional states and depressive-like behavior is highlighted by the observation that no current treatments target negative emotional/affective states in those with a history of alcoholism (Heilig & Koob 2007). Thus, KOR antagonism could be a viable pharmacotherapeutic target for the treatment of alcohol dependent individuals.
Acknowledgments
Research was supported by National Institutes of Health grants AA08459, AA012602 (GFK) and AA014723 (BMW) from the National Institute on Alcohol Abuse and Alcoholism and the Pearson Center for Alcoholism and Addiction Research. The authors are grateful to Kevin Gormley (Chemistry and Physiological Systems Research Branch, National Institute on Drug Abuse) for assistance in obtaining nor-BNI. The authors would additionally like to thank Maury Cole for his technical assistance and Mike Arends for his editorial support.
References
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. PM:7871070. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. Journal of Neuroscience. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. PM:9852601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. PM:17629579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Journal of Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. PM:18184783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. PM:11163124. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behavioural Pharmacology. 1996;7:495–504. PM:11224446. [PubMed] [Google Scholar]
- Roy A, DeJong J, Lamparski D, George T, Linnoila M. Depression among alcoholics. Relationship to clinical and cerebrospinal fluid variables. Archives of General Psychiatry. 1991;48:428–432. doi: 10.1001/archpsyc.1991.01810290040007. PM:2021295. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacology and Therapeutics. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. PM:17868902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. PM:14727002. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. PM:17473837. [DOI] [PMC free article] [PubMed] [Google Scholar]